
Research Article
Thromb Haemost Res. 2022; 6(3): 1082.
Diffuse Large B-cell Lymphoma in Adults at Chris Hani Baragwanath Academic Hospital
Patel M*, Machailo JT, Philip V, Lakha A and Waja MF
Clinical Haematology Unit, Department of Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, South Africa
*Corresponding author: Patel M, Emeritus Professor, Clinical Haematology Unit, Department of Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, P O Box 96092, Brixton, 2019, Johannesburg, South Africa
Received: September 02, 2022; Accepted: October 13, 2022; Published: October 20, 2022
Abstract
Background: Diffuse Large B-Cell Lymphoma (DLBCL) is the most common subtype of Non-Hodgkin lymphoma (NHL). DLBCL is a heterogenous disease and is the most common subtype of NHL occurring in Human Immunodeficiency Virus (HIV) seropositive individuals.
Aim: The aim of the study was to review the clinical profile as well as the outcome of adult patients presenting with DLBCL, to a tertiary public sector hospital (Chris Hani Baragwanath Academic Hospital – CHBAH) in Soweto, Johannesburg, South Africa.
Patients and Methods: The study entailed a retrospective review of 139 evaluable patients with DLBCL, over a 5 year period.
Results: Of the 139 patients reviewed, there were 73 females (53%) and 66 males (47%), with a female: male ratio of 1.1:1. The median age of the patients was 41 years (14-85). Common presenting features included advanced stage disease (83%), constitutional or ‘B’ symptoms (74%), extra-nodal disease (73%) and lymphadenopathy (64%). 81% of the patients were HIV seropositive. The median overall survival was 24 months.
Conclusion: DLBCL accounted for 35% of all the patients with NHL during the study period. HIV seropositivity, together with other factors such as significant delays in referral of the patients, late presentations with advanced stage disease, and comorbidities such as Tuberculosis, impacted negatively on the prognosis and the outcome of the patients with DLBCL. Despite the use of Combination Antiretroviral Therapy (cART), appropriate supportive care and specific modalities of treatment, DLBCL continues to pose a challenge in our clinical setting.
Keywords: HIV; DLBCL; NHL; South Africa
Introduction
Diffuse Large B-Cell Lymphoma (DLBCL) is the most common subtype of Non-Hodgkin Lymphoma (NHL) in both developed and developing countries, accounting for approximately one third of all adult patients presenting with NHL [1,2]. DLBCL is an aggressive subtype of NHL, with heterogeneity regarding the clinical presentation, morphological characteristics, immunophenotype, molecular expression and treatment outcomes [2,3].
HIV is highly prevalent in sub-Saharan Africa with South Africa serving as the epicentre for the pandemic. Based on data from statistics South Africa, there are approximately 8.2 million people living with HIV (PLWHIV) in South Africa in 2021, with an estimated overall HIV prevalence rate of 13.7% of the population [4]. DLBCL is also the most common HIV associated lymphoma, accounting for approximately 40-50% of all the NHL’s seen in HIV seropositive individuals [5].
The aim of this study was to review the clinical characteristics as well as the outcome of all adult patients with DLBCL at the Clinical Haematology Unit, Department of Medicine, CHBAH and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, over a five year period – 01-01-2008 to 31-12-2012.
The findings of this study, which formed part of the Master of Medicine dissertation of Dr JT Machailo (University of the Witwatersrand, 2016) are presented [6].
Patients and Methods
The study was a retrospective review of all evaluable adult patients with a histologically confirmed diagnosis of DLBCL, referred to and managed by the Clinical Haematology Unit, Department of Medicine, Chris Hani Baragwanath Academic Hospital over a 5-year period – 01-01-2008 to 31-12-2012.
Chris Hani Baragwanath Academic Hospital is a large, tertiary, public sector, University of the Witwatersrand linked teaching hospital, located in Soweto, Johannesburg, South Africa. It serves a population in excess of 1 million individuals who live in Soweto and the Southern part of the Gauteng Province. It is also a referral hospital for other provinces such as the Northwest Province [5].
A total of 451 patients were diagnosed with NHL between 2008 and 2012. Of these patients, 156 (35%) were diagnosed with DLBCL. However, for various reasons including inadequate information and incomplete work-up, only 139 (89%) patients were evaluated in the current study.
Data was collected retrospectively from the patient files and NHLS (National Health Laboratory Services), after obtaining permission from the relevant authorities and the Human Research Ethics Committee (HREC), University of the Witwatersrand, Clearance Certificate Number: M130828 [6].
Data collection using a questionnaire focused largely on the objectives of the study: obtaining information on the diagnosis, demographics, clinical presentation, prognostic factors, and management. The information was entered onto an Excel spreadsheet and analysed using the appropriate statistical tests and with the assistance of a statistician.
Results
A total of 139 evaluable patients with DLBCL were seen during the period 01-01-2008 and 31-12-2012. There were 73 females (53%) and 66 males (47%), with a female to male ratio of 1.1:1. The median age at presentation was 41 years, with a range of 14-85 years. Where the Performance Status (PS) was documented, 52.5% of the patients had a good PS of 0 or 1, and 27.4% of the patients had a PS of ≥2.
The symptoms and signs of the patients at presentation is shown in (Table 1). Common presenting symptoms and signs include: ‘B’ symptoms or constitutional symptoms (weight loss, night sweats, fever), with at least 1 one of these symptoms being present in 74% of the patients, extra-nodal disease (73%) and lymphadenopathy in 64% of the patients. The three most common extra-nodal sites of disease included the liver (18%), GIT (Gastrointestinal Tract/bowel) (16%) and the respiratory system (14%). In patients manifesting with extranodal disease, this was detected on imaging in 87% of the patients and clinically in 83% of the patients
Frequency
Percent
Unexplained weight loss
94
68%
Night sweats
76
55%
Fever
58
42%
‘B’ Symptoms (at least one present)
103
74%
Infections
29
21%
Pallor
23
17%
Jaundice
27
19%
Lymphadenopathy
89
64%
Splenomegaly (clinical)
2
1%
Hepatomegaly (clinical)
25
18%
GIT
22
16%
CNS
5
4%
Respiratory
19
14%
Renal and urinary tract
2
1%
Bone marrow
12
9%
Multiple sites of extra-nodal disease
29
21%
Skin
1
1%
Gynaecological (vulva, cervix, uterus)
2
1%
Breast
2
1%
Table 1: Symptoms and signs at presentation.
The laboratory results at presentation are shown in (Table 2). Anaemia (≥ grade 1) was evident in 55% of the patients, while leukopenia was present in 59% of the patients and thrombocytopenia in 5%. Leucocytosis was evident in 5% and thrombocytosis in 26% of the patients. A raised LDH (Lactate Dehydrogenase) level was evident in 98% of the patients, while a raised Beta 2 microglobulin was present in 88% of the patients. 37% of the patients had an albumin level below 30g/l, while 46% had a raised alkaline phosphatase and 37% a raised GGT (Gamma Glutamyl Transferase) level.
Variable
n
Median
Mean
Range
n(%)
Haemoglobin (g/dl)
133
10.70
11.37
4.3 -137
Anemia<11g/dl
133
73 (55%)
WhiteCellCount (x109/l)
96
3.65
4.41
0.1 -19.54
Leukopenia<4x109/l
96
57 (59%)
Normal
96
34 (35%)
Leukocytosis> 11x109/l
96
5 (5%)
Platelets (x109/l)
132
335
355.73
7-899
Thrombocytopenia<100x109/l
132
6 (5%)
Thrombocytosis>450x109/l
132
34 (26%)
LDH (U/L)
84
826.5
1254.12
174.3- 8486
Raised-ULN
84
82 (98%)
Beta 2 microglobulin (mg/l)
48
4.05
5.06
1.9 -16
Raised-ULN
48
42 (88%)
Calcium (mmol/l)
92
2.345
2.38
1.89-4.48
Hypercalcaemia> 2.75
92
2 (2%)
Urea (mmol/l)
118
4.8
6.08
1.9 -25.5
Creatinine (umol/l)
119
66
80.18
27-715
>173 umol/l
119
4 (3%)
Albumin (g/l)
99
32
31.74
14-53
<40 g/l
99
83 (84%)
<35 g/l
99
63 (64%)
<30 g/l
99
37 (37%)
Alkalinephosphatase (U/L)
97
97
149.72
40-1047
Raised-ULN
97
45 (46%)
GGT(U/L)
97
46
109.75
13-990
Raised-ULN
97
36 (37%)
CD4 Count (cells/ul)
101
144
205.37
3-1351
<350 cells/ul
101
21 (21%)
<200 cells/ul
101
31 (31%)
<100 cells/ul
101
31 (31%)
CD4-Cluster Designation 4; GGT-Gamma Glutamyl Transferase; LDH-Lactate Dehydrogenase; ULN-Upper Limit of Normal; U/L-Units Per Litre
Table 2: Laboratory results at presentation.
Based on the Ann Arbor staging classification [7], only 14.4% had stage I or stage II disease. The vast majority of the patients had advanced stage disease (stage III and IV – 83.4%) (Figure 1).
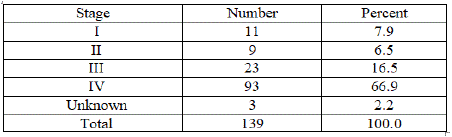
Figure 1: Ann Arbor stage.
Furthermore, using the IPI (International Prognostic Index) score [8], 32.4% of the patients had low risk disease, 4.3% high risk disease and the majority, i.e. 63.3% had intermediate risk disease (Figure 2).
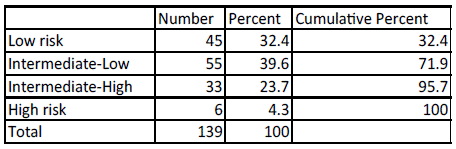
Figure 2: International Prognostic Index (IPI) score.
Of the 139 patients, 112 were HIV seropositive (81%), 21 patients (15%) were HIV seronegative and in 6 patients (4%), the HIV serology was unknown. The mean duration of HIV seropositivity prior to the lymphoma diagnosis was 22 months. Approximately one third (31.3%) of the HIV seropositive patients were not on Combination Antiretroviral Therapy (cART) at the time of the lymphoma diagnosis, despite being HIV seropositive.
The median age for the HIV seropositive patients was 39 years, while the median age for the HIV seronegative patients was 53 years. Surprisingly, the gender ratio (F:M) in the HIV seronegative patients was 1.6:1, compared to the HIV seropositive group, where it was 1.04:1. The median CD4 count in the HIV seropositive patients was 144 cells/μl, with a range of 3-1351 cells/ul. The CD4 count in 101/112 patients in whom it was documented, is shown in (Table 3).
Category
Number
Percent
CD4count (cells/ul)
<100
31
31%
100-200
31
31%
200 – 350
21
21%
350 – 500
10
10%
>500
8
7%
CD4-cluster designation 4
Table 3: CD4 counts at presentation in HIV seropositive patients.
Table 4 shows a comparison of the HIV seropositive and HIV seronegative group of patients.
All patients
Positive
Negative
P-value
Test
Conducted
139
112
21
Median age at
diagnosis
41(14 -85)
39(14 -66)
53(22 -85)
0.009
Mann-
Whitney UMale: Female
ratio
1:1.1
1:1.04
1:1.6
0.476
Chi-square
%seropositivity
81%
15%
0.000
Chi-square
‘‘B’ Symptoms
105 (76%)
84 (75.0%)
19 (90.5%)
0.159
Chi-square
Ann Arbor
Stage III/IV
116
(83.5%)
93 (83.8%)
19 (90.5%)
0.740
Chi-square
LDH increase
76 (54.7%)
64 (57.1%)
12 (57.1%)
1.000
Chi-square
PS=2
38 (27.4%)
30 (26.8%)
6 (28.6%)
1.000
Chi-square
Extra-nodal
disease
101
(72.7%)
80 (71.4%)
17 (81.0%)
0.434
Chi-square
Positive TB
Association
42 (30.2%)
38 (33.9%)
3 (14.3%)
0.120
Chi-square
IPI score
Low risk
45 (32.4%)
38 (33.9%)
3 (14.3%)
0.038
Chi-square
Intermediate
Low
55 (39.6%)
44 (39.3%)
11 (52.4%)
Intermediate
high
33 (23.7%)
27 (24.1%)
4 (19.0%)
High risk
6 (4.3%)
3 (2.7%)
3 (14.3%)
ABC/GCB
GCB
48 (34.5%)
36 (32.1%)
10 (8.9%)
0.191
Chi-square
ABC
36 (25.9%)
31 (27.7%)
3 (2.7%)
Mixed
23 (16.5%)
19 (17.0%)
2 (1.8%)
No records/
Failed
32 (23.0%)
7 (6.3%)
0 (0.0%)
Outcome
Alive
30 (21.6%)
25 (22.3%)
4 (19.0%)
0.845
Chi-square
Dead
56 (40.3%)
44 (39.6%)
10 (47.6%)
Lost to
Follow up
49 (35.3%)
41 (36.6%)
7 (33.3%)
Unknown
4 (2.9%)
2 (1.8%)
0 (0.0%)
ABC-activated B-cell; GCB-germinal centre B-cell; LDH-lactate dehydrogenase; IPI-International Prognostic Index; PS-performance status; TB-tuberculosis
Table 4: Comparison of HIV seropositive and seronegative patients.
All patients received appropriate supportive care (analgesics, allopurinol, antibiotics, blood and blood products etc.), where indicated. With regard to specific treatment, the majority of patients (124/139-89%) received combination chemotherapy.
The remaining 11% did not receive chemotherapy as they died shortly after the diagnosis, or during the work up of the NHL. In 96% of the patients, CHOP (cyclophosphamide, hydroxydaunorubicin/ Adriamycin, oncovin/vincristine, prednisone) was used as the initial chemotherapeutic regimen. A variety of other regimens were used as second line treatment, including R-CHOP (rituximab plus CHOP), CHOEP (etoposide plus CHOP), R-CHOEP (rituximab plus CHOEP), etc. The response to treatment and survival is shown in (Table 5).
Number
Percent
Response to initial treatment (complete/partial/no response)
Complete
36
26%
Partial
17
12%
Progression of disease/Died
38
27%
Lost to follow up/ less than partial response / unknown
48
35%
Table 5: Response to treatment after initial treatment.
Figure 3 & 4 shows the Kaplan-Meier survival curves for the patients in this study.
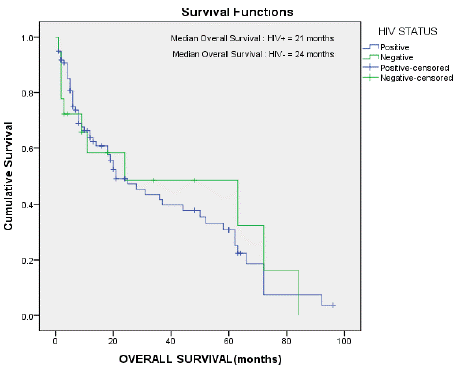
Figure 3: Median overall survival in the HIV seropositive and seronegative
patients.
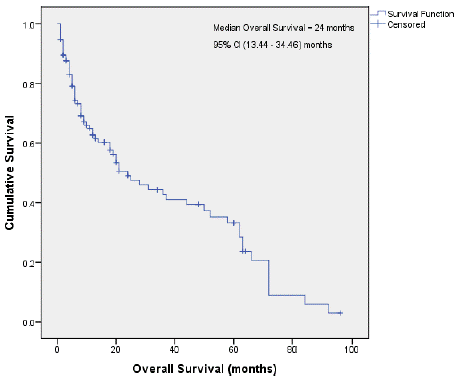
Figure 4: Median overall survival.
The median overall survival for the HIV seropositive patients was 21 months and for the HIV seronegative patients 24 months, respectively. The median survival for all the patients on the study was 24 months (Figures 3 & 4).
Discussion
Non Hodgkin Lymphoma (NHL) constitutes a hetogenous group of lymphoid malignancies with a variable clinical and biological spectrum [1-3,10]. NHL is the most common haematological malignancy in adults in South Africa [5,9]. The high prevalence of NHL in Southern Africa, including South Africa is contributed to mainly by the ongoing HIV pandemic, despite the widespread use of Combination Antiretroviral Therapy (cART). Diffuse Large B-Cell Lymphoma (DLBCL) is the most common subtype of NHL, accounting for 30-40% of NHL worldwide, and for 60-70% of the aggressive B-cell lymphomas [1,5,10,11].
NHL is the most common haematological malignancy encountered in adults at CHBAH, and DLBCL is the most common subtype of NHL seen in our patients [5]. In this study, DLBCL accounted for 35% of all the patients with NHL during the study period. Moreover, DLBCL is the most frequently observed subtype in both HIV seropositive and HIV seronegative patients, with 81% of the patients being seropositive in this study.
Of the 139 patients studied, there was a slight female predominance of 1.1:1. This supports the trend that has been noted with particular reference to HIV, where there is a higher female to male ratio. The younger median age of 41 years is in keeping with previous studies done at our institution and is a reflection of both the younger age structure of the population in Africa as well as the younger age at which HIV seropositive patients present (median age of HIV seropositive patients is 39 years versus the median age for HIV seronegative patients which is 52 years) [9,11,12].
DLBCL is an aggressive B-cell lymphoma. Moreover, the majority of the patients were HIV seropositive. In keeping with this and historically noting that patients presenting at our state sector hospital tend to present late with advanced stage disease [11,12], including what has been noted in this study, i.e. stage III and IV disease - 83%, frequent ‘B’ symptoms (74%) and more extra-nodal disease (73%). These adverse prognostic factors impact unfavorably on the outcome of our patients. Furthermore, the IPI score, a measure of prognosis showed that approximately two thirds of the patients (67%) had an intermediate or high IPI score. A review of the laboratory parameters showed that 55% of the patients had WHO grade I anaemia (Hb < 11g/dl) at presentation, 98% had a raised LDH level and 88% a raised Beta 2 microglobulin level. More than one third of patients (37%) had a low albumin level (<30g/l) and a raised GGT and alkaline phosphatase level (37% and 46%, respectively).
As indicated previously, HIV is an important risk factor for lymphomagenesis [13,14]. Lymphoma is the most common haematological malignancy encountered in HIV seropositive individuals, with subtypes such as Burkitt lymphoma, plasmablastic lymphoma and DLBCL being the most frequently encountered [5,15- 19]. In addition, an increasing association with Hodgkin lymphoma has been noted [12]. It is noteworthy that despite 87% of the study population being HIV seropositive, approximately one third (31.3%) of these patients were not on cART at the time of the lymphoma diagnosis. Moreover, 31% had a CD4 count <100 cells/μl and 62% had a CD4 count <200 cells/μl. The absence of cART and the more advanced stage of HIV in these patients contribute to an increased infection rate and a less favorable outcome, as noted in this study. A positive tuberculosis association was noted in 34% of the HIV seropositive patients compared to 14% of the HIV seronegative patients.
Table 4 shows a comparison of the HIV seropositive and HIV seronegative patients. As the number in the HIV seronegative group is small, statistically significant differences were less likely to be encountered. However, the median age at presentation (p=0.009) and the IPI score (p=0.038), were noted to be statistically significantly different.
In the updated classification of lymphoid malignancies, a clear distinction is being made between the Cell Of Origin (COO) immunophenotype in DLBCL, with the GC (Germinal Center) derived phenotype having a better prognosis than the ABC (Activated B-Cell type) [10,16,20-22]. Surprisingly, the GC subtype was noted to be more common in the whole group of DLBCL patients, irrespective of the HIV status.
Patients with DLBCL at CHBAH are treated in a similar way to other patients with DLBCL with respect to supportive care and chemotherapy as the mainstay of specific treatment. However, CHOP rather than R-CHOP was the standard of care in this cohort of patients. This practice has changed somewhat, subsequent to 2012, which is the end date of this study, with rituximab now being available to state sector hospitals and the increasing use of rituximab in HIV seropositive patients based on evidence of safety and efficacy in a number of studies and the initiation of a prospective, randomised study of R-CHOEP versus CHOEP in patients with DLBCL at CHBAH since 2014 [5,23-25].
Based on the results of the patients treated in the current study, 38% achieved a response (26% - CR (Complete Response) and 12% - PR (Partial Response). Thirty patients died (27%) and the remaining 35% includes patients who achieved less than a partial response and those who were lost to follow up.
The median overall survival for all the patients in the study was 24 months. This generally poorer survival is attributed to significant delays in diagnosis and subsequent late referrals, late presentations with more advanced stage disease, more ‘B’ symptoms, more extranodal disease as well as the significant impact of HIV on NHL, presenting with a more aggressive histological subtype, atypical clinical and laboratory features, and the attendant comorbidities such as tuberculosis and other opportunistic infections, more myelosuppression, delays in giving chemotherapy on schedule, and ultimately, a poorer prognosis.
Conclusion
NHL is the most common haematological malignancy encountered in adults at CHBAH. DLBCL accounts for 35% of all the patients with NHL. HIV seropositivity is present in 81% of the patients with DLBCL and has a significant impact with regard to the presentation and outcome of the patients in our study. More recently, with the early introduction and continuation of cART, the institution of appropriate antibiotics and CNS prophylaxis, the liberal use of growth factors and more optimal chemotherapy with the early introduction of etoposide and rituximab and (where feasible, the use of infusional regimens and novel therapeutic agents), the use of autologous stem cell transplantation in patients with relapsed, chemo-sensitive disease, it is hoped that the outcome of patients with DLBCL treated at CHBAH, will improve significantly compared the outcome of the patients in this retrospective study.
DLBCL represents a heterogeneous conglomeration of clinicopathological entities [3]. The last decade has witnessed major advances in the classification of DLBCL, including the identification of novel DLBCL subgroups having unique and distinct genetic and molecular expression profiles, beyond the known Cell-Of-Origin (COO) molecular subtypes [3,20,26]. The discovery of these new molecular targets and drivers are likely to impact on the novel and improved therapeutic landscape of DLBCL, an aggressive B-cell NHL, and the most common subtype of NHL [3,20,26].
References
- Gascoyne RD, Campo E, Jaffe ES. Diffuse large B-cell lymphoma, NOS. In: WHO classification of tumours of haematopoietic and lymphoid tissues. Eds. SHSwerdlow, E Campo, NL Harris, ES Jaffe, SPileri, H Stein, J Thiele J. 4thEd. Lyon: IARC. 2017; 291-7.
- Smith SM and Vose JM. Treatment approach to diffuse large B-cell lymphomas. In: Management of Hematologic Malignancies. Eds. S. O’Brein, J. Vose and H.M. Kantarjian. Cambridge University Press. 1st Ed, 2011; 286- 307.
- Sehn LH and Salles G. Diffuse Large B-cell lymphoma. NEJM. 2021; 384: 842-858.
- Statistics South Africa (Stats SA), 2021.
- Patel M, Philip V, Omar T, Turton D, Candy G, Lakha A, et al. The impact of Human Immunodeficiency Virus (HIV) on Lymphoma in South Africa. Journal of Cancer Therapy. 2015; 6: 527-535.
- Machailo JT. Diffuse Large B-cell lymphoma in adults at Chris Hani Baragwanath Academic Hospital. Research report in partial fulfilment of the degree of Master of Medicine (Internal Medicine), University of The Witwatersrand, 2016.
- Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer research. 1971;31(11):1860-1.
- Project TIN-HsLPF. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. NEJM. 1993; 329: 987-994.
- Patel M, Philip V, Turton D, Omar T, Kosheva S, et al. The impact of HIV on Non-Hodgkin’s Lymphoma at Chris Hani Baragwanath Hospital. Abstract No. 0731, 12th Congress of the European Hematology Association. Haematologica. 2007; 92: 273.
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127: 2375-2390.
- Patel M. Haematology. In: Baragwanath Hospital 50 years – A Medical Miscellany. Eds. K Huddle K and A Dubb, Ultra Litho. 1994; 173-190.
- Patel M, Philip V, Fazel F. Human immunodeficiciency virus infection and Hodgkin’s Lymphoma in South Africa – An emerging problem. Advances in Hematology. 2011; 2011: 578163.
- Ziegler JL, Beckstead JA, Volberding PA, et al. Non-Hodgkin’s Lymphoma in 90 Homosexual Men: Relation to Generalized Lymphadenopathy and the Acquired Immunodeficiency Syndrome. NEJM. 1984; 311: 565-570.
- Tulpule A, Levine A. AIDS-related lymphoma. Blood reviews. 1999; 13: 147- 150.
- Wiggill TM, Mantina H, Willem P, Perner Y, Stevens WS. Changing Pattern of Lymphoma Subgroups at a Tertiary Academic Complex in a High-Prevalence HIV Setting: A South African Perspective. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011; 56: 460-466.
- Pather S, Mohamed Z, McLeod H, Pillay K. Large Cell Lymphoma: Correlation of HIV Status and Prognosis with Differentiation Profiles Assessed by Immunophenotyping. Pathology & Oncology Research. 2013; 19: 695-705.
- Magangane PS, Mohamed Z, Naidoo R. Diffuse large B-cell lymphoma in a high human immunodeficiency virus (HIV) prevalence, low-resource setting. South African Journal of Oncology. 2020; 4: a104.
- Pather S, Mashele T, Willem P, Patel M, Perner Y, et al. MYC status in HIV-associated Plasmablastic Lymphoma: Dual-colour CISH, FISH and immunohistochemistry. Histopathology. 2021; 79: 86-95.
- Abayomi EA, Somers A, Grewal R, Sissolak G, Bassa F, Maartens D, et al. Impact of the HIV epidemic and Anti-Retroviral Treatment policy on lymphoma incidence and subtypes seen in the Western Cape of South Africa, 2002-2009: preliminary findings of the Tygerberg Lymphoma Study Group. Transfusion and apheresis science: official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2011; 44: 161-166.
- Pather S, Patel M. HIV-associated DLBCL: Clinicopathological factors including dual-colour chromogenic in situ hybridisation to assess MYC gene copies. Annals of diagnostic pathology. 2022; 58: 151913.
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103: 275-282.
- Cassim S, Antel K, Chetty DR, Oosthuizen J, Opie J, Mohamed Z, et al. Diffuse large B-cell lymphoma in a South African cohort with a high HIV prevalence: an analysis by cell-of-origin, Epstein-Barr virus infection and survival. Pathology. 2020; 52: 453-459.
- Patel M, Philip V, Lakha A, et al. Trends in Non-Hodgkin Lymphoma (NHL) over the past two decades at Chris Hani Baragwanath Academic Hospital (CHBAH). Proceedings of the Haematology Oncology Symposium, Johannesburg. 2013.
- Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012; 119: 3245-3255.
- A multi-centre, prospective, randomized study of the efficacy and safety of Rituximab (R) in combination with Cyclophosphamide, Hydroxydaunorubicin/ Adriamycin, Oncovin/Vincristine, Etoposide, Prednisone (i.e.R-CHOEP) in comparison to CHOEP alone, in previously untreated (newly diagnosed) adult patients with Human Immunodeficiency Virus (HIV) related lymphoma in South Africa. Protocol No: NHL 001 SA. 2014 onwards. Study completed, but not yet published.
- Papageorgiou SG, Thomopoulos TP, Katagas I, Bouchla A, Pappa V. Prognostic molecular biomarkers in diffuse large B-cell lymphoma in the rituximab era and their therapeutic implications. Therapeutic Advances in Hematology. 2021; 12: 204062072110139.