
Research Article
Thromb Haemost Res. 2022; 6(1): 1072.
Mitochondrial Superoxide is the Key for Promoting Cell Migration in Wound Healing
Ping Weidong, Wang Xin, Wang Xiaowei, Li Fei, Huang Chunyan, Zhao Qiming*
Department of Plastic Surgery, Zhejiang Hospital, Hangzhou, 310013, Zhejiang, China
*Corresponding author: Zhao Qiming, Department of Plastic Surgery, Zhejiang Hospital, No. 12 Lingyin Road, Xihu District, Hangzhou, 310013, Zhejiang, China; E-mail: zhaoqmzx@126.com
Received: December 30, 2021; Accepted: January 27, 2022; Published: February 03, 2022
Abstract
Introduction: The focus of current study was to investigate the role of mitochondrial superoxide for promoting cell migration in healing responses.
Methods: To simulate the skin wound healing process, scratch wound assay in A549 cells was utilized. Subsequently, mitochondrial superoxide and intracellular calcium flux (Ca2+) were measured by MitoSox red reagent and Fluo-4 staining. Paraquat (PQ), N-acetyl cysteine (NAC), superoxide dismutase (SOD) mimics (EUK-134), free radical scavenge (MCI-186), and diphenyliodonium (DPI) were added into medium to evaluate the influences of reactive oxygen species (ROS) and superoxide on cell migration.
Results: Compared with before wound (BW), mitochondrial superoxide increased time-dependent manner. Correspondingly, Ca2+ transients was rapidly elevated after scratch wound and gradually declined toward an steady level, as evidenced by Fluo-4 fluorescence intensity. Scratch wound assay displayed that cell migration in PQ 0.1 mM group was stronger than that of Control group, whilst it was restrained after administration of 10 mM NAC. Additionally, cell migration in EUK-134 and MCI-186 group was conspicuously lower than that of Control group and similar with the degree of inhibiting role of DPI, indicating that mitochondrial superoxide served the crux influence on promoting cell migration after scratch.
Conclusion: ROS and superoxide production during scratch wound facilitated cell migration. Importantly, mitochondrial superoxide played the positive effects on healing response.
Keywords: Reactive oxygen species; Mitochondrial superoxide; Wound healing; Cell migration
Abbreviations
ROS: Reactive Oxygen Species; mtROS: Mitochondrial ROS; Ca2+: Calcium Flux; PQ: Paraquat; NAC: N-acetyl Cysteine; SOD: Superoxide Dismutase; DPI: Diphenyliodonium
Introduction
Wound healing is a complicated regenerative process, which is indispensable for mammals to survive [1]. Currently, human wounds become a leading threat to public health, influencing millions of people worldwide [2]. In general, nonhealing wound is a main cause of morbidity and mortality. To our knowledge, cell migration exerts the crux roles on biological events including wound closure, repair and regeneration [3]. Flat membrane protrusion in the front of cells, known as lamellipodia, the first procedure of cell migration, is driven by actin polymerization modulated by Rac1 [4]. Thus, exploring the factor that influence cell migration is crucial.
It’s well known that reactive oxygen species (ROS) are produced in wound sites and serve as signaling during wound healing [5,6]. Except for being generated by membrane and cytoplasm, mitochondrion is also a principal source of ROS. Correspondingly, the role of mitochondrial ROS (mtROS) for wound closure has been verified in previous studies [7,8]. Besides, previous study has verified that endothelial cell migration is partly dependent on mitochondriagenerated ROS [9]. A similar study has also demonstrated that mtROS is closely associated with cell migration [10], supporting its promoting roles in wound closure.
Superoxide, as the member of ROS family, serves the function for cell migration, which have been pointed out. For instance, Mu et al. [11] have revealed that Chlorotyrosine boosts smooth muscle cell migration via increasing superoxide generation. Another study has uncovered that superoxide production supports epithelial cell migration, thereby promoting wound closure [12]. In terms of relation between mitochondrial superoxide and cell migration, Dias Amoedo et al. [13] have reported that inhibition of mitochondrial superoxide generation is helpful to suppress the migration of A549 cell, and then target lung adenocarcinoma. However, how mitochondrial superoxide influences migration, thereby wound healing remains poorly understood. In this study, we investigated whether mitochondrial ROS involved cell migration or not. Our results demonstrated that mitochondrial superoxide exerted the crux roles on wound healing.
Materials and Methods
Cell culture and treatment
A549 cells (non-small cell lung cancer cell line) were purchased from cell resource center of shanghai institute for life sciences (Shanghai, China), and cultured in DMEM medium (Gibco, Carlsbad, MD, USA) supplemented with 10% FBS and 1% penicillin/ streptomycin (Meilunbio, Dalian, China).
Wound healing assay
To simulate the skin wound healing process, scratch wound assay was utilized referring to previous study [14]. Briefly, A549 cells were grown to form monolayers in 6-well plates, and then scratch was performed with 200 μl pipette tip (Figure 1). After washing with DMEM medium, the scratched cells were returned to incubator for further culture up to 12 h. To detect the influences of ROS and superoxide, paraquat (PQ), N-acetyl cysteine (NAC), SOD mimics (EUK-134), free radical scavenge (MCI-186), and diphenyliodonium (DPI) were added into medium, respectively.
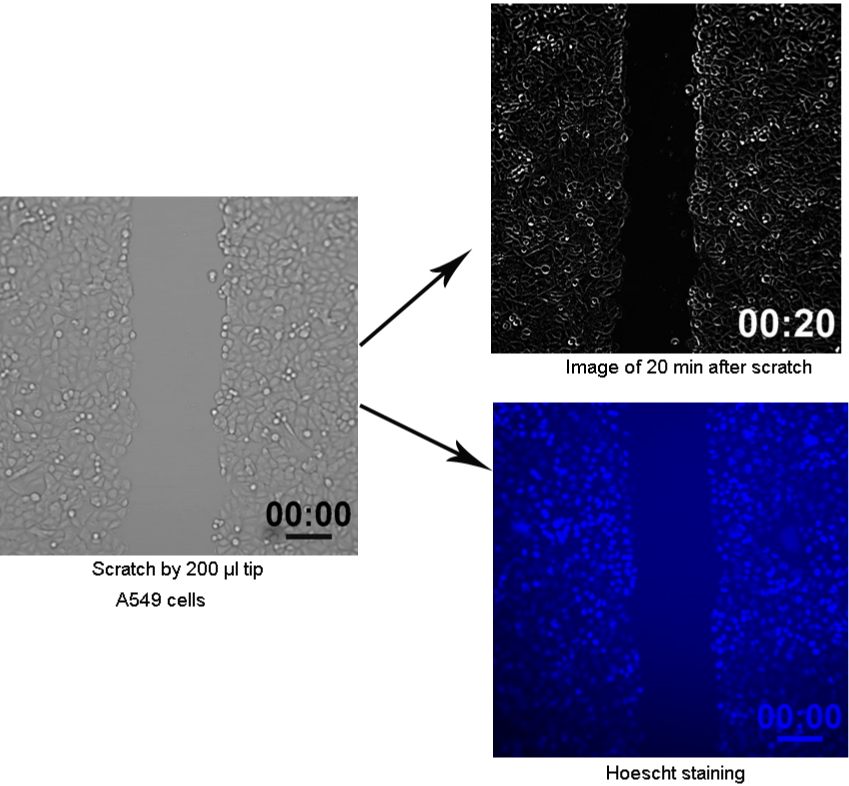
Figure 1: Scratch wound assay in vitro was established to simulate the skin wound healing process.
To estimate wound closure, images were captured by microscopy (Olympus, Tokyo, Japan), and then migration distance was evaluated by Image J software. Additionally, cells were stained with DAPI and captured by microscopy (Olympus, Tokyo, Japan). The images were analyzed using Matlab program that determined cell trajectories.
Measurement of mitochondrial superoxide
A549 cells in different states of normal and wounding were incubated with MitoSox red reagent (5 μM) (Invitrogen, Carlsbad, CA, USA) for 10 min according to the manufacturers’ instruction. After washing, the imaging was conducted using a fluorescent microscope (Olympus, Tokyo, Japan). MitoSox fluorescence level was presented as ΔF/F0, where F0 corresponded to the basal fluorescence prior to wounding, while ^ΔF was referred to the difference between fluorescence value after scratch wound (F) and F0.
Determination of intracellular calcium flux
To examine the changes in intracellular calcium flux (Ca2+) after scratch wound, A549 cells were incubated with Fluo-4 (5 μM) for 30 min. Then, A549 cells were washed to remove probe excess. Finally, the imaging was conducted using a fluorescent microscope (Olympus, Tokyo, Japan), and Fluo-4 fluorescence level was presented as ΔF/F0.
Statistical analysis
Data were presented as mean ± standard deviation (SD) and analyzed using SPSS 21.0 software package. The differences of measurement data were compared with one way ANOVA followed by LSD test. P<0.05 was considered as significant difference.
Results
Scratch wound enhanced mitochondrial superoxide production and increased cytoplasmic (Ca2+) in A549 cells
Compared with before wound (BW), mitochondrial superoxide increased time-dependent manner. Additionally, we observed about an increase of 250% in mitochondrial superoxide at post-wound 500 sec (W+ 500 sec). Cytoplasmic Ca2+ results showed that Ca2+ transients was rapidly elevated after scratch wound and gradually declined toward an steady level, as evidenced by Fluo-4 fluorescence intensity (Figure 2). Those findings indicated that mitochondrial superoxide and Ca2+ flux were activated in the healing response.

Figure 2: Wound-inducing mitochondrial superoxide production and
intracellular calcium flux around wound edge. BW: before wounding; W+ 20,
200, 500 sec, Images of at 20, 200, and 500 sec after wounding.
ROS accelerated cell migration for scratch wound closure
To investigate the effects of ROS on cell migration during wound healing, the depletion and induction of ROS by NAC and PQ were added into the medium of A549 cells, respectively. As displayed in Figure 3, cell migration in PQ 0.1 mM group was stronger than that of Control group after culturing 4 h (P<0.05). However, cell migration was restrained after administration of 10 mM NAC in contrast to Control (P<0.05). Thus, ROS exerted the facilitation on cell migration during wound healing.
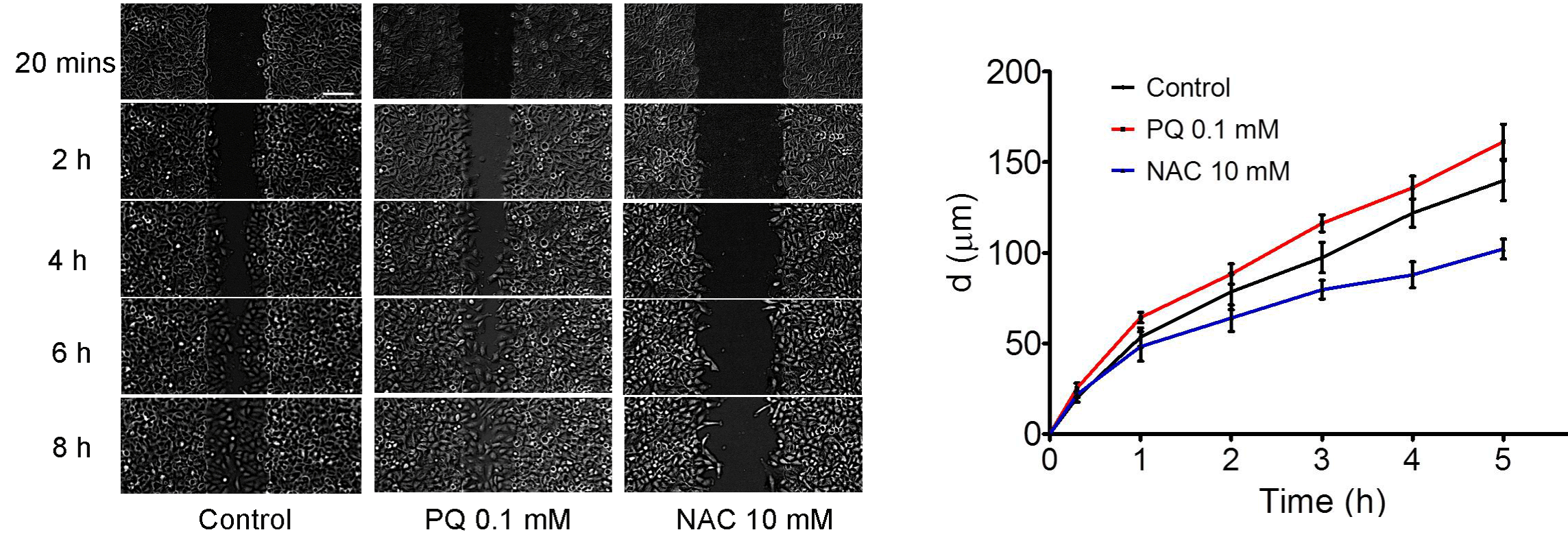
Figure 3: ROS accelerated cell migration for scratch wound closure.
Superoxide promoted cell migration after scratch wound
Following, EUK-134 and MCI-186 was utilized to inhibit the generation of superoxide. Scratch wound assay showed that the ability of cell migration in EUK-134 0.4 mM and MCI-186 2 mM group was remarkably weakened compared with Control group by 1 h after scratch (P<0.05) (Figure 4).
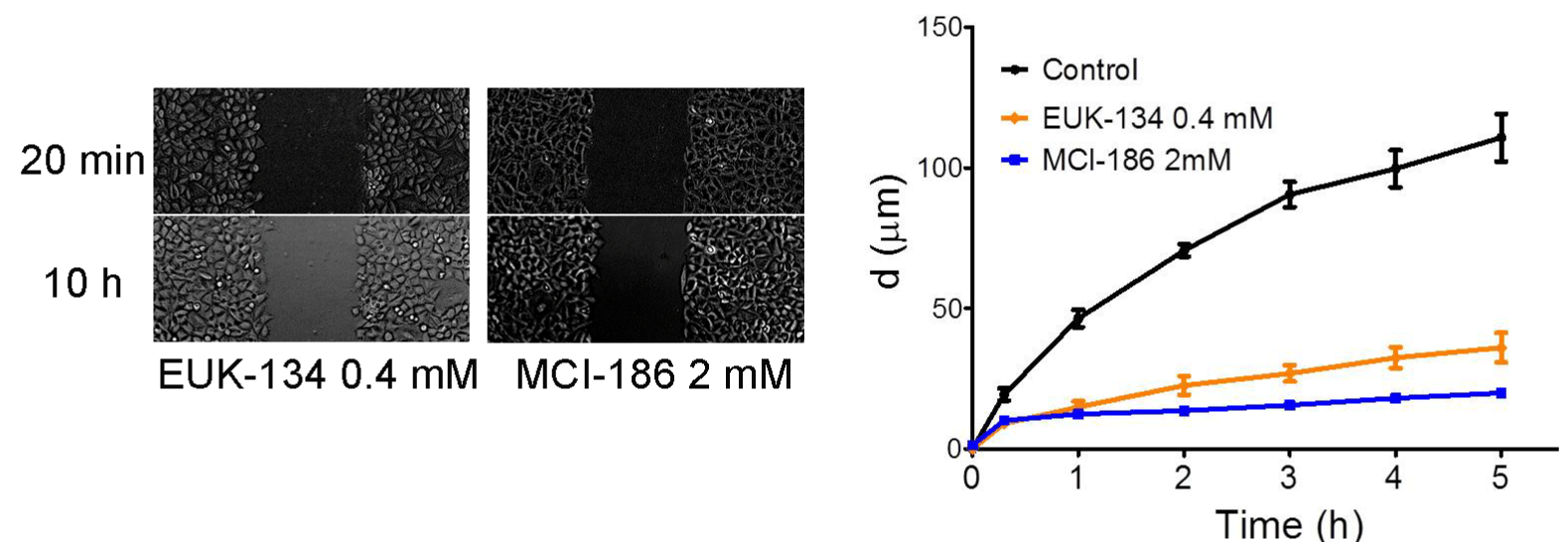
Figure 4: Superoxide promoted cell migration after scratch wound.
Mitochondrial superoxide promoted cell migration in scratch assay
DPI, as a mitochondrial complex I inhibitor, was employed for depletion of ROS in the currents study. As shown in Figure 5, administration of A549 cell with DPI obviously reduced the cell motility (P<0.001), whereas NAC treatment reduced the cell migration with a lower extent (P<0.05). Additionally, PQ elevated the ability of cell migration in contrast to Control group (P<0.05). These results indicated the pivotal role of ROS generation-mediated by mitochondria in the aspects of cell migration. Correspondingly, we also found that the cell migration in EUK-134 and MCI-186 group was conspicuously lower than that of Control group (P<0.001), and similar with the degree of inhibiting role of DPI. Thus, we concluded that mitochondrial superoxide served the crux influence on promoting cell migration after scratch.
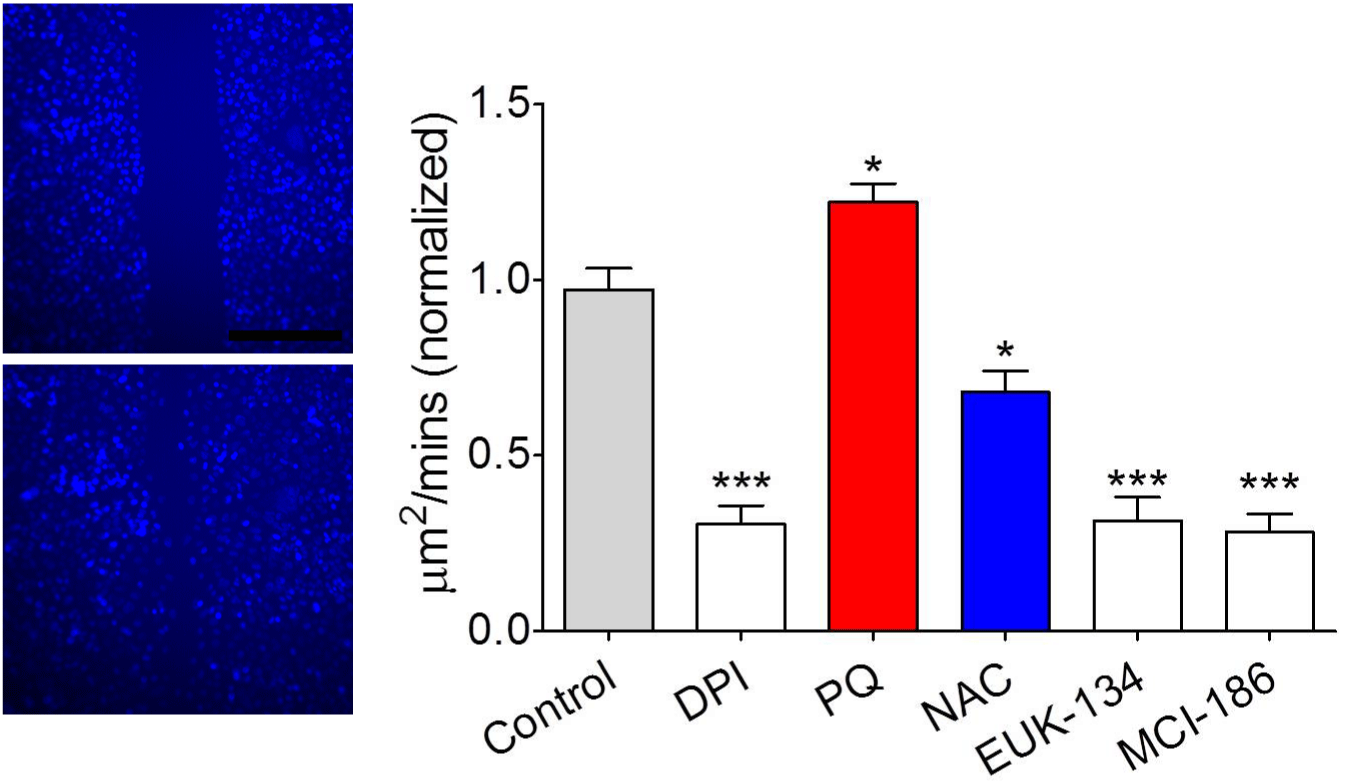
Figure 5: Matlab based cell migration quantitation showed that mitochondrial
superoxide promoted cell migration in scratch assay.
The types of protrusions indicated relationship between RHO GTPase and cell migration
As shown in Figure 6, fewer membrane protrusions were observed in normal cells. However, lamelipodia (flat cellular protrusions) were visible at the leading edges of migrating cells in the PQ group. In the NAC group, filopodia (narrow protrusions) appeared in the front of migrating cells. Taken together, migration pattern of lamelipodia served the accelerating roles on wound healing, while that of filopodia delayed the wound healing, demonstrating the relationship between RHO GTPase and cell migration.
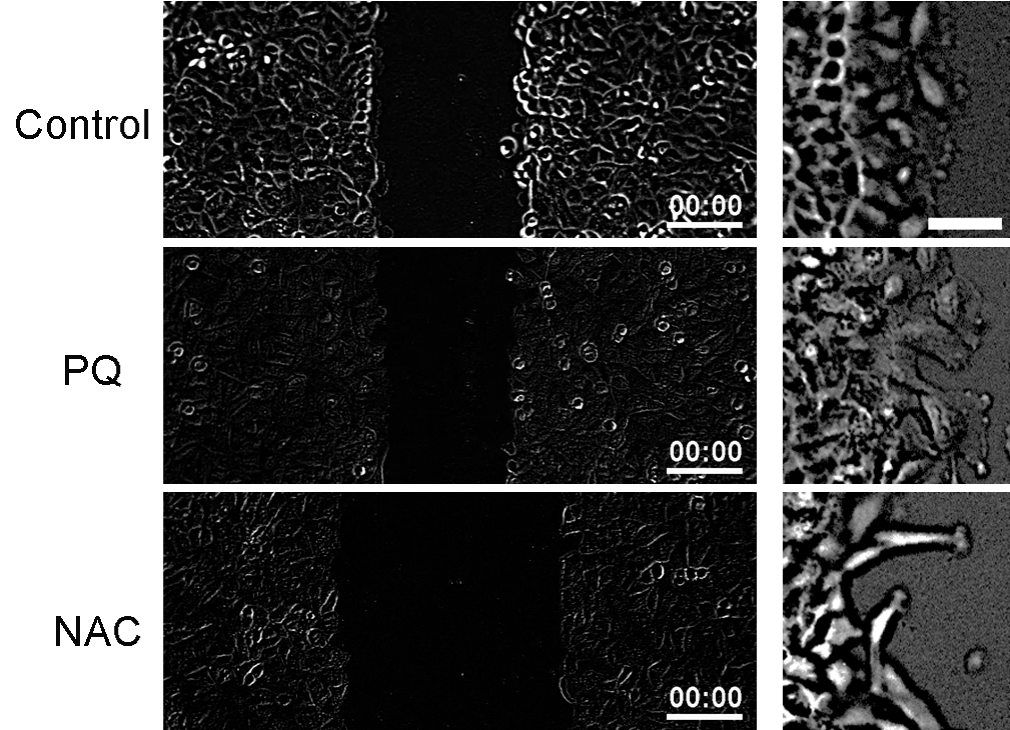
Figure 6: The types of protrusions indicated relationship between RHO
GTPase and cell migration.
Discussion
Wound healing abnormalities occurs in large portion of population, especially the old people, and patients with immunodeficiencies and chronic or acute diseases [15]. As well known, oxidative stress serves the crucial impacts on wound repair [16]. In line, mitochondrial dysfuction may contribute to the pathogenesis of non-healing wounds [17]. In the current study, we examine the roles of ROS and superoxide in the wound healing, with focus on exploring mitochondrial superoxide in healing response. As a result, our study revealed that wound-induced ROS and superoxide production facilitate cell migration. Importantly, mitochondrial superoxide played the positive effects on healing response.
Cell migration is the crux procedure for wound closure. Generally, scratch wound healing assay is utilized to estimate cell migration in vitro [18]. In terms of wound healing, accumulating evidences have confirmed that ROS is the one of potential regulatory mechanisms [5,19]. Furthermore, pervious study reported by Hunte et al. [20] has verified that ROS generation facilitates embryonic wound healing in some species. Another study has also confirmed that administration of Low-temperature Plasma treatment can induce the ROS production, thereby promoting wound closure [21]. Moreover, previous study has revealed the relationship between ROS and cell migration and it turned out that ROS-triggered signaling pathways result in cell migration [22]. Similarly, our study displayed that inducing ROS production by PQ enhanced the ability of cell migration after scratch wound, whilst cell migration was restrained after depletion of ROS. Additionally, we also observed that migration pattern of lamelipodia served the accelerating roles on wound healing, which was conformed to previous study that cell migration begins with lamellipodia formation [4]. Aforementioned findings indicated that scratch-triggered ROS generation is helpful to for cell migration and then promote the wound closure.
As everyone knows, superoxide is the indispensable regulator for cell migration [23,24]. In the current study, we showed that cell migration was restrained after inhibition of superoxide. Alternatively, several studies have verified this point. For example, Mu et al. [11] have reported that Chlorotyrosine increase superoxide production, followed by activating smooth muscle cell migration. Subsequent studies have also demonstrated that inducing cell migration depends on superoxide generation [25,26]. Additionally, superoxide functions as intracellular messengers to stimulate key step of wound healing, which has been evidenced [17]. Further, a previous study has pointed out that superoxide was suggested to motivate cell migration because its degradation can slow the chemoattractant-induced chemotaxis [27]. Those finding indicated that superoxide may be require for scratch wound closure.
On the other hand, the current study reached that mitochondrial superoxide generation was induced after scratch wound and it exerted the positive effects on cell migration. With regarding to wound impairment, previous studies have uncovered the vital impacts of mtROS on cytoskeletal and mitochondrial remodeling to enhance wound closure [7,8]. In other diseases, a previous study has uncovered that targeting mitochondrial superoxide is the promising approach for therapeutic prevention of tumor metastasis [28]. Similarly, inhibition of mitochondrial superoxide generation is helpful to suppress the migration of A549 cell, thereby targeting lung adenocarcinoma, which is in agreement with our findings that inhibitor of mitochondrial superoxide can restrain the migration of A549 cell. Besides, intracellular Ca2+ after scratch wound was obviously changed in this study. Ca2+, as a central regulator for maintaining homeostasis of epidermis, involves in the wound healing [29]. After wounding, intracellular Ca2+ is rapidly elevated around wound edge [30], and elevated Ca2+ can modulate the rate of cell migration [31]. Furthermore, our study displayed the changes of mitochondrial superoxide and intracellular Ca2+ in the time-dependent manner, which was consistent with the findings of Ishibashi et al. [32] who previously identified the relation between Ca2+ and superoxide generation. Taken together, mitochondrial superoxide is the key for promoting cell migration in wound healing.
Conclusion
ROS and superoxide facilitate cell migration during scratch wound. Importantly, mitochondrial superoxide played the positive effects on healing response. However, the specific mechanism of mitochondrial superoxide in wound healing needs to be further studied.
References
- Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb Perspect Med. 2015; 5: a023267.
- Sen CK. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv Wound Care (New Rochelle). 2019; 8: 39-48.
- Coomber BL, Gotlieb AI. In vitro endothelial wound repair. Interaction of cell migration and proliferation. Arteriosclerosis. 1990; 10: 215-222.
- Krause M, Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol. 2014; 15: 577-590.
- Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017; 14: 89-96.
- Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S, Hunt JA. Reactive oxygen species (ROS)--a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater. 2012; 24: 249-265.
- Muliyil S, Narasimha M. Mitochondrial ROS regulates cytoskeletal and mitochondrial remodeling to tune cell and tissue dynamics in a model for wound healing. Dev Cell. 2014; 28: 239-252.
- Xu S, Chisholm AD. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev Cell. 2014; 31: 48-60.
- Wang Y, Zang QS, Liu Z, Wu Q, Maass D, Dulan G, et al. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am J Physiol Cell Physiol. 2011; 301: C695-704.
- Wang L, Yu T, Lee H, O’brien DK, Sesaki H, Yoon Y. Decreasing mitochondrial fission diminishes vascular smooth muscle cell migration and ameliorates intimal hyperplasia. Cardiovasc Res. 2015; 106: 272-283.
- Mu H, Wang X, Lin PH, Yao Q, Chen C. Chlorotyrosine promotes human aortic smooth muscle cell migration through increasing superoxide anion production and ERK1/2 activation. Atherosclerosis. 2008; 201: 67-75.
- Kwon J, Wang A, Burke DJ, Boudreau HE, Lekstrom KJ, Korzeniowska A, et al. Peroxiredoxin 6 (Prdx6) supports NADPH oxidase1 (Nox1)-based superoxide generation and cell migration. Free Radic Biol Med. 2016; 96: 99-115.
- Dias Amoedo N, Dard L, Sarlak S, Mahfouf W, Blanchard W, Rousseau B, et al. Targeting Human Lung Adenocarcinoma with a Suppressor of Mitochondrial Superoxide Production. Antioxid Redox Signal. 2020; 33: 883- 902.
- Walter MN, Wright KT, Fuller HR, Macneil S, Johnson WE. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010; 316: 1271-1281.
- Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am. 2011; 19: 441-453.
- Schäfer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008; 58: 165-171.
- Cano Sanchez M, Lancel S, Boulanger E, Neviere R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants (Basel). 2018; 7.
- Martinotti S, Ranzato E. Scratch Wound Healing Assay. Methods Mol Biol. 2020; 2109: 225-229.
- Deng Z, Shi F, Zhou Z, Sun F, Sun MH, Sun Q, et al. M1 macrophage mediated increased reactive oxygen species (ROS) influence wound healing via the MAPK signaling in vitro and in vivo. Toxicol Appl Pharmacol. 2019; 366: 83-95.
- Hunter MV, Willoughby PM, Bruce AEE, Fernandez-Gonzalez R. Oxidative Stress Orchestrates Cell Polarity to Promote Embryonic Wound Healing. Dev Cell. 2018; 47: 377-387.e4.
- Shi XM, Xu GM, Zhang GJ, Liu JR, Wu YM, Gao LG, et al. Low-temperature Plasma Promotes Fibroblast Proliferation in Wound Healing by ROSactivated NF-κB Signaling Pathway. Curr Med Sci. 2018; 38: 107-114.
- Wozny AS, Vares G, Alphonse G, Lauret A, Monini C, Magné N, et al. ROS Production and Distribution: A New Paradigm to Explain the Differential Effects of X-ray and Carbon Ion Irradiation on Cancer Stem Cell Migration and Invasion. Cancers (Basel). 2019; 11.
- Sadok A, Bourgarel-Rey V, Gattacceca F, Penel C, Lehmann M, Kovacic H. Nox1-dependent superoxide production controls colon adenocarcinoma cell migration. Biochim Biophys Acta. 2008; 1783: 23-33.
- Li F, Chen T, Hu S, Lin J, Hu R, Feng H. Superoxide mediates direct current electric field-induced directional migration of glioma cells through the activation of AKT and ERK. PLoS One. 2013; 8: e61195.
- Yaghini FA, Song CY, Lavrentyev EN, Ghafoor HU, Fang XR, Estes AM, et al. Angiotensin II-induced vascular smooth muscle cell migration and growth are mediated by cytochrome P450 1B1-dependent superoxide generation. Hypertension. 2010; 55: 1461-1467.
- Ben Sghaier M, Pagano A, Mousslim M, Ammari Y, Kovacic H, Luis J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed Pharmacother. 2016; 84: 1972-1978.
- Hurd TR, Degennaro M, Lehmann R. Redox regulation of cell migration and adhesion. Trends Cell Biol. 2012; 22: 107-115.
- Porporato PE, Sonveaux P. Paving the way for therapeutic prevention of tumor metastasis with agents targeting mitochondrial superoxide. Mol Cell Oncol. 2015; 2: e968043.
- Tomás CC, Oliveira E, Sousa D, Uba-Chupel M, Furtado G, Rocha C, et al. Proceedings of the 3rd IPLeiria’s International Health Congress : Leiria, Portugal. BMC Health Serv Res. 2016; 16: 200.
- Tu CL, Celli A, Mauro T, Chang W. Calcium-Sensing Receptor Regulates Epidermal Intracellular Ca2+ Signaling and Re-Epithelialization after Wounding. J Invest Dermatol. 2019; 139: 919-929.
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996; 17: 275-285.
- Ishibashi K, Okazaki S, Hiramatsu M. Simultaneous measurement of superoxide generation and intracellular Ca2+ concentration reveals the effect of extracellular Ca2+ on rapid and transient contents of superoxide generation in differentiated THP-1 cells. Biochem Biophys Res Commun. 2006; 344: 571-580.