
Review Article
Thromb Haemost Res. 2022; 6(2): 1080.
Analytical Approaches to Wound Healing Process
Pournaki M¹, Kia SAH² and Tetik S³*
¹Department of Basic Sciences, Faculty of Pharmacy, Final International University, Cyprus
²Department of Clinical Pharmacy, Faculty of Pharmacy, Final International University, Cyprus
³Department of Medical Biochemistry, Faculty of Pharmacy, Final International University, Cyprus
*Corresponding author: Sermin TETIK, Department of Medical Biochemistry, Faculty of Pharmacy, Final International University, Cyprus
Received: July 21, 2022; Accepted: August 20, 2022; Published: August 27, 2022
Abstract
Wound healing is a complex domain that needs to solve abundant parameters. Changed protein concentration, pH effect on wound environment, mediators of immune system, and aging process are fight subjects of wound healing. Therefore, in modern approaches to improve of challenges are tending to analytical techniques for lighting dark side. Research of genetic markers, micro RNAs expression or post-translational modifications of proteins was recently evaluated in wound healing. In review article, we focused on some parameters of the healing system which are affected processes of wound healing and some analytical approaches which are using to find connect of the network.
Introduction
A wound is described as the disruption of a tissue’s normal anatomical structure and function. It also signifies the destruction of the body’s natural defenses, which facilitates the invasion of microorganisms [1]. The intricate combination of matrix destruction and reconfiguration that occurs during wound regeneration necessitates [2] well regulated mechanisms that result in the healing of damaged tissues [3]. These procedures are fusions of intricate molecular and biological processes that result in proliferation, cell migration, and remodeling and extracellular matrix deposition of scar tissues [4].
According to the type of skin damage, potential underlying disorders (such as diabetes and peripheral artery occlusive disease), local wound factors and systemic mediators, healing processes are induced. Depending on how delicately the various elements are balanced, either a physiological mode of healing (healing of acute wounds) or a pathologically delayed mode of healing (healing of chronic wounds) takes place [5].
Acute (syn. physiological) wound healing is a Four-phased, coordinated process that includes the (i) Hemostasis phase, (ii) inflammatory phase, (iii) proliferative phase (neoangiogenesis, granulation, and re-epithelialization), and (iiii) remodeling phase [Extracellular Matrix (ECM) remodeling] (Figure 1).
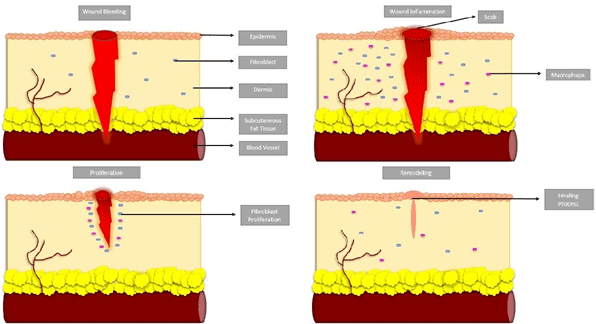
Figure 1: Phases of acut wound healing; Blood bleeding or hemostasis, inflammation of wound, proliferation, and remodeling.
During hemostasis, platelets clump together and a fibrin clot forms at the area of endothelial damage. By sticking to the damaged endothelium and releasing chemokines, platelets draw in the inflammatory phase’s cellular elements [6].
Following cutaneous injury, cytokines, chemokines, growth factors, and their effects on cellular receptors are the main mediators of the inflammatory phase. Various cell types, including granulocytes and macrophages, are attracted to the wound site by chemoattractant factors, which start the healing process. The wound milieu’s pH value gradients, which include proteinases, cytokines, chemokines, etc., may have a significant effect on cellular processes [7].
Following this, the proliferative phase that begins with an overlap in time. This phase includes re-epithelialization, granulation tissue production, and neoangiogenesis, as well as the formation of ECM. One of the most crucial steps in wound healing is neoangiogenesis because the increased metabolic activity during the proliferative phase limits the availability of nutrients and oxygen. After a suitable wound bed has been formed, an epithelial layer is applied to the wound surface, which encourages fibroblast proliferation and dramatically increased collagen synthesis and deposition. Epidermal keratinocytes, which are triggered and moved into the wound site starting from the wound margins, differentiate, proliferate, and migrate in order to reepithelialize [8].
Beginning a few days after the injury, the tissue remodeling phase can continue for up to two years. Different proteinases participate in this phase of coordinated wound healing. When compared to physiological conditions, the wound milieu itself, such as pH value variations during the various stages of wound healing, affects how these proteinases behave and how active they are [9].
Chronic wounds show inadequate repair processes that prevent the creation of a sustained anatomical and functional outcome in a reasonable amount of time [5]. Chronic wounds are described as wounds that do not heal physiologically, instead becoming stuck in an uncontrolled and self-sustaining phase of inflammation, and do not follow the well-known step-by-step process of physiological healing. This damages anatomical and functional integrity over a physiologically acceptable period of time [10].
Effect of pH on Wound Healing
Changes in pH can influence or be affected by the complicated process of wound healing at each stage [11-13]. According to early research, a chronic wound has a pH between 7.11 and 8.9 [14,15] and Compared to wounds with a pH closer to neutral, wounds with a high alkaline pH has a slower rate of healing [16].
Effect of pH on Skin Cells in Wound Healing
Changing of pH is affected skin cells behavior in during wound healing. Using ex vivo and in vitro skin cell models, researchers looked at how pH affected keratinocyte and fibroblast attachment, proliferation, and migration.Additionally, the effect of pH on keratinocyte differentiation was measured by the expression of cytokeratins, the proteins of keratin intermediate filaments found between epithelial cells, in particular cytokeratins 1 and 5. It was determined that low pH values promote a differentiated keratinocyte phenotype. Furthermore, the best pH range for fibroblast and keratinocyte is between 7.2 and 8.3, on the other hand the optimal pH value for ex vivo skin explants development was 8.4. Indicates that skin cells and explants reproduce and move at pH level above the physiological range [18].
Described how pH influences cell migration and how cell density creates changes in pH suited to healing: Studies have demonstrated that cellular proliferation is less susceptible to pH lowering in sparse chick embryo cell cultures than dense cell culture. Experiments on wounds showed similar outcomes, with low pH preventing cell migration. But those cells that migrated into the wound area proliferated just as quickly at low pH as they did at high pH, illustrating the combination impacts of pH and cell density [19,20].
Effects of Aging on Wound Healing
The alterations in aging skin are a result of both intrinsic and extrinsic aging. The alterations in skin that take place in areas shielded from the sun without reference to external aggressors are referred to as intrinsic aging. Extrinsic aging is the result of long-term environmental exposure, especially to sunlight’s UV radiation, which causes cumulative alterations in the body [21]. Increased sensitivity to the environment, a decline in homeostatic capacity, and a cumulative loss of function are the overall effects of intrinsic and extrinsic aging [22].
Comparing older humans to younger ones, there is a noticeable decrease in cutaneous blood flow [23]. Age-related changes in blood flow are accompanied by a decrease in cutaneous lymphatic drainage, which impairs the ability to rid the wound of pathogens and also prevents wound contraction [24].
Age-Related Changes in Phases of Healing
Alterations in Hemostasis and Inflammation
With endothelial damage exposes collagen, which helps platelets attach to the damaged endothelium. Aged subjects have increased platelet adhesion to the endothelium [25,26].
Age also causes platelets to release more alpha-granules, which are composed of TGF-β, TGF-a, and Platelet-Derived Growth Factor (PDGF) [27].
Elderly endothelial cells release less nitric oxide, a vasoactive mediator [28]. As a result, the diapedesis of neutrophils is reduced, and capillary permeability is reduced at the site of damage. On the other hand, leukocytes exhibit an age-related increase in the secretion of and responsiveness to several inflammatory mediators [29,30]. Macrophage and B-lymphocyte infiltration into wounds is postponed in middle-aged and elderly mice using wound healing models. Aged animals similarly have a delay in T-lymphocyte entry at the wound bed, but the overall level is higher than in young animals. Lymphocytes from elderly animals exhibit a reduced proliferative response, a reduced number of naive cells, and an increased number of memory cells [31,32].
Alterations in Proliferation
In older animals, the proliferative response of fibroblasts, keratinocytes, and vascular endothelial cells is diminished [33,34]. Age-related delays can be seen in angiogenesis, collagen production, and re-epithelialization [31]. Dermal fibroblast size and number generally decline with age [35]. Additionally, maturated fibroblasts are representedless sensitivity to growth factors and have less replication potential than young fibroblasts [35,36]. Both in animal models and in human wounds, these changes cause an age-related delay in the healing of the wound [37,38]. In aged animals [39, 40] as well as humans [41,42], whole wound studies have revealed lower rates of epithelialization and contraction.
The process of angiogenesis is thought to be crucial for the best possible healing of wounds because the epidermis needs nutrients to move and multiply [43].
Alterations in Resolution
Collagenase activity is higher in young animals than in older animals, allowing for more collagen remodeling and turnover [44]. In older wounds, keloids, hypertrophic scars and other hyperproliferative wound-healing disorders are uncommon. This may be caused by lower amounts of circulating TGF-β, according to some studies [45]. In adults, severe scarring can be avoided by blocking TGF-, a protein that promotes the synthesis of collagen [46].
Have demonstrated that aged animals have diminished strength and gain wound strength more slowly than younger animals. It has been demonstrated that these findings also apply to human beings; incisional wounds in individuals older than 70 years old showed lower tensile strength than those in patients younger than 70 years old [47].Infection rates and medical consequences rise as a result of the delayed wound closure [48-51].
Analytical Approaches to Wound Healing
When trying to understand wound healing, it is important to monitor changes due to comorbidities (e.g., diabetes, vascular diseases). More biological materials are needed for these studies [52]. Analyzing wound fluid helps us understand the microenvironment of the wound.
In recent years, inflammatory mediators, growth factors, cytokines [53,54], proteases [55,56] and oxidative stress proteins [57] have been analyzed in wound fluid, and in this way the effects of proteins in different phases of wound healing have been tried to be characterized. However, since the mechanism underlying wound healing has not yet been fully elucidated, a diagnostic marker cannot be recommended in routine clinic.
In wound healing studies, immunoblotting, microbeads arrays, and immunoassays methods are used. These methods allow the analysis of a relatively small fraction of selected proteins.
With mass spectrometry based proteomics, a diagnostic point of view can be developed by detecting potential biomarkers that will allow us to monitor wound healing [58]. Proteins from different tissues can be used in these studies [59-60].
However, despite the large number of proteins, which are the handicaps of proteomic studies, issues such as insufficient concentrations and the predominance of abundant protein groups limit these studies [61,62].
A gold standard method cannot currently be recommended for reproducible analyzes of proteins that are modified or expressed/ depressed in wound healing and derived from wound fluid.
In addition, chronic ulcers, diabetic wounds and pathological features due to venous stasis ulceration cause delay in the healing process. At the pathological level, abnormal formations such as fibrotic responses and adhesions also occur. When we add the contribution of cytokines and enzymes, controlling and optimizing the physiological responses to wound healing becomes complicated. Therefore, analytical research methodologies developed are of great importance
Microdialysis for Following of Wound Healing
Microdialysis is a method used to analyze low molecular weight proteins from the extracellular interstitium. A semipermeable membrane is designed like a blood capillary (mimic) and inserted into the wound. Dialysate obtained as wound fluid is used for protein analysis in situ studies. [59,61,63,64]. Samples collected from a specific site can help explain a dynamic physiological and pathophysiological process. For this reason, samples taken from tissue, skin, brain, eye and liver by microdialysis method are increasingly used to understand the molecular mechanism [59, 61].
The samples consist of molecules that cross the semi-permeable membrane with a concentration gradient. In fact, although the biological activities of cytokines are high, their concentrations in body fluids are low [64]. Therefore, analysis of cytokines and chemokines is useful as a direct method. It can be said that the most important benefit of microdialysis is that it allows the removal of wound fluid from the same place with a non-invasive method and the continuation of sample collection during the healing process [64].
The resulting micro dialysate is subjected to further investigation with traditional analysis methods such as radioimmunoassay or Enzyme Linked Immunosorbent Assays (ELISAs) [65].
These analytical methods have some limitations. However, characterization of many molecules can be done with “omics” techniques. It makes it possible to analyze molecules <2 kDa with metabolomics, which is among the omics techniques [66].
Microdialysates collected from samples not from the skin, but especially from various tissues, can be applied to metabolomics techniques. Among these analytical techniques, separation methods such as mass spectrometry liquid and gas chromatography or Nuclear Magnetic Resonance (NMR) spectroscopy are used [67].
Omics for Following of Wound Healing
Because the complex molecular mechanisms of wound healing have not been fully elucidated, we need tools to optimize the analysis of the healing process. To understand the molecular pathways involved in wound healing and to see the cascading links of different stages of the healing process, we need to know the regulatory mechanisms. Regulatory mechanisms of wound healing include genes, proteins, microRNAs, metabolites and drug molecules. All these molecules interact with each other through chemical reactions. In addition, the concentrations and interactions of molecules increase the complexity of the healing process.
MicroRNAs (miRNAs): miRNAs are important post transcriptional regulators and are being investigated as potential markers in the diagnosis and treatment of diseases. Our knowledge of miR-mediated gene regulation in wound healing is limited and “omics” research is gaining in importance.
miRs bind to the 3’ untranslated region of target mRNAs, causing destabilization and translational repression in mRNA [68].
Since miRNAs are regulators of the complex gene network, it has been suggested that they have essential biological functions in wound healing [69,70].
In addition, miR-based therapeutics constitutes a promising approach in the wound healing process [71].
Imbalance in the response to inflammation during wound healing may cause chronic inflammation and delay healing. It is suggested that miRNAs regulate inflammation in wound healing by target specific genes (Table 1). Table 1 lists 6 key mediators involved in the inflammation stage during wound healing and miRNAs regulating these proteins; TNF, transforming growth factor receptor 1, IL10, TNF receptor-associated factor 6, interleukin receptor-associated kinase, monocyte chemotactic protein 1, miR-125b, miR-128a, miR- 466l, miR-146a, and miR-124a [72].
Cytokine
miRNA
References
TNFa
miR-125b
28
TRAF6
miR146a
36
IRAK
miR146a
36
TGF-βR1
miR128a
47
IL-10
miR-466l
50
MCP-1
miR124a
32
Table 1: miRNA regulation of major proteins involved with wound inflammation [72].
TNF-a may have beneficial or detrimental effects on tissue repair depending on the concentration. Suppression of miR-125b expression controls TNF-a production [73]. miR-146 negatively affects Toll-Like Receptors (TLRs) by targeting TRAF and IRAK [74]. The gene expression of Macrophage Chemoattractant Protein (MCP- 1) increases approximately 70-times when the wound is formed [75]. IL-10, on the other hand, inhibits the pro-inflammatory response by inhibiting the STAT3-dependent pathway. On the other hand, when chronic and non-chronic wounds are evaluated to decide a biomarker, there was indicated that a lot of some Interleukines (IL-) entity such as IL-11, IL-1A, IL-1B, IL-12B, IL-8, IL-15, IL-23. However, target gene analyzes of these interleukins have not been performed [76].
The route used for proteomic analysis of proteins in wounds is generally described in (Figure 2).
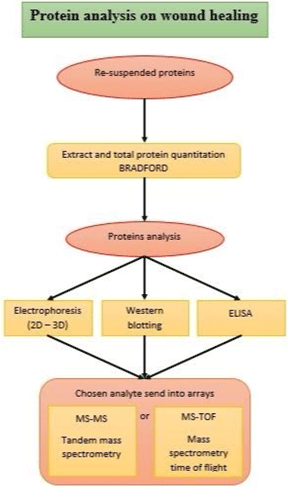
Figure 2: The routes of proteomic analysis as ordinary.
Potential biomarkers are determined by proteome analyzes applied for proteins that are involved in wound healing and that are expressed or depressed. Proteomics enables the analysis of specific proteins using Mass Spectrometry (MS), a fast and reliable method. It is possible to quantify the analytes in the analyzes made with MS, at the same time the analysis of protein mixtures is carried out sensitively [77]. Protein analyzes in biological tissue samples were presented in studies using the Imaging MS (MALDI IMS) technique [78].
While analysis is performed on minimal samples in biological processes, protein analysis is also possible in frozen or paraffin-fixed tissue samples, thus detecting the healing process of the damaged tissue. MALDI IMS studies on chronic wounds caused by pathological features are promising for therapeutic approaches [79].
Nanofibrous biomaterial for wound healing: Nanotechnological approaches emerge as one of the current methodologies in the healing of acute and chronic wounds [80].
Studies on the variety of synthetic or natural nanomaterials such as nanofiber mats and scaffolds in wound healing have come to the fore in recent years. The aim here is to develop a treatment approach that will assume the role of a blood clot in the wound area. In natural functioning, during the healing of the wound under in vivo conditions, fibrinogen, a plasma protein, is formed by blood coagulation, and then fibrin degradation process (fibrinolysis) occurs with enzymatic degradation [81,82].
Meanwhile, the fibrous blood clot behaves like an Extracellular Matrix (ECM) and fibroblasts and endothelial cells migrate to the wound site [83,84].
Because of this relationship, the use of nanoscaffolds as an accelerating factor in blood coagulation and wound healing, that is, the use of fibrinogen nanofibers, has gained importance. Although it is possible to use nanofibrous fibrinogen in different sizes with electrospining, this method has not yet become optimal because it can cause changes in the natural protein structure [85,86].
One of the important handicaps is that the biological function of nanofibrous scaffolds can change due to electro spinning and protein degradation occurs. For the use of nanofibrous scaffold in wound healing, it would be appropriate to eliminate problems such as organic solvents and acidic conditions [87]. Changed total protein concentrations is other problem because of collection process of fluid of wound affects total protein concentration. We know that total protein concentration is not change just depend on sample collection method, meanwhile there is occurred protein oxidation. Oxidation on proteins of skin or fluid of wound is formed loss of total protein concentration, actually carbonylated proteins are formed after oxidation.
Conclusion
Wound healing process has complex molecular pathways, intermediators, and it is open to different pathological challenges at the same time. We need to understand all parameters such as pathological conditions, loss of total protein concentration, pH effect, changed aging condition to improve healing process of wound. Modern approaches are focused on omics technology. These approaches will allow the identification of biomarkers of molecular pathways.
Author Disclosure
The authors declare have no commercial associations and no competing financial interest.
Acknowledgements
This work was not supported by grant.
References
- Boateng JS, Pawar HV, Tetteh J. Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. International journal of pharmaceutics. 2013; 441: 181-191.
- Wu SC, Marston W, Armstrong DG. Wound care: the role of advanced wound healing technologies. Journal of vascular surgery. 2010; 52: 59S-66S.
- S. Enoch, K. Harding, Wound Bed Preparation: The Science Behind the Removal of Barriers to Healing. Wounds. 2003; 15: 17-25.
- Falanga V. Wound healing and its impairment in the diabetic foot. The Lancet. 2005; 366: 1736-1743.
- Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clinics in dermatology. 2007; 25: 19-25.
- DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. The Journal of clinical investigation. 1998; 101: 1693-1698.
- Shah A, Amini-Nik S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. International Journal of Molecular Sciences. 2017; 18: 1068.
- Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on woundhealing: a new perspective for wound-therapy?. Archives of Dermatological Research. 2006; 298: 413-420.
- Rippke F, Schreiner V, Schwanitz H. The acidic milieu of the horny layer: new findings on the physiology and pathophysiology of skin pH. American journal of clinical dermatology. 2002; 3: 261-72.
- Izadi K, Ganchi P. Chronic wounds. Clinics in plastic surgery. 2005; 32: 209- 222.
- Tsukada K, Tokunaga K, Iwama T, Mishima Y. The pH changes of pressure ulcers related to the healing process of wounds. Wounds. 1992; 4: 16–20.
- Leveen H, Falk G, Borek B, Diaz C, Lynfield Y, Wynkoop B, et al. Chemical acidification of wounds—adjuvant to healing and unfavorable action of alkalinity and ammonia. Ann Surg. 1973; 178: 745–53.
- Dissemond J, Witthoff M, Brauns T, Haberer D, Goos M. pH values in chronic wounds. Evaluation during modern wound therapy. Hautarzt. 2003; 54: 959– 65.
- Wilson IA, Henry M, Quill RD, Byrne PJ. The pH of varicose ulcer surfaces and its relationship to healing. VASA. Zeitschrift fur Gefasskrankheiten. 1979; 8: 339-42.
- Romanelli M, Schipani E, Piaggesi A, Barachini P. Evaluation of surface ph on venous leg ulcers under Allevyn Dressings. London: Royal Society of Medicine Press, 1997.
- Roberts G, Hammad L, Creevy J, Shearman C, Mani R. Physical changes in dermal tissues around chornic venous ulcers. 7th European Conference on Advances in Wound Management 1997 Harrogate, UK: 104–5.
- Gethin G. The significance of surface pH in chronic wounds. Wounds UK. 2007; 3: 52–6.
- Sharpe JR, Harris KL, Jubin K, Bainbridge NJ, Jordan NR. The effect of pH in modulating skin cell behaviour. British Journal of Dermatology. 2009; 161: 671-673.
- Rubin H. pH and population density in rehulation of animal cell manipulation. J Cell Biol. 1971; 51: 686–702.
- Percival SL, McCarty S, Hunt JA, Woods EJ. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair and Regeneration. 2014; 22: 174-186.
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. The New England journal of medicine. 1997; 337: 1419-1429.
- Gilchrest BA, Garmyn M, Yaar M. Aging and photoaging affect gene expression in cultured human keratinocytes. Archives of dermatology. 1994; 130: 82.
- Tsuchida Y. The effect of aging and arteriosclerosis on human skin blood flow. Journal of dermatological science. 1993; 5: 175-181.
- Gniadecka M, Serup J, Sondergaard J. Age-related diurnal changes of dermal oedema: evaluation by high-frequency ultrasound. Br J Dermatol. 1994; 131: 849–85.
- Grigorova-Borsos AM, Bara L, Aberer E, Grochulski A, André J, Mozère G, et al. Aging and diabetes increase the aggregating potency of rat skin collagen towards normal platelets. Thrombosis and haemostasis. 1988; 60: 075-078.
- Silverman EM, Silverman AG. Granulocyte adherence in the elderly. American journal of clinical pathology. 1977; 67: 49-52.
- Yonezawa Y, Kondo H, Nomaguchi TA. Age-related changes in serotonin content and its release reaction of rat platelets. Mechanisms of Ageing and Development. 1989; 47: 65-75.
- Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. Agedependent impairment of angiogenesis. Circulation. 1999; 99: 111-120.
- Doria G, Frasca D. Regulation of Cytokine Production in Aging Mice. Annals of the New York Academy of Sciences. 1994; 741: 299-304.
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual review of medicine. 2000; 51: 245-270.
- Ginaldi L, Martinis MD, D’Ostilio A, Marini L, Loreto F, Modesti M, et al. Changes in the expression of surface receptors on lymphocyte subsets in the elderly: Quantitative flow cytometric analysis. American Journal of Hematology. 2001; 67: 63-72.
- Plackett TP, Schilling ME, Faunce DE, Choudhry MA, Witte PL, Kovacs EJ. Aging enhances lymphocyte cytokine defects after injury. The FASEB Journal. 2003; 17: 688-689.
- Reed MJ, Ferara NS, Vernon RB. Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mechanisms of Ageing and Development. 2001; 122: 1203- 1220.
- Puolakkainen PA, Twardzik DR, Ranchalis JE, Pankey SC, Reed MJ, Gombotz WR. The enhancement in wound healing by transforming growth factor-beta 1 (TGF-beta 1) depends on the topical delivery system. The Journal of surgical research. 1995; 58: 321-9.
- Puolakkainen P, Twardzik DR, Ranchalis JE, Pankey SC, Reed MJ, Gombotz WR. The Enhancement in Wound Healing by Transforming Growth Factor-β1 (TGF-β1) Depends on the Topical Delivery System. Journal of Surgical Research. 1995; 58: 321-329.
- Plisko A, Gilchrest BA. Growth factor responsiveness of cultured human fibroblasts declines with age. Journal of gerontology. 1983; 38: 513-518.
- West MD. The cellular and molecular biology of skin aging. Arch. Dermatol. 1994; 130: 87–95.
- Bruce SA, Deamond SF. Longitudinal study of in vivo wound repair and in vitro cellular senescence of dermal fibroblasts. Experimental Gerontology. 1991; 26: 17-27.
- Butcher EO, Klingsberg J. Age, gonadectomy and wound healing in palatal mucosa of the rat. Oral Surg. 1963; 16: 482–492.
- Billingham RE, Russell PS. Studies on wound healing with special reference to the phenomenon of contraction in experimental wounds in rabbits’ skin. Ann. Surg. 1956; 144: 961–981.
- Cuthbertson AM. Contraction of full thickness skin wounds in the rat. Surg. Gynecol. Obstet. 1959; 108: 421–432.
- Fatah MF, Ward CM. The morbidity of split-skin graft donor sites in the elderly: the case for mesh-grafting the donor site. British journal of plastic surgery. 1984; 37: 184-190.
- Orentreich N, Salmanowitz VJ. Levels of biological functions with aging. Trans N Y Acad Sci. 1969; 2: 992–1012.
- Strigini L, Ryan T. Wound healing in elderly human skin. Clinics in dermatology. 1996; 14: 197-206.
- Platt D, Rühl W. An age-dependent determination of lysosomal enzyme activities, as well as the measurements on the incorporation of 14-C-proline and 14-C-glucosamine in a subcutaneously implanted polyether sponge. Gerontologia. 1972; 18: 96-112.
- S Younai, L S Nichter, T Wellisz, J Reinisch, M E Nimni, T L Tuan. Modulation of collagen synthesis by transforming growth factor-beta in keloid and hypertrophic scar fibroblasts. Ann Plast Surg. 1994; 33: 148–151.
- Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet 1992; 339: 213–214.
- Puolakkainen P, Twardzik DR, Ranchalis JE, Pankey SC, Reed MJ, Gombotz WR. The Enhancement in Wound Healing by Transforming Growth Factor-β1 (TGF-β1) Depends on the Topical Delivery System. Journal of Surgical Research. 1995; 58: 321-329.
- Halasz NA. Dehiscence of laparotomy wounds. American journal of surgery. 1968; 116: 210-214.
- Capewell S, Reynolds S, Shuttleworth D, Edwards C, Finlay AY. Purpura and dermal thinning associated with high dose inhaled corticosteroids. British Medical Journal. 1990; 3001: 548-1551.
- Gosain A, DiPietro LA. Aging and Wound Healing. World Journal of Surgery. 2003; 28: 321-326.
- Salbach J, Rachner TD, Rauner M, Hempel U, Anderegg U, Franz S, et al. Regenerative potential of glycosaminoglycans for skin and bone. Journal of Molecular Medicine. 2011; 90: 625-635.
- Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair and Regeneration. 2000; 8: 13-25.
- Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. Journal of vascular surgery. 2009; 49: 1013-1020.
- Utz ER, Elster EA, Tadaki DK, Gage F, Perdue PW, Forsberg JA, et al. Metalloproteinase expression is associated with traumatic wound failure. The Journal of surgical research. 2010; 159: 633-639.
- GP Ladwig, MC Robson, RAN Liu, MA Kuhn, DF Muir, GS Schultz. Ratios of activated matrix metalloproteinase- 9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair and Regeneration, 2002; 10: 26–37.
- Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair and Regeneration. 2009; 17: 832-839.
- Broadbent J, Walsh T, Upton Z. Proteomics in chronic wound research: Potentials in healing and health. PROTEOMICS – Clinical Applications. 2010; 4: 204-214.
- Aksoy H, Demirbag, Sen A, Sekerler T, Özakpinar, Sener A, et al. Evaluation of biochemical parameters in Rubus tereticaulis treated rats and its implications in wound healing. Molecular and Cellular Biochemistry. 2020; 472: 67-78.
- Maurer MH, Berger C, Wolf M, Fütterer CD, Feldmann RE, Schwab S, et al. The proteome of human brain microdialysate. Proteome Science. 2003; 1: 7-7.
- Yang CS, Tsai PJ, Chen WY, Liu L, Kuo JS. Determination of extracellular glutathione in livers of anaesthetized rats by microdialysis with on-line high-performance liquid chromatography. Journal of chromatography. B, Biomedical applications. 1995; 667: 41-48.
- Förster Y, Gao W, Demmrich A, Hempel U, Hofbauer LC, Rammelt S. Monitoring of the first stages of bone healing with microdialysis. Acta Orthopaedica. 2013; 84: 76-81.
- G Wei, P-T Ding, J Zheng, W Lu. Pharmacokinetics of timolol in aqueous humor sampled by microdialysis after topical administration of thermosetting gels. Biomedical Chro- matography. 2006; 20: 67–71.
- Gill C, Parkinson E, Church MK, Skipp P, Scott D, White AJ, et al. A Qualitative and Quantitative Proteomic Study of Human Microdialysate and the Cutaneous Response to Injury. The AAPS Journal. 2011; 13: 309-317.
- Petersen LJ, Sørensen MA, Codrea MC, Zacho HD, Bendixen E. Large pore dermal microdialysis and liquid chromatography-tandem mass spectroscopy shotgun proteomic analysis: a feasibility study. Skin Research and Technology. 2013; 19.
- U. Microdialysis--principles and applications for studies in animals and man. J Intern hMed 1991; 230: 365–73.
- Schenk T, Irth H, Marko-Varga G, Edholm LE, Tjaden UR, Greef JVD. Potential of on-line micro-LC immunochemical detection in the bioanalysis of cytokines. Journal of pharmaceutical and biomedical analysis. 2001; 26: 975-985.
- Shippenberg TS, Thompson AC. Overview of Microdialysis. Current Protocols in Neuroscience. 2001; 7.
- Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, McLean JA. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. Journal of The American Society for Mass Spectrometry. 2016; 27: 1897-1905.
- Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS. Quantitative Metabolomics by 1H-NMR and LC-MS/MS Confirms Altered Metabolic Pathways in Diabetes. PLoS ONE. 2010; 5: e10538.
- Stavast CJ, Erkeland SJ. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells. 2019; 8: 1465.
- Herter E K, Xu Landen N. Non-Coding RNAs: New Players in Skin Wound Healing. Adv Wound Care (New Rochelle), 2017; 6: 93-107.
- Meng Z, Zhou D, Gao Y, Zeng M, Wang W. miRNA delivery for skin wound healing. Advanced Drug Delivery Reviews. 2018; 129: 308-318.
- Pastar I, Marjanovic J, Stone RC, Chen V, Burgess JL, Mervis JS, et al. Epigenetic regulation of cellular functions in wound healing. Experimental Dermatology. 2021; 30: 1073-1089.
- Roy S, Sen CK. miRNA in Wound Inflammation and Angiogenesis. Microcirculation. 2012; 19: 224-232.
- Tili E, Michaille J, Cimino A, Costinean S, Dumitru CD, Adair B, et al. Modulation of miR-155 and miR-125b Levels following Lipopolysaccharide/ TNF-a Stimulation and Their Possible Roles in Regulating the Response to Endotoxin Shock1. The Journal of Immunology. 2007; 179: 5082-5089.
- Taganov KD, Boldin MP, Chang K, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences. 2006; 103: 12481-12486.
- Nakamachi Y, Kawano S, Takenokuchi M, Nishimura K, Sakai Y, Chin T, et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis and rheumatism. 2009; 60: 1294- 1304.
- Laura E Edsberg, Jennifer T Wyffels, Michael S Brogan DPT, Kristin M Fries. Analysis of the proteomic profile of chronic pressure ulcers. Wound Repair and Generation. 2012; 20: 378-401.
- Seeley EH, Caprioli RM. Molecular imaging of proteins in tissues by mass spectrometry. Proceedings of the National Academy of Sciences. 2008; 105: 18126-18131.
- Tetik S, Kaya K, Yardimci T. Effect of Oxidized Fibrinogen on Hemostatic System: In Vitro Study. Clinical and Applied Thrombosis/Hemostasis. 2011; 17: 259-263.
- Schwartz SA, Caprioli RM. Imaging mass spectrometry: viewing the future. Methods in molecular biology. 2010; 656: 3-19.
- Vickerman JC. Molecular imaging and depth profiling by mass spectrometry- -SIMS, MALDI or DESI?. The Analyst. 2011; 136: 2199.
- Hamdan S, Pastar I, Drakulich S, Dikici E, Tomic-Canic M, Deo S, et al. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Central Science. 2017; 3: 163-175.
- Taverna D, Pollins AC, Sindona G, Caprioli RM, Nanney LB. Imaging Mass Spectrometry for Assessing Cutaneous Wound Healing: Analysis of Pressure Ulcers. Journal of Proteome Research. 2015; 14: 986-996.
- LAURENS N, KOOLWIJK P, MAAT MPMD. Fibrin structure and wound healing. Journal of Thrombosis and Haemostasis. 2006; 4: 932-939.
- Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Advanced drug delivery reviews. 2007; 59: 1413-1433.