
Special Article - Coagulopathy
Thromb Haemost Res. 2021; 5(3): 1063.
Measurement of Bivalirudin Thrombin Inhibition Activity in Plasma with Clotting or Chromogenic Assays and Dedicated Calibrators and Controls
Amiral C¹, Dunois C¹ and Amiral J²*
¹Hyphen BioMed, Neuville sur Oise, France
²SH (Scientific-Hemostasis), Franconville, France
*Corresponding author: Jean Amiral, SH (Scientific-Hemostasis), Franconville, France
Received: March 26, 2021; Accepted: May 11, 2021; Published: May 18, 2021
Abstract
Bivalirudin is a parenteral direct thrombin inhibitor anticoagulant and does not induce any impairment of the Protein C pathway, which function remains preserved. This drug meets increasing applications for cardiac surgery and heart diseases, especially when heparin is contra-indicated in presence of heparin-induced thrombocytopenia. Major indications concern Extra Corporeal Circulation, PCI/PTCA, and myocardial infarction. Drug clearance occurs partly through kidney. Patients with moderate or severe renal dysfunctions are exposed to drug accumulation and subsequent bleeding, the major adverse effect reported. This study presents 2 automated assays, a clotting method, and a kinetics chromogenic technique, proposed for the quantitative measurement of bivalirudin in citrated plasma. Both assays need a specific bivalirudin calibration, are fully automatable on coagulation instruments, and can be available at any time in specialized clinical laboratories for an on time monitoring of treated patients. Assay ranges are from 0.3 to 5.0 μg/ml (clotting assay) or to 6.0μg/ml (chromogenic assay), and up to 20.0μg/ml with an additional automatic plasma dilution. These methods offer excellent performances, with good reproducibility and repeatability. This study reports the results obtained with both assays on bivalirudin measurements in 26 treated patients collected at 4 timings. Both methods are fully consistent and contribute to facilitate and secure the use of this anticoagulant when it is indicated.
Keywords: Bivalirudin; Thrombin inhibition; Clotting method; Chromogenic assay; Monitoring; Dosage adjustment
Introduction
Hirudin is a small protein anticoagulant synthesized by Hirudo Medicinal is leeches, and present in salivary glands. The anticoagulant activity was first identified at the end of the XIXth century [1,2], and the active principle was isolated at the very early of last century [1]. Hirudin was purified to a crystalline form by Markward at the middle of the XXth century [3,4]. Its mode of action is a direct and specific inhibition of thrombin, and this suggested that this protein could become a promising anticoagulant drug [5]. However, the low concentration extracted from leech saliva glands highly restricted its potential use. Hirudin is composed of 65 Amino Acids (AA), with a Molecular Weight (MW) of 7044Da. Genetic engineering permitted the development of recombinant hirudin, available at large amounts and at an affordable price [6]. This opened the possibilities to use this product as a parenteral anticoagulant for the control of thrombotic diseases in emergency situations [5,7,8]. In practice, a slightly modified recombinant hirudin, called lepirudin, was produced through yeast fermentation (Pichia pastoris) and was purified from culture medium. Like hirudin, lepirudin has 65 AA, but a slightly lower MW of 6979Da, a modified N-terminal AA and lacks a sulfate group. It was introduced as an anticoagulant (with the Refludan® brand) for patients with Heparin Induced Thrombocytopenia (HIT), when heparin is contra-indicated in cardiac surgery with Extra Corporeal Circulation (ECC) [7,9]. Many adverse anaphylactic and bleeding complications were reported for lepirudin, although its use in some clinical indications was considered as safe as or even safer than heparin [8,10-14]. In addition, no antidote was available. Its half-life in blood circulation is short and increases in presence of renal insufficiency. If bleeding occurred during lepirudin treatment, use of procoagulant drugs like Novoseven®, i.e. activated factor VII (FVIIa), was recommended [15]. In 2012, lepirudin manufacturer withdraw this drug as the consequence of raw material supply discontinuation. Concomitantly, various hirudin-derived molecules were developed, and expected to be easier to handle. Among them, bivalirudin became the most promising [16-18]. This drug is a much smaller molecule, composed only of the 2 AA sequences required for thrombin inhibition and exocite I binding, and is now available as Angiox® or Angiomax®.
Bivalirudin is a synthetic 20 AA peptide, with a thrombin inhibitory N-terminal sequence (D-Phe-Pro-Arg-Pro) linked through 4 glycines to a C-Terminal sequence, corresponding to the 11 AA located at positions 53 to 64 of lepirudin. That sequence binds to the fibrinogen-binding region (exocite I) of thrombin [16,17,19,20]. Bivalirudin inhibits thrombin in a highly specific, non-competitive, manner, and is cleaved by thrombin itself or other blood proteases. This cleavage results in the release of the inhibitory N-terminal sequence and decreases the C-terminal sequence affinity for thrombin exocite I. Cleaved bivalirudin then becomes a competitive inhibitor of thrombin, as represented on Figure 1. Conversely, to lepirudin, which forms a stable irreversible complex with thrombin inhibiting its Protein C (PC) activation capacity, bivalirudin has a controlled inhibition of thrombin, and PC pathway is preserved. In addition, thanks to its small size, it penetrates clots for inhibiting trapped thrombin, what heparin is unable to achieve [21]. Bivalirudin is widely used for heart surgery, especially when Extra-Corporeal- Circulation (ECC) is required [22-25], including Cardio-Pulmonary Bypass (CPB) and Extra Corporeal Membrane Oxygenation (ECMO). Preferred indications concern patients developing Heparin Induced Thrombocytopenia (HIT), for whom heparin is contra-indicated [26,27]. In addition, some studies reported a safer anticoagulation obtained with bivalirudin for Percutaneous Coronary Interventions (PCI/PTCA) and treatment of myocardial infarction with elevated ST segment [28,29]. A well-controlled and safe use of bivalirudin requires monitoring its anticoagulant activity in plasma. Two laboratory methods, already reported for testing dabigatran, a direct oral thrombin inhibitor, have been adjusted for measuring this drug [30]. Dedicated plasma calibrators and controls are required. Both assays allow monitoring bivalirudin concentration in plasma, without any interference from other factors influencing clotting times.
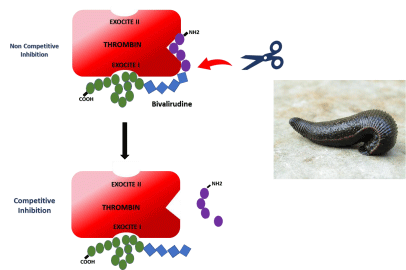
Figure 1: Mode of action of Bivalirudin, a synthetic 20 AA peptide, derived from the “Hirudo Medicinalis” leech anticoagulant hirudin, for the inhibition of thrombin:
first bivalirudin binds to and inhibits the thrombin active site, and also reacts with its exocite I; inhibition is noncompetitive; then, the amino-terminal sequence is
cleaved by thrombin itself or other blood proteases, and the thrombin active site is re-exposed, whilst a competitive weaker inhibition remains through the binding
to exocite I; this exquisite mechanism allows preserving the protein C activation capacity of thrombin, when bound to the endothelial cell thrombomodulin in
microcirculation.
Materials and Methods
Laboratory instruments
Clotting assays were performed using the following coagulation instruments: CS-5100 from Sysmex Corp. (Kobe, Japan) and STARMax from Diagnostica Stago (Asnières, France). Clot formation is detected optically for CS-5100, through the measurement of the change in absorbance induced by clotting and measured at a wavelength of 660nm. Clot detection is mechanical for STA-R: a small metal ball introduced in the assay milieu moves in a magnetic field and immobilizes when clotting occurs.
Clotting and chromogenic assays for measurement of thrombin inhibitors
HEMOCLOTTM Thrombin Inhibitors (HTI) is a clotting method designed as follows: the assayed diluted patients’ plasma is first mixed with a normal plasma pool, then clotting is triggered by the addition of a constant concentration of a-thrombin, human, in presence of calcium, and Clotting Time (CT) is recorded. This method involves 2 reagents: a lyophilized normal plasma pool; and lyophilized thrombin with calcium, used at about 1NIH/ml, the exact concentration being adjusted for each lot. Both reagents are restored with distilled water just before use. The test is performed at 37°C: in the test cuvette, 50μl of the assayed plasma, diluted 1:10 with physiological saline, are mixed with 100μl of the plasma pool, then 100μl of the thrombincalcium reagent are added; the clotting time is measured. Higher is the bivalirudin concentration, and higher is the amount of thrombin inhibited, and CT is prolonged in a dose-dependent manner. CT is then a direct relationship of bivalirudin concentration in the tested sample. The method can be used with mechanical or optical clot detection instruments.
BIOPHENTM Direct Thrombin Inhibitor (DTI) is a kinetics chromogenic assay performed at 37°C. The protocol for CS 5100 instrument is as follows: in the assay cuvette, 80μl of the assayed plasma sample diluted 1:2 with physiological saline are incubated with 80 μl of the thrombin substrate at a 1.5 mg/ml concentration, then 80μl of the thrombin reagent, at 3NIH/ml, are added. Thrombin cleaves the chromogenic substrate, and releases para-Nitro-Aniline (pNA), with an inverse relationship of the bivalirudin concentration present in the tested sample. The change in absorbance resulting from pNA release is measured kinetically at 405nM from 15 to 45 seconds following addition of thrombin. A similar protocol is used for STA-R instrument, but sample dilution is 1:3, and reagent volumes are of 100μl each. Measurement wavelength is also at 405nM. The exact reactants’ concentrations are adjusted for each lot to ensure constant performances. Color development measured is an inverse relationship of bivalirudin concentration.
Bivalirudin plasma calibrators and controls
Bivalirudin plasma calibrators were obtained by adding bivalirudin, at concentrations ranging from 0 to 5.0 μg/ml, to citrated plasma, then distributed at 1.0ml into siliconized glass vials and lyophilized. Five levels are currently prepared. Plasmas are bulked with glycine, hepes and mannitol excipients for lyophilization. Before use, vials are restored with 1.0ml of distilled water.
Quality control plasmas were prepared in a similar way at 2 final concentrations of bivalirudin (QC1 and QC2) of about 1.0 and 3.5 μg/ml (measured exactly for each lot). They are used in each measurement series to check the right performance of the assay, and must be within the established acceptance ranges.
Traceability of plasma bivalirudin calibrators is ensured through a reference preparation, established using 2 independent sources of bivalirudin raw material: the first one from Polypeptide (Strasbourg, France) and the second one from Selleck (Houston, USA), both obtained through peptide synthesis. Both products are provided with the full analysis characteristics and purity grade, which allows knowing accurately the amount of bivalirudin in the weighted powder. A stock solution is prepared by dissolving peptides in 1% Bovine Serum Albumin (BSA) and 0.15M sodium chloride diluent, and supplemented in the plasma pool at the expected concentrations. Various lots from the 2-bivalirudin sources were used for verifying the product homogeneity and lot-to-lot variability.
Interferences
Interferences were evaluated using hemoglobin, bilirubin, and triglycerides/intra lipids, from Sigma-Aldrich, St Louis, Mo, USA. Rivaroxaban was from Bayer (Berlin, Germany), Apixaban from BMS (Princeton, NJ, USA), Edoxaban from Daichii (Basking Ridge, NJ, USA), UFH and LMWH were from Sanofi (Paris, France).
Normal and patients’ plasmas
Normal plasmas were supplied either by Establishment Français du Sang (EFS, Strasbourg, France), or by BioMex (Heidelberg, Germany), and were obtained from blood gift pouches or through apheresis. All donors gave their informed consent. Patient’s plasmas, anonymized, were from the remaining leftover tube of citrated samples from an Angiox® clinical study performed at Lariboisière University Hospital (Paris, France), and were kindly provided by professor L. Drouet and Dr C. Bal Dit Sollier. Plasma samples were from 26 patients, with 4 different sampling times for each patient, i.e. a total of 104 samples.
Statistics
Statistical analysis was performed using the Analyze-it software, especially for calculating CVs, LOQ, and comparison studies.
Results
Quantitative clotting assay
The HTI assay allows measuring bivalirudin concentrations in citrated plasma over a dynamic range from 0 to 5.0 μg/ml, with the standard protocol used. For higher concentrations, a complementary plasma dilution can be prepared. As calibration curve example for the assay, Figure 2A shows the standard dose-response curve obtained for the HTI assay, using the CS-5100 instrument. The dynamic assay range is from 0.1 to 5.0 μg/ml, or up to 20.0μg/ml with automatic redilution. The Lower Limit of Quantitation (LOQ) is of 0.3μg/ml. The assay offers a good reproducibility and reliability, and Table 1 shows the intra-and inter-series Coefficients of Variability (CV), which remain always below 4%. No interference was noted from bilirubin, hemoglobin, or intra-lipids. In addition, there was no interference of direct or indirect anti-FXa drugs, including Rivaroxaban, Apixaban, Edoxaban, UFH and LMWH. As expected, interferences were observed with Anti-Thrombin drugs, like Dabigatran. The assay can be used on any automated coagulation instrument.
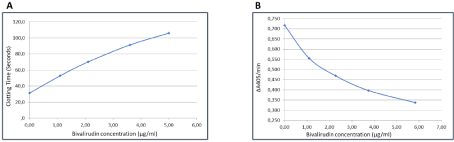
Figure 2: Bivalirudin dose response curves obtained with HemoclotTM Thrombin Inibitors (A) or BiophenTM DTI (B) using the Sysmex CS 5100 instrument, with a
dynamic range from 0 to 5.0 μg/ml for HTI, and 0 to 6.0μg/ml for BDTI.
Precision
BIOPHENTM DTI
HEMOCLOTTM Thrombin Inhibitors
CS-5100
STA-R® MAX
CS-5100
STA-R® MAX
Intra-assay
N
40
10
5
40
QC1
Mean (μg/mL)
1.57
1.50
1.60
1.39
CV%
3.2
5.1
2.1
3.6
QC2
Mean (μg/mL)
4.05
3.80
3.89
4.07
CV%
3.2
1.2
1.5
2.1
Inter-assay
N
30
8
8
30
QC1
Mean (μg/mL)
1.64
1.70
1.64
1.59
CV%
2.4
6.3
4.6
3.3
QC2
Mean (μg/mL)
4.10
4.36
4.10
4.14
CV%
2.6
2.2
2.1
3.1
Table 1: Performance of BIOPHENTM DTI and HEMOCLOTTM Thrombin Inhibitors reagents on various analyzers and evaluated using 2 control plasmas, with a low (QC1) and a high (QC2) bivalirudin concentration; the mean values, and intra- and inter-series CVs are presented; for inter-series: n=30 for 10 runs, 5 days or n=8 for 4 runs, 4 days.
Kinetics chromogenic method
DTI method is designed for measuring all direct thrombin inhibitors, and a dedicated drug-specific calibrator is needed for each product tested. It is a kinetics chromogenic assay, with a dynamic range from 0 to 6.0 μg/ml. An example of the calibration curve is shown on figure 2B for the test performed on CS-5100. For higher bivalirudin concentrations, a redilution can be performed using a normal plasma pool, to avoid any matrix effect. The method can be automated on any laboratory instrument. The lowest limit of detection is of 0.3mg/ml, and intra-assay or inter-assay CVs remain below 4%, as shown on Table 1.
For both assays, concentrations higher than the dynamic range can be measured up to 20.0μg/ml by including a manual redilution or using an automatic redilution depending on the instrument used, as shown on Figure 3A and 3B. Redilution is performed with physiological saline for HTI and with a normal plasma pool for DTI to keep the assay matrix requested by the method.
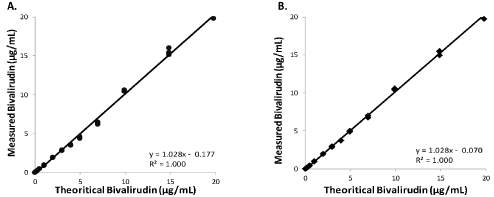
Figure 3: Linearity ranges for the BIOPHENTM DTI - Bivalirudin (A) and HEMOCLOTTM Thrombin Inhibitors - Bivalirudin (B) from 0 to 20 μg/ml. Diagrams present
the linear regression analysis for bivalirudin following preparation of a concentration range, obtained by spiking the drug in plasma.
Plasma calibrators and controls
Assays’ calibrations are currently performed with freeze-dried bivalirudin plasma calibrators, restored with 1.0ml distilled water. The exact bivalirudin concentration in each plasma calibrator is assigned after lyophilization, using a plasma pool spiked with concentrations of well-defined bivalirudin material. This evaluation allowed validating the accurate recovery of bivalirudin supplemented in normal citrated plasma (recovery of 100 +/- 8 % on the range 0.5 to 5.0μg/ml). There is no WHO or Pharmacopeia reference material available at this stage. To ensure the right and accurate concentration for each new calibrator lot, 3 different bivalirudin lots from 2 different suppliers were used: for each lot, the exact concentration was established by weight and according to the purity grade provided on the analysis certificate. Finally, the 2 Polypeptide lots and the Selleck’s one allow measuring the same concentrations, in line with the supplemented bivalirudin amounts in lyophilized plasmas. This process allowed defining an internal reference preparation, which was then used to assign the bivalirudin concentrations for all other lots.
Correlation studies
Bivalirudin was measured in citrated left-over plasmas from the 26 patients included in an Angiox clinical study and from healthy donors. Kinetics courses of bivalirudin concentrations were tested in these patients (with 4 kinetics times per patient, i.e.; a total of 104 samples). Concentrations were measured with DTI chromogenic assay, and HTI method on either STA-R Max or CS 5100 instruments. Correlation diagrams for bivalirudin concentrations measured with both assays on CS-5100 instrument or STA-R are presented on Figure 4. The same results’ homogeneity and comparability was obtained for each method, whether used on CS-5100 or STA-R. As expected, bivalirudin was measured always below LOQ in plasmas from healthy individuals. On study samples, almost all concentrations measured ranged from 0.0 to 12.0 μg/ml, with few samples [7] presenting concentrations >24μg/ml. Equivalent results were obtained whether the samples were tested with DTI or HTI methods and using the CS- 5100 or STAR-Max instruments. Both assay methods are robust and reliable for measuring bivalirudin anti-thrombin activity in citrated plasma.
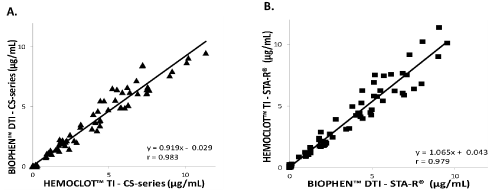
Figure 4: Correlation results of tested samples in this study (26 patients collected at 4 different timings) and shown by regression analysis, using both methods
on CS-5100 and STA-R® Max. (A) Correlation of BIOPHENTM DTI-bivalirudin and HEMOCLOTTM Thrombin Inhibitors-bivalirudin on CS-5100 (B) Correlation of
BIOPHENTM DTI - Bivalirudin and HEMOCLOTTM Thrombin Inhibitors - Bivalirudin on STA-R® Max. Statistical significance was defined with hypothesis test yielding
a P-value <0.05. Both assays measure bivalirudin activity similarly.
Discussion
Bivalirudin is a parenteral anticoagulant with increasing applications for patients with open-heart surgery and ECC, either CPB or ECMO and PCI/PTCA, but also for some myocardial infarctions [18,22,26,34,35]. The preferred application concerns especially cases where heparin must be withdrawn in presence of HIT [26,27]. However, bivalirudin indications are now extended to other heart and vascular interventions or diseases [22,23,28,35], more especially in North America. This drug does not present the undesired side effects of lepirudin.
In presence of bivalirudin, the thrombin capacity to activate protein C when it binds to thrombomodulin in microcirculation is preserved. Furthermore, in contrast with heparin, a catalytic indirect inhibitor requiring Antithrombin (AT) that cannot penetrate fibrin clots for neutralizing trapped thrombin, hirudin and bivalirudin can migrate into clots and inhibit its activity. This prevents from generation of procoagulant potential with clot lysis, as demonstrated by the lack of generation of Thrombin-Antithrombin (TAT) complexes [21]. Bivalirudin is reported to be as safe as or safer than UFH. Its major advantage is its limited time of action, and the preservation of protein C activation pathway. In addition, thrombin inhibition is reversible because bivalirudin can be cleaved by thrombin itself or other proteases. It then becomes a competitive inhibitor, through the interaction of cleaved peptide with the thrombin exocite I. This represents a high superiority as compared to the former hirudin derived drug, lepirudin, which formed an irreversible complex with thrombin, and blocked the protein C pathway. However, use of bivalirudin remains essentially restricted to emergency and intensive care units in hospital settings, although its indications tend to extend. No immediate antidote is available for this drug, and procoagulant drugs must be used in case of bleeding [15].
Laboratory methods are necessary for controlling the anticoagulation activity of bivalirudin to prevent bleeding risk [31-33]. Especially, adjusted dosages are necessary for patients with impaired renal clearance, as this drug is partly eliminated by kidney. Patients with moderate or elevated kidney dysfunction are exposed to drug accumulation and increased hemorrhagic risk (36). Nevertheless, clinical use of bivalirudin is reported to be safe [37]. In current practice, many clinical indications require an immediate availability of a decentralized evaluation of drug anticoagulant activity. This activity can be measured in blood or citrated plasma with global coagulation assays and specific instruments including. Methods available are: the Activated Clotting Time (ACT), performed directly on blood [31,32]; Thromboelastography (TEG), restricted to specialized investigations in labs familiar enough with this technique [32]; the Activated Partial Thromboplastin Time (APTT), performed on citrated plasma. Which gives a useful indication on the hypocoagulability level obtained through the exploration of the intrinsic coagulation pathway but does not correlate closely with its plasma concentration. In addition, use of Thrombin Time (TT) is possible to test the residual anticoagulant activity following drug withdrawal: it is a very sensitive test to verify elimination of any trace amount of bivalirudin. Lastly, Liquid Chromatography / Mass Spectrometry (LC:MS/MS) is used in highly specialized and equipped laboratories for pharmacokinetics’ studies and in pharmaceutical industry. Cassette devices used for ACT give immediate and individual results, and are the most appropriate in intensive care units, although they only provide indications on the global anticoagulation level reached and not on the drug concentration or its specific anticoagulant activity. ACT is measured directly on blood and was already introduced for testing heparin anticoagulation in these settings [31,32]. This assay can be designed with a blood coagulation activator, like silica particles, and is associated to small instruments with individual cassettes. The global assay used is Thromboelastography (TEG), but it remains restricted to specialized investigations, and research studies. Few labs currently use this technique [32]. The semi-global assay available for testing bivalirudin anticoagulant activity, APTT, is performed on citrated plasma. It needs optimized reagents, a clot detection instrument, and is performed in central coagulation laboratories. APTT gives a useful indication on the hypocoagulability level obtained by exploring the intrinsic coagulation pathway. However, APTT is not always representative of drug concentration, as various factors can influence clotting time, like the citrate concentration or the presence of a patient’s inflammatory state with high factor VIII (FVIII) and high fibrinogen concentrations. Thrombin Time (TT) is used to test the absence of residual anticoagulant activity. Among the other assays described, Liquid Chromatography / Mass Spectrometry (LC:MS/ MS) is available in highly specialized laboratories [33]. This method is useful for pharmacokinetics’ studies and for pharmaceutical industry, but it cannot be implemented in emergency situations or for closely monitoring patients.
More precise, automated, and quantitative assays are now available, like those reported in this article and previously reported for other direct thrombin inhibitors [30]. These assays are fully automated on laboratory instruments, can be pre-calibrated, and they need only controls for validating the right assay performances. These methods are performed in most clinical laboratory settings, but usually not at the patient’s bedside. Nevertheless, fast and efficient plasma sample transportation and permanently available testing possibilities are now more and more available in specialized hospitals. This allows implementing quantitative and accurate methods for generating results within a short time frame. The two assays presented in this report fulfill all these requirements. They are automated and offer the possibility of accurate, fast, and reliable measurements of bivalirudin concentrations within few minutes. Although no WHO standard or reference material is yet available, the standardization approach proposed is robust enough for ensuring the right accurateness and reliability over time. Furthermore, the tendency to extend the clinical indications of bivalirudin brings this drug outside the intensive care units, in other clinical settings. The automated laboratory testing methods proposed for monitoring treated patients meet all this rationale and allow individual adjustment of drug dosage when needed. The 2 assays presented offer the required expectations for this application, with full traceability of calibration, exactness, accuracy, and reliability. These methods are undergoing all compliance with regulatory requirements and are presently available with CE mark. Next, they will be upgraded in accordance with IVDR and FDA regulation for the new EU requirements and 510(k) release.
Conclusions
The increasing use of bivalirudin in patients with heart diseases requires its monitoring for exactly adjusting the individual dosages according to patients’ characteristics. The 2 quantitative methods presented in this report, the chromogenic BIOPHENTM DTI and the clotting HEMOCLOTTM Thrombin Inhibitor assays, offer all the practicability and automation needed for methods available 24/24 and 7/7.
References
- Fields WS. The history of leeching and hirudin. Haemostasis. 1991; 21: 3-10.
- Markwardt F. Hirudin as alternative anticoagulant--a historical review. Semin Thromb Hemost. 2002; 28: 405-414.
- Markwardt F. Die Isolierung und chemische Charakterisierung des Hirudins [Isolation and chemical characterization of hirudin]. Hoppe Seylers Z Physiol Chem. 1957; 308: 147-156.
- Markwardt F. Past, present and future of hirudin. Haemostasis. 1991; 21: 11-26.
- Markwardt F. The comeback of hirudin as an antithrombotic agent. Semin Thromb Hemost. 1991; 17: 79-82.
- Harvey RP, Degryse E, Stefani L, et al. Cloning and expression of a cDNA coding for the anticoagulant hirudin from the bloodsucking leech, Hirudo medicinalis. Proc Natl Acad Sci USA. 1986; 83: 1084-1088.
- He S, Blombäck M, Bark N, Johnsson H, Wallén NH. The direct thrombin inhibitors (argatroban, bivalirudin and lepirudin) and the indirect Xa-inhibitor (danaparoid) increase fibrin network porosity and thus facilitate fibrinolysis. Thromb Haemost. 2010; 103: 1076-1084.
- Greinacher A, Völpel H, Janssens U, Hach-Wunderle V, Kemkes-Matthes B, Eichler P, et al. Recombinant hirudin (lepirudin) provides safe and effective anticoagulation in patients with heparin-induced thrombocytopenia: a prospective study. Circulation. 1999; 99: 73-80.
- Parissis H. Lepirudin as an alternative to “heparin allergy” during cardiopulmonary bypass. J Cardiothorac Surg. 2011; 6: 44.
- Bircher AJ, Czendlik CH, Messmer SL, Müller P, Howald H. Acute urticaria caused by subcutaneous recombinant hirudin: evidence for an IgG-mediated hypersensitivity reaction. J Allergy Clin Immunol. 1996; 98: 994-996.
- Song X, Huhle G, Wang L, Hoffmann U, Harenberg J. Generation of antihirudin antibodies in heparin-induced thrombocytopenic patients treated with r-hirudin. Circulation. 1999; 100: 1528-1532.
- Greinacher A, Lubenow N, Eichler P. Anaphylactic and anaphylactoid reactions associated with lepirudin in patients with heparin-induced thrombocytopenia. Circulation. 2003; 108: 2062-2065.
- Cardenas GA, Deitcher SR. Risk of anaphylaxis after reexposure to intravenous lepirudin in patients with current or past heparin-induced thrombocytopenia. Mayo Clin Proc. 2005; 80: 491-493.
- Veach SA, Franks AM, Allan MC. Severe anaphylactic reaction after repeated intermittent exposure to lepirudin. Pharmacotherapy. 2007; 27: 760-765.
- Oh JJ, Akers WS, Lewis D, Ramaiah C, Flynn JD. Recombinant factor VIIa for refractory bleeding after cardiac surgery secondary to anticoagulation with the direct thrombin inhibitor lepirudin. Pharmacotherapy. 2006; 26: 569-577.
- Shammas NW. Bivalirudin: pharmacology and clinical applications. Cardiovasc Drug Rev. 2005; 23: 345-360.
- Van De Car DA, Rao SV, Ohman EM. Bivalirudin: a review of the pharmacology and clinical application. Expert Rev Cardiovasc Ther. 2010; 8: 1673-1681.
- Berlioz BE, Sanghavi D Bivalirudin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2021.
- Bates SM, Weitz JI. Direct thrombin inhibitors for treatment of arterial thrombosis: potential differences between bivalirudin and hirudin. Am J Cardiol. 1998; 82: 12P-18P.
- Scatena R. Bivalirudin: a new generation antithrombotic drug. Expert Opin Investig Drugs. 2000; 9: 1119-1127.
- Weitz JI, Hudoba M, Massel D, Maraganore J, Hirsh J. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990; 86: 385-391.
- Veale JJ, McCarthy HM, Palmer G, Dyke CM. Use of bivalirudin as an anticoagulant during cardiopulmonary bypass. J Extra Corpor Technol. 2005; 37: 296-302.
- Cho JH, Parilla M, Treml A, Wool GD. Plasma exchange for heparin-induced thrombocytopenia in patients on extracorporeal circuits: A challenging case and a survey of the field. J Clin Apher. 2019; 34: 64-72.
- Cho HJ, Kim DW, Kim GS, Jeong IS. Anticoagulation Therapy during Extracorporeal Membrane Oxygenator Support in Pediatric Patients. Chonnam Med J. 2017; 53: 110-117.
- Netley J, Roy J, Greenlee J, Hart S, Todt M, Statz B. Bivalirudin Anticoagulation Dosing Protocol for Extracorporeal Membrane Oxygenation: A Retrospective Review. J Extra Corpor Technol. 2018; 50: 161-166.
- Joseph L, Casanegra AI, Dhariwal M, Smith MA, Raju MG, Militello MA, et al. Bivalirudin for the treatment of patients with confirmed or suspected heparininduced thrombocytopenia. J Thromb Haemost. 2014; 12: 1044-1053.
- Bain J, Meyer A. Comparison of bivalirudin to lepirudin and argatroban in patients with heparin-induced thrombocytopenia. Am J Health Syst Pharm. 2015; 72: S104-S109.
- Mehran R, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet. 2009; 374: 1149-1159.
- Verdoia M, Schaffer A, Barbieri L, Suryapranata H, De Luca G. Bivalirudin as compared to unfractionated heparin in patients undergoing percutaneous coronary revascularization: A meta-analysis of 22 randomized trials. Thromb Res. 2015; 135: 902-915.
- Amiral J, Dunois C, Amiral C, Seghatchian J. An update on laboratory measurements of Dabigatran: Smart specific and calibrated dedicated assays for measuring anti-IIa activity in plasma. Transfus Apher Sci. 2016; 54: 428-437.
- Weeks PA, Alkhateeb HM, Michaud SE, Diez JG. Evaluation of bivalirudin hyper- and hypo-ACT responses in the setting of percutaneous coronary intervention. J Invasive Cardiol. 2013; 25: 250-253.
- Carroll RC, Chavez JJ, Simmons JW, Snider CC, Wortham DC, Bresee SJ, et al. Measurement of patients’ bivalirudin plasma levels by a thrombelastograph ecarin clotting time assay: a comparison to a standard activated clotting time. Anesth Analg. 2006; 102: 1316-1319.
- Pan G, Wang X, Huang Y, Gao X, Wang Y. Development and validation of a LC-MS/MS method for determination of bivalirudin in human plasma: Application to a clinical pharmacokinetic study. J Pharm Biomed Anal. 2010; 52: 105-109.
- Tardy B, Lecompte T, Boelhen F, Tardy-Poncet B, Elalamy I, Morange P, et al; GEHT-HIT Study Group. Predictive factors for thrombosis and major bleeding in an observational study in 181 patients with heparin-induced thrombocytopenia treated with lepirudin. Blood. 2006; 108: 1492-1496.
- Stone GW, Clayton T, Deliargyris EN, Prats J, Mehran R, Pocock SJ. Reduction in cardiac mortality with bivalirudin in patients with and without major bleeding: The HORIZONS-AMI trial (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction). J Am Coll Cardiol. 2014; 63: 15-20.
- Robson R, White H, Aylward P, Frampton C. Bivalirudin pharmacokinetics and pharmacodynamics: effect of renal function, dose, and gender. Clin Pharmacol Ther. 2002; 71: 433-439.
- Mehrzad M, Tuktamyshov R, Mehrzad R. Safety, efficiency and cost effectiveness of Bivalirudin: A systematic review. World J Cardiol. 2017; 9: 761-772.