
Short Communication
Thromb Haemost Res. 2023; 7(1): 1087.
Application of Nano-HydroxyApatite (n-HAp) for Wound Healing and Tissue Bioengineering
Haidar ZS
1BioMAT’X (HAiDARI+D+i LAB), Santiago, Chile
2Clínica Universidad de los Andes, Santiago, Chile
3Facultad de Odontología, Universidad de los Andes,Santiago, Chile
4Programa de Doctorado en BioMedicina, Facultad deMedicina, Universidad de los Andes, Santiago, Chile
5Centro de Investigacion e Innovación Biomedica (CiiB), Facultad de Medicina, Universidad de los Andes, Santiago, Chile
*Corresponding author: Ziyad S. Haidar (Haidar ZS)Dentist (DDS), Implantologist (Cert Implantol), Oral and Maxillofacial Surgeon (MSc OMFS), FRSC (Canada), FICD, FICS, MBA, PhD. Professor and Scientific Director, Faculty of Dentistry, Universidad de los Andes, Santiago de Chile. Founder and Head/Director of BioMAT’X R&D&I Research Group and Laboratory, (Laboratorio de Biomateriales, Farmacéuticos y Bioingeniería de Tejidos Cráneo-Máxilo-Facial), Biomedical Research and Innovation Center / Centro de Investigación e Innovación Biomédica (CiiB), Faculty of Medicine, Department for Research, Development and Innovation, Universidad de los Andes, Avenida Mons. Álvaro del Portillo 12.455 - Las Condes, Santiago de Chile.
Received: December 20, 2022; Accepted: January 24, 2023; Published: January 31, 2023
Introduction
PRéCIS- A wound, or tissue defect can be defined as a disruption in the normal structure and function that occurs due to internal or external injury. In tissue bioengineering, natural hemostasis, and the inflammatory phase stand at the beginning of the wound and defect healing cascade. Briefly, the restorative, regenerative or reparative response to tissue injury is governed/driven by resident and circulating cells, homing to the injury site that release signals (soluble mediators) generated from the extracellular matrix. For bone defects, optimal healing can be simply classified as either primary (1o) or secondary (2o). It undergoes acascade of complex, orderly, and predictable events, that include four/five over-lapping phases: hemostasis/haematoma formation, inflammation, proliferation, callus formation (or not), and remodeling (Haversian/osteonal bone remodelling). Herein, the bone healing type is dependent on the removal of a fracture haematoma and the fixation (stabilization) strategy performed. For example, optimal stability results in 1o bone healing, with no callus formation, normal bone architecture and functional integrity restored within 3 months. On the other hand, in 2o healing, where fixation is not done, fracture haematoma, callus formation and healing via endochondral ossification ensues.
Despite limitations, the main alternative for damaged or lost bone tissue replacement, restoration, regeneration, reconstruction, or repair is the autogenous bone graft, to date.
Keywords: Biomaterials; Bioengineering; Wound healing; Growth factors; Nano-hydroxyapatite; Nanotechnology.
Bone and Bone Bioengineering
Is a natural organic–inorganic ceramic composite consisting of collagen fibrils containing embedded, well-arrayed, nano-crystalline and rod-like inorganic materials (25–50 nm in length). The restoration, regeneration, repair and/or reconstruction of orthopaedic as well as cranio-maxillo-facial and oral bone defects continues to represent one of the greatest challenges in clinical practice, today. Indeed, various localized and systemic bone defects can arise from wounds, tumors, infections, and ageing, amongst other factors; a major health care burden, World-wide, as well as a challenging clinical and surgical concern regarding the identification, selection and use/application of an appropriate bone biomaterial and/or substitute. Herein, tissue bio-engineering, an inter-, intra-, and multi-disciplinary field that applies the principles of engineering and life sciences, aims to develop and introduce bio-solutions that have the potential to restore, maintain or improve tissue function and reduce the complications related to current or traditional treatment methods. Thus, organic–inorganic composite scaffold and matrix materials, incorporating hydroxyapatite (HAp), are deemed attractive. Indeed, bone tissue bio-engineering solutions are to combine, in situ, at least: (a) isolated cells or cell substitutes (to replace the limited functions of the defected tissue or bone wound); (b) tissue-inducing substances (such as growth factors and cytokines, preferably incorporated into release-controlled drug delivery systems); and (c) scaffolds (suitable to favor/support and direct proper tissue development). The scaffold should be biocompatible, preferably biodegradable, mechanically-stable (and supportive), exhibit favorable surface properties (promoting adhesion, proliferation, and differentiation of cells and their phenotype), and be able to mimic the structure and biological function of the native extracellular matrix in terms of both, physical structure and chemical composition.
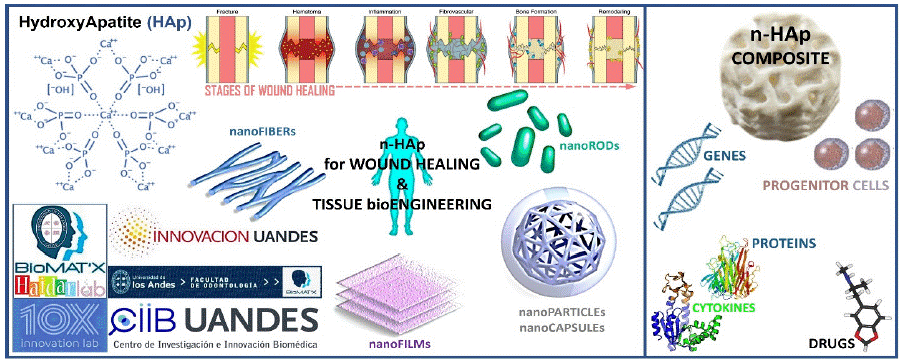
Graphical abstract: TOP LEFT: Chemical structure of Hydroxy Apatite.
TOP CENTER: The phases/stages of wound/bone healing (fracture model): A “haematoma” is formed at the injury/fracture site, followed with “inflammation” where haematoma granulates and osteoclasts remove necrotic tissue (bone), then “proliferation” of periosteal and endosteal cells, “tissue formation” where callus forms and transforms from a soft callus or osteoid to a hard callus, and finally, “remodelling” of tissue-woven bone to lamellar bone. In bone healing, osteocytes and osteoblasts help form de novo/new bone and maintain calcium homeostasis.
BOTTOM CENTER: Distinctive formats/morphologies of nano-scaled/-sized n-HAp solutions.
RIGHT: Synthesis of a n-HAp composite scaffold biomaterial satisfying tissue bioengineering requirements.
n-HAp can be synthesized using a range of R&D&I methods, which dictate the properties and behaviour of the final product. Indeed, n-HApparticle size, chemical composition (+/- carbonated groups), and crystallinity, for example, will have an impact onceintroduced into a defect, in terms of biodegradability rate.
Bio-Ceramics and HAp
Bio-ceramics is the class of ceramics used for repair and replacement of diseased and damaged parts of musculo-skeletal systems. In trauma, the most widely used materials include calcium phosphates, HAp, octa-calcium phosphate, calcium pyrophosphate dihydrate, and β-tricalcium phosphate. Briefly, calcium phosphates are often classified according to the molar ratio of calcium and phosphorus (Ca:P) ranging from 0.5 to 2.0. In our bones as well as in teeth/dentition, HAp is the main inorganic component. Due to its close chemical similarity to natural bone, exhibited biocompatibility (including soft tissues such as skin, muscle, and gingivae) and favorable bio-efficacy; to intrinsically stimulate wound healing and tissue regenerative responses (osseo-conduction/-induction), HAp-based strategies and solutions have been developed forextensive use in tissue bioengineering. Indeed, HAp, with the general formula [Ca10(PO4)6(OH)2], has been suggested as an ideal candidate for orthopaedic, or o-dental and cranio-maxillo-facial implants (or implant components). Synthetic HAp, alternatively, with improved mechanical strength and defect affinity to host hard tissue(s), has been commonly-investigated for chemical bonding and coating implant surfaces, load-bearing applications (orthopedic and dental), the repair of hard tissues including bone repair and/or augmentation (bone and tooth fillers). Herein, recent advancements in materials, biomaterials, nano-science, and -biotechnology have re-boosted the R&D&I (research, development, and innovation) with a focus on (particle) size and crystal morphology control, amongst others, to design, formulate/fabricate, evaluate and optimize (then translate from bench-top to market/end-user) nano-sized, -scaled, and/or -crystalline HAp (n-HAp), with greater surface area, enhanced sinter-ability and densification, improved biocompatibility and biodegradability (timed), better fracture toughness and superiGraphical or bio-activity/-efficacy, in situ osseo-integration/-regeneration and wound healing properties; extending to nano-formulations and -matrices for drug(s) release control and modulation.
n-HAp
Various nano-sized and -crystalline calcium orthophosphates and tetra-calcium phosphates, with different morphologies and porous structures-materials, have been designed, synthesized, characterized, and evaluated in an array of bio-medical/-dental and other clinical applications; offering a great R&D&I potential for tissue bioengineering. In addition to or in combination with nHAp, other biological agents have been reported, for soft/hard tissue bioengineering (histiogenesis) including the concentrated blood derivatives of platelets (platelet-rich plasm, platelet-rich fibrin, leukocyte-and platelet rich fibrin, and recombinant human platelet-derived growth factor- BB); and proteins/cytokines (fibroblast growth factor, enamel matrix derived protein and DNA recombination of DNA, mainly bone morphogenic proteins or BMPs-2, -4, -7, -9 and growth differential factor-5).
In our BioMAT’X (HAiDAR I+D+i) LAB, n-HAp : calcium phosphate based bio-ceramics, in distinctive morphologies, can be synthesized via several powder, cement and ceramic (bio-ceramic) processing techniques, including aqueoussol–gel synthesis, solid state reactions, co-precipitation, hydro-thermal reactions, and mechano-chemical processing, to formulate nano-wires and-rods, as well as nano-particles and -capsules, extending to micro-spheres and -sheets (via the in-situ polymerization and step-wise polysaccharide layer-by-layer self-assembly composite technique/method, electro-phoretic deposition and plasma spraying with/or reinforcement with Cnanotubes).Overall, nHAp-based nanostructure composites (crystal particle size ranging from 1 nm to 100 nm)with high surface free energy and binding energy (alongside the physico-chemical macro quantum tunnelling effect), have been demonstrated to enhance osteoblastic functions (including adhesion, proliferation, synthesis of bone-related proteins and deposition of calcium-containing minerals), in vitro and in vivo. Calcium phosphate nanoparticles are degradable at low pH.Further, was shown to promote early osteogenic gene expression and upregulate the late osteogenic gene expression. The high stability, structural flexibility and tissue affinity of nHAp, as bone analog, despite some limitations that can be fine-tuned, offers promising biological advantages and have been widely recognized as a good hard tissue bioengineering candidate for incorporation and application in clinical bone graft substitutes(natural osseo-ceramic). Porous three-dimensional (3-D) scaffolds based on HAp and n-HAp are perhaps a fine example, herein. Indeed, the size and shape/morphology of the n-Hap nanosystem (nanocapsules or nanospheres, etc…) and the in situ behavior of the scaffold can be further optimized today using 3-D modeling and computed simulation, via alignment with the size and anatomical form and shape of the defect undergoing treatment. Furthermore, computational modeling can also simulate (and help the surgeon predict) response(s) to loads and other environmental/area stresses. It is noteworthy to mention that the efficiency of n-Hap biomaterials is associated with the material-to-cell (cell membrane) interaction, which basically induces an increase in protein and growth factor production that helps in wound/defect reconstruction and/or repair. Similarly herein, for better composite materials, molecular dynamic simulation can be a useful tool for exploring or assessing the interactions between cells, bio-polymers and -ceramics. Finally, nHAp-based composites are rapidly gaining attention for incorporation in bone prosthesis and graft coatings and proposed to carry and deliver anti-cancer drugs (nucleic acids, proteins, and enzymes) and employed in cell culture substrates, enzymatic immobilization, nerve tissue graft production, wound protection, and anti-infection dental tissue repair scaffold biomaterials. Certainly, through doping the biomaterial using an array of metals, biological characteristics such as improving the localized and in situ bacteriostatic or bactericidal action, can be enhanced.
Ongoing R&D&I studies focus on analyzing, characterizing, and optimizing cell–material interaction, bio-mechanical strength, and biological response(s) of n-HAp–based nanocomposite biomaterials; crucial for translation through evidence-based clinical trials.
Overall, optimizing production and scale-up to create cost-effective biomaterials are key.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding and Acknowledgments
This work was supported by operating grants provided to the HAiDAR R&D&I LAB/BioMAT’X (Laboratorio de Biomateriales, Farmaceìuticos y Bioingenieriìa de Tejidos Cráneo-Máxilo-Facial), member of CiiB (Centro de Investigación e InnovaciónBiomédica), Faculties of Medicine and Dentistry, Universidad de los Andes, Santiago de Chile, through the ANID-NAM (Agencia Nacional de Investigación y Desarrollo, Chile and National Academy of Medicine, USA) Grant código # NAM21I0022 (2020-2022), CORFO Crea y ValidaI+D+i Grant código # 21CVC2-183649 (2021-2023), CORFO Crea y Valida – Proyecto de I+D+iColaborativo - Reactivate” Grant código # 22CVC2-218196 (2022-2024), and FONDEF Concurso IDEA de I+D, ANID, Grant código # ID22I10215 (2022-2024). The author wishes to acknowledge the exceptional F-ODO students behind inspiring this piece: Yr3 (Andrea Bustos, Ismael Valenzuela and Zabdiel Faundez), Yr4 (Alondra Beniscelli) and Yr6 (Ignacio Fernández).
References
- Damsaz M, Castagnoli CZ, Eshghpour M, Alamdari DH, Alamdari AH, et al. Evidence-Based Clinical Efficacy of Leukocyte and Platelet-Rich Fibrin in Maxillary Sinus Floor Lift, Graft and Surgical Augmentation Procedures. Front Surg. 2020; 7: 537138.
- Eskitoros-Togay SM, Bulbul YE, Dilsiz N. Combination of Nano-Hydroxyapatite and Curcumin in a Biopolymer Blend Matrix: Characteristics and Drug Release Performance of Fibrous Composite Material Systems. International Journal of Pharmaceutics. 2020; 590: 119933.
- Haidar ZS. Bio-Inspired/-Functional Colloidal Core-Shell Polymeric-Based Nano Systems: Technology Promise in Tissue Engineering, Bioimaging and NanoMedicine. Polymers. 2010; 2: 323-352.
- Hassan M, Sulaiman M, Yuvaraju PD, Galiwango E, Rehman Iu, et al. Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration. Journal of Functional Biomaterials. 2022; 13: 13.
- Järbrink K, Ni G, Sönnergren H. Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Syst Rev. 2016; 5: 152.
- Järbrink K, Ni G, Sönnergren H. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017; 6: 15.
- Jawadi Z, Yang C, Haidar ZS, Santa Maria PL, Massa S. Bio-Inspired Muco-Adhesive Polymers for Drug Delivery Applications. Polymers. 2022; 14: 5459.
- Joo V, Ramasamy T, Haidar ZS. A Novel Self-Assembled Liposome-Based Polymeric Hydrogel for Cranio-Maxillofacial Applications: Preliminary Findings. Polymers. 2011; 3: 967-974.
- Lebre F, Sridharan R, Sawkins MJ, Kelly DJ, O’Brien FJ, Lavelle EC. The Shape and Size of Hydroxyapatite Particles Dictate Inflammatory Responses Following Implantation. Scientific Reports. 2017; 7: 2922.
- Mardia T Elsayed, Abeer A Hassan, Said A Abdelaal, Mohamed M Taher, Mohamed khalaf Ahmed, et al. Morphological, antibacterial, and cell attachment of cellulose acetate nanofibers containing modified hydroxyapatite for wound healing utilizations. Journal of Materials Research and Technology. 2020; 9: 13927-13936.
- Mingzu Du, Jingdi Chen, Kaihua Liu, Huaran Xing, Cui Song. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Composites Part B: Engineering. 2021; 215: 108790.
- Ramos AP, Cruz MAE, Tovani CB, Ciancaglini P. Biomedical Applications of Nanotechnology. Biophysical Reviews. 2017; 9: 79-89.
- Razieh Khalifehzadeh, Hamed Arami. Biodegradable calcium phosphate nanoparticles for cancer therapy. Advances in Colloid and Interface Science. 2020; 279: 102157.
- Shu X, Liao J, Wang L, Shi Q, Xie, X. Osteogenic, Angiogenic, and Antibacterial Bioactive Nano-Hydroxyapatite Co-Synthesized Using γ-Polyglutamic Acid and Copper. ACS Biomaterials Science & Engineering. 2020; 6: 1920-1930.
- Sidra W, Misbah S, Tahir J, Tousif H. Comparative Analysis of Hydroxyapatite Synthesized by Sol-gel, Ultrasonication and Microwave Assisted Technique. Materials Today: Proceedings. 2015; 2: 5477-5484.
- Torres F, Sousa E, Cipreste M. A Brief Review on Hydroxyapatite Nanoparticles Interactions with Biological Constituents. Journal of Biomaterials and Nanobiotechnology. 2022; 13: 24-44.
- Wang R, Hu H, Guo J, Wang Q, Cao J, et al. Nano-Hydroxyapatite Modulates Osteoblast Differentiation through Autophagy Induction via mTOR Signaling Pathway. Journal of Biomedical Nanotechnology. 2019; 15: 405-415.
- Ziyad S Haidar, Lucy Di-Silvio, Ziad EF Noujeim, John E Davies, Frédéric Cuisinier, et al. Engineering Solutions for Cranio-Maxillo-Facial Rehabilitation and Oro-Dental Healthcare. Journal of Healthcare Engineering. 2019; 5387305.
- Ziyad S Haidar, Reggie C Hamdy, Maryam Tabrizian. Protein release kinetics for core–shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials. 2008; 29: 1207-1215.
- Zumarán CC, Parra MV, Olate SA, Fernández EG, Muñoz FT, et al. The 3 R’s for Platelet-Rich Fibrin: A “Super” Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration. Materials. 2018; 11: 1293.