
Research Article
Thromb Haemost Res. 2023; 7(1): 1088.
The LIPL-PLATELET Study Postprandial LIPid Panels and PLATELET Activity in Coronary Heart Disease after Treatment with PCSK9-Inhibitors
Pogran E1,2*, Ahmed A1, Burger AL1, Kaufmann CC1, Kaun GR4, Fasching P4, Hübl W5 and Huber K1,2
13rd Medical Department, Cardiology and Intensive Care Medicine, Clinic Ottakring (Wilhelminen hospital), Vienna, Austria
2Medical University Vienna, Vienna, Austria
35th Medical Department for Endocrinology, Rheumatology and Acute Geriatry, Clinic Ottakring (Wilhelminen hospital), Vienna, Austria
4Central Laboratory, Clinic Ottakring (Wilhelminen Hospital), Vienna, Austria
*Corresponding author: Edita Pogran3rd Medical Department of Cardiology and Intensive Care Medicine, Klinik Ottakring, Montleart Strasse 37, A-1160 Vienna, Austria
Received: January 09, 2023; Accepted: February 14, 2023; Published: February 21, 2023
Abstract
Reports about a pleiotropic potential of pro-protein convertase subtilisin/kexin type 9 inhibitor are scarce. This hypothesis-generating study investigates the platelet reactivity after standardized Oral Fat Tolerance Testing (OFTT) under an optimized lipid-lowering therapy (statin plus ezetimibe) alone and during the add-on treatment with the alirocumab.
We investigated ten patients with Chronic Coronary Syndrome (CCS). Lipid variables and markers of platelet function were assessed during the fasting state and 3 and 5 hours after OFTT using a milkshake with 90 g of fat. Measurements were performed in the same CCS patients under Dual Lipid-Lowering Therapy (DLLT) alone and after three months of add-on therapy with alirocumab.
Postprandial inflammatory reaction did not change, irrespective of alirocumab. Neutrophile to lymphocyte ratio increased during the OFTT more significantly when on dual lipid-lowering therapy (p=0.021). The multiplate electrode aggregometry test with ASPI reagents (p=0.037) showed a paradoxically higher platelet reactivity five hours after OFTT with addition of alirocumab compared to the DLLT only. Platelet reactivity remained unchanged during OFTT in CCS patients before or after alirocumab therapy.
Altogether, alirocumab showed a trend of decreased postprandial inflammation and increase in platelet reactivity.
Keywords: Platelet activity; Standardizedoral fat tolerance test; PCSK9-inhibitors; Alirocumab; Postprandial inflammation
Abbreviations: ADP: Adenosine Diphosphate; AU: Aggregation Units; ASPI: Arachidonic Acid; CCS: Chronic Coronary Syndrome; HDL-C: High-Density Lipoprotein; Lp (a): Lipoprotein (a); LDL: Low-Density Lipoprotein; MEA: Multiplate Electrode Aggregometry; NLR: Neutrophile to Lymphocyte Ratio; OFTT: Oral Fat Tolerance Test; PCSK9-I: Proprotein Convertase Subtilisin/Kexin type 9 Inhibitors; TGs: Triglycerides
Introduction
Lipid variables are evaluated mainly in the fasting state after a fasting period of up to 12 hours [1]. This can be not meaningful as, in reality, most of the patients are in a non-fasting state for the whole day, as it is suggested that atherosclerosis develops most frequently under the influence of non-fasting lipids [2-4]. It has been reported that postprandial Triglycerides (TGs) can better predict the presence of coronary artery disease than TGs measured during the fasting state [5-10]. Accordingly, evaluating the lipid profile independently of a fasting or postprandial state, i.e. after a defined lipid load, might be beneficial.
Very low-density lipoprotein and Low-Density Lipoprotein (LDL) play a role in platelet activation by changing the lipid composition and interactions between lipoprotein and lipoprotein receptors on the platelet membrane [11,12]. Moreover, an increase in platelet cholesterol enhances the sensitivity of platelets to aggregating agents [13]. On the contrary, High-Density Lipoprotein (HDL-C) removes cholesterol from peripheral tissues, limits the content of intra-platelet cholesterol and modulates signals of the platelet pathway, thus preventing platelet activation [14].
Hypercholesterolemia can be treated with well-investigated lipid-lowering agents. Statins represent the first line medication within those agents and are considered to exert pleiotropic effects that may be explained by lipid-lowering and/or other mechanisms [15-17]. Recently, a new group of lipid-loweringtreatment, the Proprotein Convertase Subtilisin/Kexin type 9 inhibitors (PCSK9-I) alirocumab and evolocumab, both humanized antibodies, became available. So far, only a few reports of pleiotropic effects beyond their intense LDL-lowering effect have been reported [18-20] especially not in the postprandial phase. We assumed that measuring the impact of a defined lipid meal on lipid variables and platelet activation in Chronic Coronary Syndrome (CCS) patients, who are already on dual lipid-lowering agents, i.e. statins and ezetimibe, without or with additional use of PCSK9-I could contribute to a better understanding of potential anti-atherosclerotic pleiotropic effects of the PCSK9-I, alirocumab.
Methods
This study is an explorative and hypothesis-generating longitudinal analysis of the potential pleiotropic effect of alirocumab in the same CCS population during an Oral Fat Tolerance Test (OFTT). The study was designed and performed according to the Helsinki criteria and evaluated and approved by the local ethics committee (EC Number: 17-093-0318). The study was registered in Eudra CT as a clinical trial (Eudra CT number: 2017-003483-12). All patients undersigned an informed consent.
The study was conducted at the 3rd Medical Department, Cardiology and Intensive Care Medicine, at Clinic Ottakring (Wilhelminen hospital), Vienna, Austria.
Study Population
The studied population consisted of ten symptom-free patients with CCS defined as a history of elective or acute percutaneous coronary interventions at least two months prior to study inclusion. Study subjects were on dual antiplatelet therapy (aspirin and clopidogrel) and optimal lipid-lowering therapy (a combination of high-dosed, highly effective statins, or a maximally tolerated dose of statins, plus ezetimibe) at the time of inclusion. In case LDL-C was >55 mg/dl despite three months of lipid-lowering combination treatment, patients received a third lipid-lowering strategy, alirocumab (150 mg SC every two weeks for 12 weeks). Patients with relevant hematologic disorders (haemoglobin<10 g/dl, platelet count <100 × 109 cells/l or platelet count >600 × 109 cells/l), with a known platelet function disorder, a history of alcohol abuse or drug addiction, and patients on oral anticoagulation were not included.
Study Conduction
Study subjects underwent two visits: Visit 1 was completed on dual lipid-lowering therapy with statins and ezetimibe (baseline therapy). Visit 2 was performed three months after the first alirocumab injection. The baseline therapy stayed unchanged during the whole course of the study. An OFTT was performed at each visit and included three blood analyses: The first blood collection was performed after at least ten hours of fasting between 7 and 8 am. The morning medication was taken approximately one hour before the blood draw. Afterwards, subjects consumed a standardized high-fat meal in the form of a milkshake. Further blood samples were taken three and five hours later (Figure 1). The standardized high-fat meal consisted of whipping cream and contained 90 g fat, 62,35 g saturated fatty acid, and 42,7 g carbohydrates, respectively, with a corresponding caloric intake of 1032, 8 kcal. The milkshake was consumed within 10 minutes under direct staff supervision, and patients were not allowed to perform any physical activity during the whole course of OFTT. Moreover, only beverages without sugar or fat were allowed during the five hours after the fat loading. The second blood draw was performed three hours and the third blood draw was performed five hours after the consumption of standardized high-fat meal.
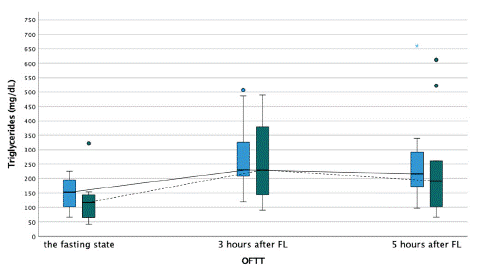
Figure 1: Triglyceride concentration during the oral fat tolerance test.
OFTT- oral fat tolerance test, FL- fat loading
Blue boxplot- at baseline, no therapy with PCSK9-inhibitors, and solid line connecting each time point during the OFTT. Green boxplot- after three months of therapy with PCSK9-inhibitors, and dashed line connecting each time point during the OFTT.
Analysis of Laboratory Parameters
Blood samples for routine lipid parameters and blood count were analyzed at the general laboratory of Clinic Ottakring within 20 minutes after each blood draw. Blood collections were performed using vacuum tubes (Vacuette, Greiner. Bio-one GmbH), and the blood was centrifugated using the following program: 5°C, 4000 rpm for 30 minutes. Lipoprotein (a) (Lp (a)) was evaluated from the serum using particle-enhanced immunonephelometry with the Atellica® NEPH 630 System. The rest of the lipid profile (HDL, TGs, total cholesterol, apolipoprotein A, apolipoprotein B) was estimated from the serum with Siemens Dimension Vista. Leukocyte, neutrophilcount and platelet activation markers (mean platelet volume, platelet distribution width, reticulated thrombocytes) were investigated based on florescence flow cytometry using XN-3000, Sysmex.
Platelet function was assessed by the use of Multiplate Electrode Aggregometry (MEA). Blood for the MEA assay was drawn into hirudin prepared blood tubes (Multiplate® Hirudin Blood Tube (Double-Wall), Roche Diagnostics GmbH, Mannheim, Germany). The tests were performed using the Multiple Analyzer system (Roche Diagnostics GmbH, Mannheim, Germany) within ten minutes after the blood draws from the whole blood. The MEA assay detects the change in electrical impedance when platelets aggregate on metal electrodes in the test cuvette. The increase in impedance as aggregation occurs was transformed and presented in this study as Aggregation Units (AU). We used Adenosine Diphosphate (ADP) (concentration of 6.5 μmol/L, ADP test Reagent Kit, Roche Diagnostics GmbH, Mannheim, Germany) and arachidonic acid (ASPI) (concentration of 0.5 mmol/L, ADP test Reagent Kit, Roche Diagnostics GmbH, Mannheim, Germany) as agonists in MEA.
Statistical Analysis
Parametric variables are presented as mean ± standard deviation. Non-parametric variables are shown as median with interquartile range. Normality analysis of laboratory values was performed visually through boxplot, and the Kolmogorov-Smirnov. Differences in the laboratory parameters within the groups were determined using one-way repeated-measures ANOVA for normally distributed variables. Friedman test was used for not normally distributed variables. One patient was not included in the final analysis of platelet reactivity because of low response to P2Y12-inhibitors.
For all tests, a p-value of <0.05 was statistically significant. However, due to multiple measurements of laboratory parameters, Bonferroni correction was performed. Calculations were performed using the SPSS 27 statistical package for Macintosh (BM Corp. Released 2020, IBM SPSS Statistics for Macintosh, Version 26.0. Armonk, NY: IBM Corp).Only one author had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
The mean age of the study population was 58.9 years (±12.13), and 80% of the populations were males. All patients were on concomitant therapy with 100mg acetylsalicylic acid and the P2Y12-inhibitor clopidogrel 75 mg daily during the whole course of the study (Table 1).
PCSK9-inhibitors group
N=10Age, yrs (mean, ±SD)
58.9 (±12.13)
Male, n (%)
8 (80.0)
BMI, kg/m² (median, IQR)
27.54 (±2.66)
Comorbidities
Diabetes mellitus Type 2
2 (20.0)
- on OAD
2 (20.0)
- on insulin
0
Arterial hypertension, n (%)
8 (80.0)
History of acute coronary syndrome, n (%)
4 (40.0)
History of PCI, n (%)
10 (100.0)
History of CABG, n (%)
4 (40.0)
Positive family history for CAD, n (%)
3 (30.0)
Peripheral artery occlusive disease, n (%)
1 (10.0)
Cerebral occlusive disease, n (%)
3 (30.0)
Chronic kidney disease, n (%)
1 (10.0)
Previous smoking, n (%)
10 (100)
Therapy
Beta blockers, n (%)
5 (50.0)
ACE-inhibitors, n (%)
4 (40.0)
Calcium channel blockers, n (%)
1 (10.0)
Sartans, n (%)
3 (30.0)
Diuretics, n (%)
4 (40.0)
Acetylsalicyl acid, n (%)
10 (100.0)
Clopidogrel, n (%)
10 (100.0)
Table 1: Baseline characteristics of the study population.
Lipid Profile and Glucose
Under dual lipid-lowering therapy only, OFTT caused a statistically significant increase of TG concentration from baseline 153.0 mg/dL (IQR=103.5) to 230.0 mg/dL (IQR=173.5) at three hours (p=0.035) after intervention, respectively. After three months of additional intake of alirocumab, TG concentration significantly increased from 116.5 mg/dL (IQR=83.0) at baseline to 229.0 mg/dL (IQR=263.8) three hours (p<0.001) after fat loading (p=0.003). Five hours after fat loading, there was no difference in TG concentration irrespective of alirocumab treatment (Figure 2). Other lipid variables such as total cholesterol, HDL-C, non-HDL-C, apolipoprotein A1, apolipoprotein B, Lp (a) were not impacted by OFTT before and after alirocumab treatment.
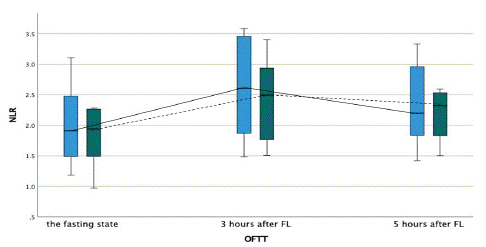
Figure 2: Neutrophile to lymphocyte ratio during the oral fat tolerance test.
OFTT- oral fat tolerance test, FL- fat loading
Blue boxplot- at baseline, no therapy with PCSK9-inhibitors, and solid line connecting each time point during the OFTT. Green boxplot- after three months of therapy with PCSK9-inhibitors, and dashed line connecting each time point during the OFTT.
After three months of alirocumab treatment we observed a statistically significant decrease in total cholesterol from 163.8 mg/dL (±34.4) at baseline to 101.3 mg/dL (±25.2) under alirocumab (F (5,45)=39,829, p<0.001), non-HDL-Cfrom 142.0 mg/dL (IQR=90.0) at baseline to 55.0 mg/dL (IQR=41.5) under alirocumab (p<0.001), and apolipoprotein B from 0.69 g/dL (IQR=0.59) at baseline to 0.37 g/dL (IQR=0.22) under alirocumab (p<0.001), respectively (Figures 3-5). In contrast, HDL-C showed a trend for increase after three months of treatment with alirocumab compared to dual lipid-lowering therapy only (Figure 6). However, this difference was not statistically significant. TG levels were significantly elevated under dual lipid-lowering therapy only compared with additional alirocumab treatment, but the delta increase in TGs caused by OFTT was similar without or with PCSK9-I therapy. Moreover, there was no statistically significant change in apolipoprotein a concentration after three months of treatment with alirocumab.
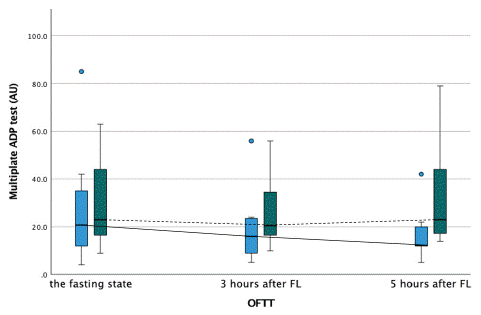
Figure 3: Platelet activity measured by multiplate ADPtest during the oral fat tolerance test.
AU-aggregation units, OFTT- oral fat tolerance test, FL- fat loading
Blue boxplot- at baseline, no therapy with PCSK9-inhibitors, and solid line connecting each time point during the OFTT. Green boxplot- after three months of therapy with PCSK9-inhibitors, and dashed line connecting each time point during the OFTT.
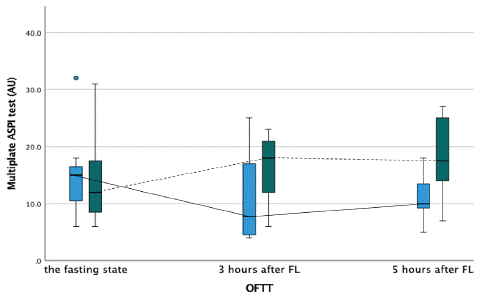
Figure 4: Platelet activity measured by multiplate ASPI test during the oral fat tolerance test.
AU-aggregation units, OFTT- oral fat tolerance test, FL- fat loading
Blue boxplot- at baseline, no therapy with PCSK9-inhibitors, and solid line connecting each time point during the OFTT. Green boxplot- after three months of therapy with PCSK9-inhibitors, and dashed line connecting each time point during the OFTT.
The glucose level remained stable during the OFTT when on dual lipid-lowering therapy. There was no difference in glucose level during the OFTT between the two treatment strategies. After three months of additional intake of alirocumab, the level of glucose decreased statistically significantly from 91 mg/dL (IQR=19.0) during the fasting state at baseline to 85.0 mg/dL (IQR=11.5) five hours after fat loading (p=0.005) (Figure 7).
Postprandial Inflammation
OFTT-induced inflammatory reaction was reflected by a statistically significant increase of absolute leucocyte (p=0.002) and neutrophil counts (p<0.001) after fat loading inpatients irrespective of the alirocumab therapy. The leucocyte count raised from 6.4 G./L (IQR=2.2) at baseline to 7.7 G./L (IQR=3.4) three hours and 8.5 G./L (IQR=3.0) five hours after fat loading when on dual lipid-lowering therapy. During the add-on alirocumab therapy, leucocyte count increased from 6.2 G./L (IQR=1.7) at baseline to 7.5 G./L (IQR=2.2) three hours, and 7.8 G./L (IQR=2.9) five hours after fat loading. However, after Bonferroni correction there was no difference between each of the leucocyte count measurements. Moreover, under dual lipid-lowering therapy only, neutrophile count increased from baseline 3.4 G./L (IQR=1.2) to 4.5 G./L (IQR=2.0) at three hours and 4.7G./L (IQR=1.9) at five hours after the intervention, respectively. After Bonferroni correction, there was no difference in neutrophile count. After 3 months of additional intake of alirocumab, neutrophile count increased from 3.5G./L (IQR=0.9) at baseline to 4.4G./L (IQR=1.4) three hours (p=0.019) and to 4.6G./L (IQR=2.0) five hours (p=0.042) after fat loading, respectively. Although not statistically significant, there was nominally a decreasing trend in OFTT-induced inflammatory reaction presented by decreased leucocyte and neutrophile count, when on add-on alirocumab therapy (Figures 8,9).
Moreover, Neutrophile to Lymphocyte Ratio (NLR) increased significantly during the OFTT from 1.9 (IQR=0.99) at baseline to maximum of 2.6 (IQR=1.12) three hours after Fat Loading (FL) when on dual lipid-lowering therapy only (p<0.001). On the other hand, after three months of additional alirocumab treatment NLR increased from 1.9 (IQR=0.77) at baseline to maximum of 2.5 (IQR=1.17) three hours after FL (p<0.001), (Figure 10). The increase of NLR during the OFTT was statistically significantly higher before the treatment with alirocumab (p=0.021). There was no difference in level of NLR between the two treatment strategies.
Platelet Function
Markers of platelet activation such as platelet count, mean platelet volume and platelet distribution width did not show any significant difference during OFTT in patients before and after add-on alirocumab therapy. Platelet aggregation during the OFTT measured by MEA revealed comparable results at baseline and 3 hours after OFTT in patients without or with alirocumab therapy, showing stable postprandial platelet activity as measured by ADP- and ASPI test, respectively. Interestingly, platelet aggregation measured five hours after OFTT by MEA with ASPI reagent showed a paradoxically higher platelet reactivity in patients on alirocumab 24.5 AU (IQR=64) vs 15.3 AU (IQR=15) without alirocumab, (p=0.037). Platelet aggregation measured with ADP reagent five hours after fat loading did not show any difference between two treatment strategies (Figures 11,12). ACE- angiotensin-converting enzyme, BMI- body mass index, CABG- coronary artery bypass graft, CAD- coronary artery disease, OAD- oral antidiabetic drug, PCI- percutaneous coronary intervention, yrs- years.
Discussion
To our knowledge, this study analyzed for the first time the possible pleiotropic effect of alirocumab therapy during the non-fasting state after a standardized fat loading. We observed that treatment with alirocumab leads to 1) a significant improvement of the lipid profile with no significant effect on postprandial lipid profile; 2) a decreasing trend of postprandial inflammatory reaction, and 3) no significant change in platelet activation but a paradoxical increased platelet aggregation 5 hours after OFTT under add-on alirocumab therapy compared to dual lipid-lowering therapy only.
While other lipid variables as total cholesterol, HDL-C, non-HDL-C, apolipoprotein A1, apolipoprotein B, Lp (a) were not influenced by OFTT, which agrees with previously published studies [8,21], OFTT caused a significant increase of TGs with maximum concentrations three hours thereafter. As shown previously, postprandial hypertriglyceridemia results in oxidative stress and inflammation and correlates with a higher risk for coronary heart disease [22-25]. In our hands, alirocumab nominally reduced TG concentration but did not prevent TG increase post-OFTT.
The main reason for additional treatment with PCSK9-I is to reach the treatment goal of less than 55 mg/dL in very-high risk patients with cardiovascular disease [26]. Previous studies showed that alirocumab could effectively reduce the LDL-C and non-HDL-C, apolipoprotein B, Lp (a) and TG while increasing HDL-C [27-29], results that could partially also be shown in our study.
Postprandial inflammation plays an essential role in endothelial dysfunction and the development of atherosclerosis and consecutive thrombo-ischemic event rates [30,31]. Moreover, previous studies identified NLR as outcome predictor for several cardiovascular diseases [32-34]. In our study, NLR increased more significantly in the postprandial phase during the dual lipid-lowering treatment. Hence, we can suggest that there is a decreasing trend of postprandial inflammation presented by leucocyte and neutrophile count, and NLR under alirocumab in CCS patients.
Marques et al. showed impaired leukocyte activation and reduced plasma levels of mediators involved in the activation and chemotaxis of neutrophils and eosinophils in patients with familial hypercholesterolemia after eight weeks of treatment with alirocumab [19]. In contrast, the meta-analysis published by Cao et al. found no reduction in hs-CRP levels under short-term treatment with PCSK9-I, irrespective of the agent (alirocumab, evolocumab, bococizumab) [35]. Moreover, Metzner et al. did not detect a change in systemic inflammatory biomarkers (CRP and MCP-1) after ten weeks of treatment with alirocumab [36].
Previously, several studies have focused on postprandial platelet reactivity. Most of them have shown higher platelet activation by means of an increased platelet count, increased P-select in levels, or increased platelet monocyte aggregates [37-40]. However, these studies were performed primarily on healthy males or patients with newly diagnosed diabetes mellitus type 2 on a diet without any concomitant pharmacotherapy. In our hands, platelet activity and platelet aggregation remained stable during OFTT in CCS patients. We assume that the reason for the unchanged platelet function was the effect of the concomitant dual antiplatelet therapy. An additional antiplatelet action of statins can only be assumed but not proven [41-44]. The paradoxically higher platelet aggregation measured by MEA five hours after OFTT under therapy with alirocumab is hard to explain and might be coincidentally and a matter of chance, especially as studies performed in the fasting state support a particular antiplatelet effect of alirocumab. They showed reduced TNFa-induced leukocyte-platelet adhesion and decreased platelet aggregation detected in platelet-rich plasma by light transmission aggregometry, decreased platelet membrane expression of CD62P and plasma levels of the in vivo platelet activation markers soluble CD40 lig and, platelet Factor-4, and soluble P-Selectin [18-20,45]. Another reason for this unexpected finding may be a postprandial hyperinsulinemia, which has been described to cause platelet hyperactivity [46]. In our study, the addition of alirocumab led to a significant decrease in glucose level during the OFTT, which is usually accompanied by hyperinsulinemia. However, we did not measure the insulin level in our study population. Hence, we can only speculate that the reason for significant glucose reduction during OFFT was higher postprandial hyperinsulinemia under alirocumab, which caused a temporary higher platelet aggregation after five hours from fat loading with little clinical evidence if at all.
In contrast to our finding, data from larger and omized placebo-controlled trials showed that levels of glycated haemoglobin and fasting plasma glucose remained unchanged under alirocumab, and there was no safety concern concerning new-onset of diabetes mellitus type 2 [47-50].
Limitations and Strengths
This longitudinal pilot study was performed in a small patient group under comparable clinical conditions. It is strength of this study that the results obtained with or without alirocumab were obtained from the same and extremely well-defined study population.
Conclusion
As expected, the treatment with alirocumab significantly improved the lipid profile dramatically. However, there was no change in the postprandial lipid profile. Decreasing trend in the level of postprandial inflammation in patients on alirocumab therapy is of potential interest. Furthermore, a potential temporary increase in platelet aggregation five hours after the fat loading measured by MEA under alirocumab was shown in this study. Due to the pilot- design of our study conclusions must be taken with care, can be seen only hypothesis-generating and would need confirmation by future investigation in bigger patient cohorts.
Acknowledgment
This study was supported by a grant from Sanofi-Aventis and the Österreichischer Herzfonds (Project number: 201701). Sponsors did not have any role in study design, in the collection, analysis and interpretation of data, and in the decision to submit the article for publication.
References
- Warnick GR, Nakajima K. Fasting versus nonfasting triglycerides: implications for laboratory measurements. Clin Chem. 2008; 54: 14–6.
- Karpe F. Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med. 1999; 246: 341–55.
- Zilversmit DB. GEORGE LYNIAN DUFF NMEN40RIAL LECTURE Atherogenesis : A Postprandial Phenomenon. 2016.
- Kolovou GD, Anagnostopoulou KK, Daskalopoulou SS, Mikhailidis DP, Cokkinos D V. Clinical relevance of postprandial lipaemia. Curr Med Chem. 2005; 12: 1931–45.
- Nordestgaard BG, Benn M. Fasting and nonfasting LDL cholesterol: to measure or calculate? Clin Chem. 2009; 55: 845–7.
- Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008; 118: 993–1001.
- Oka R, Kobayashi J, Miura K, Nagasawa S, Moriuchi T, Hifumi S, et al. Difference between fasting and nonfasting triglyceridemia; the influence of waist circumference. J Atheroscler Thromb. 2009; 16: 633–40.
- Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and Nonfasting Lipid Levels: Influence of Normal Food Intake on Lipids, Lipoproteins, Apolipoproteins, and Cardiovascular Risk Prediction. Circulation. 2008; 118: 2047–56.
- Abdel-Maksoud MF, Hokanson JE. The complex role of triglycerides in cardiovascular disease. Semin Vasc Med. 2002; 2: 325–33.
- Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996; 3: 213–9.
- Pedreño J, Hurt-Camejo E, Wiklund O, Badimón L, Masana L. Platelet function in patients with familial hypertriglyceridemia: evidence that platelet reactivity is modulated by apolipoprotein E content of very-low-density lipoprotein particles. Metabolism. 2000; 49: 942–9.
- Korporaal SJ a, Akkerman J-WN. Platelet activation by low density lipoprotein and high density lipoprotein. Pathophysiol Haemost Thromb. 2006; 35: 270–80.
- Ravindran R, Krishnan LK. Increased platelet cholesterol and decreased percentage volume of platelets as a secondary risk factor for coronary artery disease. Pathophysiol Haemost Thromb. 2007; 36: 45–51.
- Nofer J-R, Brodde MF, Kehrel BE. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin Exp Pharmacol Physiol. 2010; 37: 726–35.
- Wassmann S. Cellular Antioxidant Effects of Atorvastatin In Vitro and In Vivo. Arterioscler Thromb Vasc Biol. 2002; 22: 300–5.
- Palinski W. New evidence for beneficial effects of statins unrelated to lipid lowering. Arterioscler Thromb Vasc Biol. 2001; 21: 3–5.
- Undas A, Brummel-Ziedins KE, Mann KG. Statins and blood coagulation. Arterioscler Thromb Vasc Biol. 2005; 25: 287–94.
- Cammisotto V, Baratta F, Castellani V, Bartimoccia S, Nocella C, D’erasmo L, et al. Proprotein convertase subtilisin kexin type 9 inhibitors reduce platelet activation modulating ox-LDL pathways. Int J Mol Sci. 2021; 22.
- Marques P, Domingo E, Rubio A, Martinez-Hervás S, Ascaso JF, Piqueras L, et al. Beneficial effects of PCSK9 inhibition with alirocumab in familial hypercholesterolemia involve modulation of new immune players. Biomed Pharmacother. 2022; 145.
- Barale C, Bonomo K, Frascaroli C, Morotti A, Guerrasio A, Cavalot F, et al. Platelet function and activation markers in primary hypercholesterolemia treated with anti-PCSK9 monoclonal antibody: A 12-month follow-up. Nutr Metab Cardiovasc Dis. 2020; 30: 282–91.
- Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: A cross-sectional study. Arch Intern Med. 2012; 172: 1707–10.
- Masuda D, Yamashita S. Postprandial hyperlipidemia and remnant lipoproteins [Internet]. Vol. 24, Journal of Atherosclerosis and Thrombosis. Japan Atherosclerosis Society. 2017; 95–109.
- O’Keefe JH, Bell DSH. Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) Is a Cardiovascular Risk Factor. Am J Cardiol. 2007; 100: 899–904.
- M B, CM M, GW D, JCW B, MH M. Preceding exercise and postprandial hypertriglyceridemia: effects on lymphocyte cell DNA damage and vascular inflammation. Lipids Health Dis. 2019; 18.
- Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, et al. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004; 53: 701–10.
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019; 1–78.
- Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018; 379: 2097–107.
- Bays HE, Leiter LA, Colhoun HM, Thompson D, Bessac L, Pordy R, et al. Alirocumab treatment and achievement of non-high-density lipoprotein cholesterol and apolipoprotein B goals in patients with hypercholesterolemia: Pooled results from 10 phase 3 ODYSSEY trials. J Am Heart Assoc. 2017; 6.
- Blom DJ, Harada-Shiba M, Rubba P, Gaudet D, Kastelein JJP, Charng MJ, et al. Efficacy and Safety of Alirocumab in Adults With Homozygous Familial Hypercholesterolemia: The ODYSSEY HoFH Trial. J Am Coll Cardiol. 2020; 76: 131–42.
- de Vries MA, Klop B, Eskes SA, van der Loos TLJM, Klessens-Godfroy FJM, Wiebolt J, et al. The postprandial situation as a pro-inflammatory condition. Vol. 26, Clinica e Investigacion en Arteriosclerosis. Elsevier Doyma; 2014: 184–92.
- B K, SD P, JC M, KM B, M CC. Understanding postprandial inflammation and its relationship to lifestyle behaviour and metabolic diseases. Int J Vasc Med [Internet]. 2012 [cited 2021 Oct 21];2012. Available from: https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/21961070/
- Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther [Internet]. 2016 May 3 [cited 2022 Mar 24];14(5):573–7. Available from: https://pubmed-ncbi-nlm-nih-gov.ez.srv.meduniwien.ac.at/26878164/
- Dentali F, Nigro O, Squizzato A, Gianni M, Zuretti F, Grandi AM, et al. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: A systematic review and meta-analysis of the literature. Int J Cardiol. 2018; 266: 31–7.
- Chen C, Cong BL, Wang M, Abdullah M, Wang XL, Zhang YH, et al. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr Med Res. 2018; 7: 192–9.
- Cao YX, Li S, Liu HH, Li JJ. Impact of PCSK9 monoclonal antibodies on circulating hs-CRP levels: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2018; 8: e022348.
- Metzner T, Leitner DR, Dimsity G, Gunzer F, Opriessnig P, Mellitzer K, et al. Short-Term Treatment with Alirocumab, Flow-Dependent Dilatation of the Brachial Artery and Use of Magnetic Resonance Diffusion Tensor Imaging to Evaluate Vascular Structure: An Exploratory Pilot Study. Biomedicines. 2022; 10: 152.
- Wiens L, Lutze G, Luley C, Westphal S. Platelet count and platelet activation: Impact of a fat meal and day time. Platelets. 2007; 18: 171–3.
- Bröijersén A, Karpe F, Hamsten A, Goodall AH, Hjemdahl P. Alimentary lipemia enhances the membrane expression of platelet P- selectin without affecting other markers of platelet activation. Atherosclerosis. 1998; 137: 107–13.
- Hyson DA, Paglieroni TG, Wun T, Rutledge JC. Postprandial lipemia is associated with platelet and monocyte activation and increased monocyte cytokine expression in normolipemic men. Clin Appl Thromb Hemost [Internet]. 2002; 8: 147–55.
- Sinzinger H, Berent R. Platelet function in the postprandial period. Thrombosis Journal. Thromb J. 2012; 10: 19.
- Puccetti L, Pasqui AL, Auteri A, Bruni F. Mechanisms for anti-platelet action of statins. Curr Drug Targets Cardiovasc Haematol Disord. 2005; 5: 121–6.
- Verdoia M, Pergolini P, Rolla R, Nardin M, Schaffer A, Barbieri L, et al. Impact of high-dose statins on vitamin D levels and platelet function in patients with coronary artery disease. Thromb Res. 2017; 150: 90–5.
- Violi F, Carnevale R, Pastori D, Pignatelli P. Antioxidant and antiplatelet effects of atorvastatin by Nox2 inhibition. Trends Cardiovasc Med. 2014; 24: 142–8.
- Ali FY, Armstrong PCJ, Dhanji ARA, Tucker AT, Paul-Clark MJ, Mitchell JA, et al. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler Thromb Vasc Biol. 2009; 29: 706–11.
- Di Minno A, Orsini RC, Chiesa M, Cavalca V, Calcaterra I, Tripaldella M, et al. Treatment with pcsk9 inhibitors in patients with familial hypercholesterolemia lowers plasma levels of platelet-activating factor and its precursors: A combined metabolomic and lipidomic approach. Biomedicines. 2021; 9: 1–16.
- Spectre G, Östenson CG, Li N, Hjemdahl P. Postprandial platelet activation is related to postprandial plasma insulin rather than glucose in patients with type 2 diabetes. Diabetes. 2012; 61: 2380–4.
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017; 376: 1713–22.
- Ray KK, Del Prato S, Müller-Wieland D, Cariou B, Colhoun HM, Tinahones FJ, et al. Alirocumab therapy in individuals with type 2 diabetes mellitus and atherosclerotic cardiovascular disease: Analysis of the ODYSSEY DM-DYSLIPIDEMIA and DM-INSULIN studies. Cardiovasc Diabetol. 2019; 18: 1–10.
- Müller-Wieland D, Rader DJ, Moriarty PM, Bergeron J, Langslet G, Ray KK, et al. Efficacy and Safety of Alirocumab 300 mg Every 4 Weeks in Individuals with Type 2 Diabetes on Maximally Tolerated Statin. J Clin Endocrinol Metab. 2019; 104: 5253–6.
- Leiter LA, Cariou B, Müller-Wieland D, Colhoun HM, Del Prato S, Tinahones FJ, et al. Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: The ODYSSEY DM-INSULIN randomized trial. Diabetes Obes Metab. 2017; 19: 1781–92.