
Research Article
Thromb Haemost Res. 2024; 8(1): 1097.
Zinc Increases Platelet Activation in Sickle Cell Disease
Grace K Ababio1*; Lawrence Ababio Boateng1; Eugenia V Asare2; Robert Reeks1; Anthony Dongdem3
1University of Ghana Medical School, Medical Biochemistry, Korle-Bu, Accra, Ghana
2Ghana Institute of Clinical Genetics (sickle cell clinic), Korle-Bu, Accra, Ghana
3University of Health and Allied Health Sciences, Epidemiology & Biostatistics, Fred N. Binka School of Public Health, Ho, Volta Region, Ghana
*Corresponding author: Grace K Ababio, University of Ghana Medical School, Medical Biochemistry, Korle-Bu, Accra, Ghana. Email: gkababio@ug.edu.gh
Received: July 18, 2024 Accepted: September 04, 2024 Published: September 12, 2024
Abstract
Background: Despite suggestions that zinc therapy may lessen the pain discomfort implicated by zinc – induced thrombotic crisis, zinc levels has not been linked to platelet indices in SCD pain. Hence the focus.
Aim: To determine [zinc] and its relation to platelet counts in sickle cell disease patients.
Methodology: Ghana Institute of Clinical Genetics (sickle cell clinic) was the site for the case-control study. After ethical clearance from College of Health Sciences (CHS-Et/M.1-P5.12/2023-2024), a validated Tampa scale kinesiophobia (TSK) pain assessment questionnaires were used for data collection. Ten (10) mls of blood was collected, four (4) mL into EDTA tube for full blood count and Hemoglobinopathy on cellulose acetate electrophoresis, while six (6) mL was placed in a Serum Separating Tube (SST) for the determination of zinc. The data was analyzed using the Statistical Package for the Social Science (SPSS) version 21 and Microsoft Excel 2016.
Results: Under zinc above threshold stratifications, the average platelet count (p = 0.000) and platelet distribution width (PDW; p = 0.002) were increased in SCD. [Zn] stratifications also related statistically to heart rate, SCD pain in fear avoidance model (TSK), SpO2 and weight.
Conclusion: Zinc activated certain platelet differentials causing an increase in platelet counts and Platelet Distribution Width (PDW) in SCD.
Keywords: Zinc; Platelet; Sickle cell disease; Atomic absorption spectroscopy
Introduction
The link between zinc and platelet emanated from animal studies, importantly, rats subjected to Zn deficiency [1,2]. In these studies, prolonged bleeding time and decreased platelet aggregation [3] were the prominent findings. Other animal modelled experiment has asserted that zinc deficiency could impair platelet reactivity to certain agonists [4].
Platelets cannot synthesize fibrinogen [5]. The conversion of soluble fibrinogen to insoluble fibrin fibers is best achieved by thrombin activation, an essential component of hemostasis [6]. Thrombin and other regulating factors like zinc and calcium act on fibrin clot structure and mechanical stability [2].
Similar to existing literature on calcium, few studies have indicated zinc to also simulate thrombin-induced fibrin clot formation [7,8]. It has been shown that the association of fibrin monomers to protofibrils is greatly enhanced by zinc to a greater extent than calcium [2]. Other studies have indicated plausible facts that zinc deficiency could impair calcium uptake by platelet [9,10] and thus, delay clotting time and prolong the bleeding time in rats [12].
Bleeding abnormality linked to Zn deficiency has been implicated in Sickle Cell Disease (SCD) [13]. This deficiency is widespread among SCD patients [14]. These patients do benefit from zinc supplements as it has been suggested to decrease vaso-occlussive crises in SCD [15]. Routine oral zinc supplementation has also been shown to decrease the prevalence of diarrhea-related mortality in subjects at high risk of zinc deficiency [16].
One such high risk zinc deficiency is SCD [15]. In West Africa, Ghana has the second highest prevalence (2%) of SCD [17]. Even though SCD can cause intravascular and extravascular hemolysis [18], it is also, characterized by normocytic intrinsic hemolytic anemia [19,20] brought on by defective hemoglobin [21]. Despite suggestions that zinc therapy may lessen the discomfort of painful crisis due to zinc – induced reactive oxidant species [22], zinc levels has not been definitively linked to platelet differentials in SCD pain.
SCD patients do suffer thrombotic crisis [23]. Recent studies have suggested that abnormal platelet function could be involved in thrombotic crises [24]. Abnormal platelet includes increased platelet count, shortened platelet survival and an increase in platelet activation [25]. Platelet indices such as Mean Platelet Volume (MPV), Plateletcrit (PCT), and Platelet Distribution Width (PDW) are biomarkers of platelet activation [26]. These indices, are parameters determined together in an automated hematological analyzer. The indices relate to morphology and platelet proliferation kinetics.
In this paper, it is anticipated that the link between these platelet indices under zinc level stratification in SCD will be unraveled. Hence the focus.
Aim
To determine zinc levels and its relation to platelet indices in sickle cell disease.
Methodology
Study Design
Case-control study.
Study Site
Ghana Institute of Clinical Genetics (sickle cell clinic), Korle-Bu, Accra.
Study Population
Sickle cell disease patients attending the Ghana Institute of Clinical Genetics (sickle cell clinic) and control subjects at the blood bank, Korle-Bu.
Inclusion Criteria
• Ballas 2012 criteria was utilized.
Exclusion Criteria
• The following were excluded from this studyPatients who declined to respond to the questionnaire were excluded from the study.
Sample Size Determination and Sampling Strategy
For precision level in outcome of interest, Cochran formula was used; n = [z2(p)(1-p)]/e2. At 95% confidence interval, z =1.96, desired precision ‘e’ of 0.05, and SCD prevalence ‘p’ being 0.02, the minimum sample size was calculated.
On the field, seventy-three (73) controls and 150 SCD cases were obtained, making a total of 223.
Sampling Strategy
Convenient sampling
Data Collection, Tools and Methods
After obtaining ethical clearance from College of Health Sciences (CHS-Et/M.1-P5.12/2023-2024) and patient’s consent, tampa scale kinesiophobia pain rating as well as venous blood samples were taken for analysis of full blood count, hemoglobin electrophoresis and assessment of zinc levels. These are briefly described below:
Tampa scale of kinesiophobia
This is a 17-item questionnaire used to analyze fear associated with movement due to pain. Individual responses are based on the extent to which they agree with the statements which ranges from strongly disagree, somewhat disagree, somewhat agree and strongly agree. These correspond to a score of 1, 2, 3 and 4 respectively.
Blood Processing
Four (4) mL of blood for full blood count was obtained in EDTA tube from participants while the other 6mL of blood for zinc level determination was taken by laboratory assistants at the study sites with a syringe into Serum Separating Tube (SST). The blood samples were transported on ice to the laboratory. After allowing the SST blood to coagulate at room temperature, it was centrifuge for five minutes at 3000rpm. The serum was kept at -22°C till analysis.
Cellulose Acetate Electrophoresis
EDTA whole blood sample was centrifuged, red blood cells (rbc) obtained and washed 3 times in 0.85% saline and once in distilled water. Cellulose acetate membrane was marked and moistened with the buffer prior to the start of the experiment. Rbc(s) were placed at vantage points on the membrane; time and voltage were then set for the electrophoretic run. The migration of hemoglobin was determined by comparison to a reference hemoglobinopathy.
The Atomic Spectrophotometer for Assessment of Zinc Levels
The atomic spectrophotometer at Ecolab, University of Ghana, Geography Department, as well as standards of operation protocol in the Lab was carefully followed to the latter for the determination of zinc in study subjects.
Data Handling, Analysis and Presentation
The data was analyzed using the software Statistical Package for the Social Science (SPSS) version 21 and Microsoft Excel 2016. The mean and standard deviation for continuous data was determined and student T-test and Analysis of Variance (ANOVA) were used for statistical association between two means and three or more mean values respectively.
Results
Study Participants
The study investigated 150 Sickle Cell Disease (SCD) patients and 73 control group (Figure 1 & Figure 2).
Figure 1: Flowchart for study participants used in data analysis.
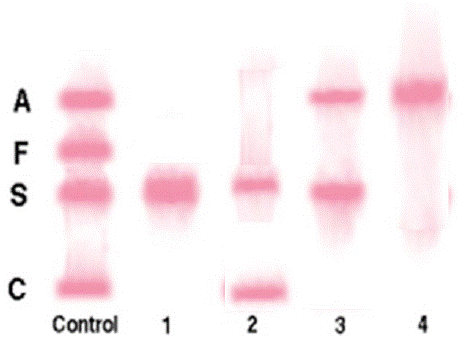
Figure 2: Cellulose acetate electrophoretogram for the hemoglobinopathy identified in this study.
Socio-Demographics
The most recorded clinical manifestations were acute chest syndrome (n = 67), severe pain (51), infection (n = 4) and avascular necrosis (n = 7) with relatively few having varying manifestations. Major areas where SCD subjects felt much pain were at their back, shoulders, chests, arms, elbow, hip, knee, abdomen, wrist and ankle. The mean age for the SCD group was 29.7±12.2(150) while the mean age for the control group was 26.3±9.0(73). Majority of subjects in both SCD and controls never smoked. However, few SCD subjects (18.7%) have taken alcohol before.
Zinc Levels
Average zinc levels for SCD VOC, SCD steady state and control subjects were 2.6±1.1(46), 2.5±1.1(104) and 1.4±1.3(73) μg/ml respectively. Reference range for [Zn] was 0.7 to 1.8 μg/ml (Yokokawa et al., 2020).
Study Characteristics
Statistical significance was seen in platelet count, heart rate, after zinc level stratification (Table 1) as well as other unique statistical finding under increased zinc level stratification (Table 1).
Zn levels <1.8µg/ml
Zn levels >1.8µg/ml
Controls
Steady state
VOC
Controls
Steady state
VOC
AA(38)
AS(9)
SS(12)
SC(7)
SS(7)
SC(2)
p-value
AA(18)
AS(8)
SS(63)
SC(22)
SS(28)
SC(9)
p-value
Weight (kg)
74.8±11.5
71.4±16.3
61.3±7.5
61.0±10.7
53.7±7.3
65.5±14.8
0.00
72.4±13.9
67.3±7.4
59.0±9.0
62.9±10.3
6.0±10.0
64.2±18.7
0.000
HR (bpm)
74.30±8.0
73.8±11.4
82.8±6.8
82.6±7.8
90.6±10.6
86.0±5.7
0.000
78.6±6.7
74.4±7.0
84.7±8.2
81.5±10.2
88.3±9.9
85.4±10.9
0.000
SpO2
87.9±11.9
88.9±11.2
94.3±6.1
96.0±4.5
94.3±2.6
97.0±1.4
0.142
98.2±1.2
97.9±0.6
95.8±2.1
97.5±1.5
95.5±2.1
96.7±2.0
0.000
TSK
20.7±6.0
1.3±5.1
35.3±7.8
41.1±5.8
42.1±12.3
23.5±0.7
0.000
25.2±6.4
22.1±3.2
35.3±6.2
35.0±8.0
36.7±6.3
39.6±6.3
0.000
Plt (103/mm3)
192.0±148.0
198.9±140.7
352.3±109.6
272.7±84.2
555.6±267.8
224.5±72.8
0.000
217.3±72.6
251.3±52.9
417.3±139.7
284.8±77.0
443.3±134.5
268.0±139.0
0.000
MPV
7.2±3.6
8.2±3.5
8.5±0.8
8.3±0.4
7.8±0.6
7.9±0.3
0.761
9.1±0.5
9.5±0.5
8.1±0.7
7.9±1.1
8.0±0.7
7.7±0.5
0.000
PCT
0.3±0.1
0.3±0.2
0.3±0.2
0.2±0.1
0.4±0.2
0.2±0.1
0.192
0.2±0.1
0.2±0.1
0.3±0.1
0.2±0.1
0.4±0.1
0.2±0.1
0.000
PDW
17.9±5.7
20.7±8.5
17.8±1.4
16.1±0.9
17.0±1.
17.6±1.1
0.588
18.4±1.6
19.1±2.5
16.1±3.3
17.2±1.5
17.0±1.3
16.9±2.0
0.002
Legend: *mean ± sd; HR: Heart Rate; Plt: Platelet; MPV: Mean Platelet Volume; rbc: Red Blood Cell; VOC: Vaso-Occlussive Crisis; PCT: Plateletcrit; SpO2: Oxygen saturation; PDW: Platelet distribution width
Table 1: Study characteristics stratified by zinc levels.
Normal ranges:
Platelet count (PLT) = 150 to 400 × 109/L
Plateletcrit (PCT) = 0.22–0.24%
Platelet distribution width (PDW) = 15.1% to 17.9%
Mean platelet volume (MPV) = 7 to 11.5 fL
Discussion
The current study presented a novel insight on zinc above threshold stratification levels and showed statistical significance with study parameters, most importantly, platelet indices among subjects.
It is therefore, presented here for the first time, platelet indices, a biomarker of platelet activation and how zinc affects these indices in SCD. This study emanated from our working hypothesis that indicated a model by which endothelial injury from SCD vasoocclusive crisis could lead to the release of zinc from damaged cells, thus, enabling the potentiation of platelet activation in response to thrombin. Other studies also suggested an increase in zinc levels within the area of a growing thrombus [2,12]. Zinc, released by platelets act as a co-factor for the initiation of procoagulant assembly on surfaces of platelets in the endothelial system. Zinc is thereby shown to modulate platelet activity by also, acting as an intracellular second messenger and thus, influence membrane receptor activity [3].
Zinc - induced platelet activation is actually an integrin aIIbβ3-dependent. Recent work also suggested that exogenous zinc can access platelet cytosol through non – selective transporters and cation channels [28]. The presence of significantly increased zinc – induced - circulating platelets during acute vaso-occlusion in SCD patients could have important implications [29]; since it is plausible for red blood cells to sludge or cause stasis in an anoxic SCD patient. In SCD vascular injury, exposed collagen and von Willibrand factor in the endothelial system allows platelet adhesion, in such a way that this process causes the release of thromboxane A, adenosine phosphate and thrombin for the formation of microaggregates [30,31]. The formed aggregates might occlude blood vessels and compromise tissue perfusion [32]. Platelets, therefore, play a major role in inflammation. Numerous studies have found significant relationship between platelet indices and systemic inflammatory reaction syndrome, activation of the coagulation system, thrombotic diseases, infection and trauma [33].
Whenever there is a decreased platelet production, MPV levels may increase [34] and vice versa. Under physiological conditions, MPV directly relates to PDW in the same direction (De Luca et al., 2014); while the number of platelets in SCD blood is maintained at equilibrium by elimination and regeneration. In this study, platelet counts and PDW were increased in SCD while PCT and MPV were within normal limits under zinc stratification. Genetic and some environmental factors might have modified these indices [35]. Increased platelet count in SCD has been well established by several studies [36-38] with limited information of PDW in SCD. One study indicated an increase of PDW in SCD patients experiencing vasoocclussive crisis [39]. Others associated PDW with cerebrovascular events and mortality [40]. In our study, PDW levels were rather increased in all groupings, whether control or steady state SCD or SCD with Vaso occlusive crisis. Thus, indicating the influence of zinc on PDW.
Subjects experiencing severe pain or vasocclusive crisis had relatively higher TSK values indicating their fear of movement and establishing SCD hallmark of sudden onset of excruciating musculoskeletal painful episodes.
The study population’s characteristics data conformed to the existing literature that SCD subjects were relatively lean [37] and had increased platelet counts. Leanness may be related to retarded growth brought about by perhaps, zinc deficiency (Table 1).
Limitations
The present study was unable to indicate whether increased platelet indices were primary or secondary, even though there was some level of understanding that platelet activation might be secondary to the sludging of red blood cell and/or tissue anoxia.
Conclusion
Zinc increased platelet activation in SCD and this contributed to the progression of thrombotic crisis in SCD pain.
Recommendation
Future studies are required for the understanding of platelet - zinc signaling pathways that contribute to zinc - induced platelet activation.
Author Statements
Acknowledgement
The authors duly acknowledge the staff of the Sickle Cell Clinic, Korle-Bu, Accra, Ghana and Medical Biochemistry, Univ. of Ghana Medical School.
Funding Disclosure
This work was financed by AGK and EVA.
Medical writing by Ababio, GK and Ababio Boateng L
Benchwork by Ababio Boateng L, Reeks R, Ababio GK
Analysis of results by Ababio, GK, Dongdem A and Ababio Boateng L
The authors declare that there is no conflict of interest.
References
- Gaffney-Stomberg E. The impact of trace minerals on bone metabolism. Biological trace element research. 2019; 188: 26-34.
- Mammadova-Bach E, Braun A. Zinc homeostasis in Platelet-Related diseases. International Journal of Molecular Sciences. 2019; 20: 5258.
- Ahmed NS, Lopes-Pires M, Pugh N. Zinc: an endogenous and exogenous regulator of platelet function during hemostasis and thrombosis. Platelets. 2020; 32: 880–887.
- Elgheznawy A, Öftering P, Englert M, Mott K, Kaiser F, Kusch C, et al. Loss of zinc transporters ZIP1 and ZIP3 augments platelet reactivity in response to thrombin and accelerates thrombus formation in vivo. Frontiers in Immunology. 2023; 14: 1197894.
- Ghoshal K, Bhattacharyya M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. The Scientific World JOURNAL. 2014: 1–16.
- Gaule TG, Ajjan RA. Fibrin(ogen) as a Therapeutic Target: Opportunities and Challenges. International Journal of Molecular Sciences. 2021; 22: 6916.
- La Corte ALC, Philippou H, Ariëns RAS. Role of fibrin structure in thrombosis and vascular disease. Advances in Protein Chemistry and Structural Biology. 2011; 83: 75–127.
- Chaudhry SA, Serrata M, Tomczak L, Higgins S, Ryu J, Laprise D, et al. Cationic zinc is required for factor XII recruitment and activation by stimulated platelets and for thrombus formation in vivo. Journal of Thrombosis and Haemostasis. 2020; 18; 2318–2328.
- O’Dell BL, Browning JD. Zinc Deprivation Impairs Growth Factor-Stimulated Calcium Influx into Murine 3T3 cells Associated with Decreased Cell Proliferation. Journal of Nutrition. 2011; 141: 1036–1040.
- O’Dell BL, Browning JD. Zinc deficiency induced in Swiss 3T3 cells by a Low-Zinc medium impairs calcium entry and two mechanisms of entry are involved. Biological Trace Element Research. 2013; 152: 98–104.
- Ballas SK. More definitions in sickle cell disease: steady state v base line data. American journal of hematology. 2012; 87: 338.
- Taylor KA, Pugh N. The contribution of zinc to platelet behaviour during haemostasis and thrombosis. Metallomics. 2016; 8: 144–155.
- Datta D, Namazzi R, Conroy AL, Cusick SE, Hume HA, Tagoola A, et al. Zinc for Infection Prevention in Sickle Cell Anemia (ZIPS): study protocol for a randomized placebo-controlled trial in Ugandan children with sickle cell anemia. Trials. 2019; 20: 460.
- Ababio G, Boateng LA, Asare EV, Reeks R, Dongdem A. Sickle Cell Disease Pain and Zinc, Any Link? A Case-Control Study among Patients with Sickle Cell Disease In Korle-Bu. International Journal of Scientific Research and Management (IJSRM). 2024; 12: 1119–1129.
- De Oliveira Fernandes Miranda CT, Vermeulen-Serpa KM, Pedro ACC, Brandão-Neto J, De Lima Vale SH, Figueiredo MS. Zinc in sickle cell disease: A narrative review. Journal of Trace Elements in Medicine and Biology. 2022; 72: 126980.
- Dhingra U, Kisenge R, Sudfeld CR, Dhingra P, Somji S, Dutta A, et al. Lower-Dose zinc for childhood diarrhea — a randomized, multicenter trial. New England Journal of Medicine. 2020; 383: 1231–1241.
- Sims AM, Bonsu KO, Urbonya R, Farooq F, Tavernier F, Yamamoto M, et al. Diagnosis patterns of sickle cell disease in Ghana: a secondary analysis. BMC Public Health. 2021; 21: 1719.
- Conran N. Intravascular hemolysis: a disease mechanism not to be ignored. Acta Haematologica. 2014; 132: 97–99.
- Kavanagh PL, Fasipe TA, Wun T. Sickle cell disease. JAMA. 2022; 328: 57-68.
- Ababio GK. Book Chapter. Emerging Trends in Sickle Cell Disease and CRISPR/Caspases. IntechOpen. 2024: 1-9.
- Alrayyes S, Baghdan D, Haddad RY, Compton A, Mohama S, Goreishi R, et al. Sickle cell disease; An overview of the disease and its systemic effects. Disease-a-Month. 2018; 64: 283–289.
- Prasad AS. Impact of the discovery of human zinc deficiency on health. Journal of Trace Elements in Medicine and Biology. 2014; 28: 357–363.
- Nikparvar M, Evazi MR, Eftekhari T, Moosavi F. Intracardiac thrombosis in sickle cell disease. DOAJ (DOAJ: Directory of Open Access Journals). 2016; 41: 150–153.
- De Franceschi L, Cappellini MD, Olivieri O. Thrombosis and sickle cell disease. Seminars in Thrombosis and Hemostasis. 2011; 37: 226–236.
- Krishnegowda M, Rajashekaraiah V. Platelet disorders. Blood Coagulation & Fibrinolysis. 2015; 26: 479–491.
- Samuel D, Bhat AN, Prabhu VM. Platelet indices as predictive markers of prognosis in Critically Ill patients: a prospective study. Indian Journal of Critical Care Medicine. 2020; 24: 817–822.
- Ahmed NS. Zinc is an intracellular secondary messenger in platelets. 2019.
- Lin L. Comprehensive identification of nitroxyl-reactive cysteines in human platelet proteins by quantitative mass spectrometry (Doctoral dissertation, University of British Columbia). 2011.
- Conran N, Franco-Penteado CF, Costa FF. Newer Aspects of the Pathophysiology of Sickle Cell Disease Vaso-Occlusion. Hemoglobin. 2009; 33: 1–16.
- Eisinger F, Patzelt J, Langer HF. The platelet response to tissue injury. Frontiers in medicine. 2018; 5: 317.
- Berna-Erro A, C Redondo P, Lopez E, Albarran L, A Rosado J. Molecular interplay between platelets and the vascular wall in thrombosis and hemostasis. Current Vascular Pharmacology. 2013; 11: 409-430.
- Scharf R. Platelet signaling in primary haemostasis and arterial thrombus formation: Part 1. Hämostaseologie. 2018; 38: 203–210.
- Levi M. Pathogenesis and diagnosis of disseminated intravascular coagulation. International Journal of Laboratory Hematology. 2018; 40: 15–20.
- Beyan C, Kaptan K, Ifran A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. International journal of clinical practice. 2010; 64: 827-827.
- Bozzi L. Genetic and Environmental Determinants of Quantitative Platelet Markers in the Pharmacogenomics of Antiplatelet Intervention (PAPI) Study (Master’s thesis, University of Maryland, Baltimore). 2014.
- Thakkar CC, Sailaja I. Study of Implication of Wbc and Platelet Count among Sickle Cell Disease Patients in Waghodia Region, Vadodara, Gujarat. Journal of Pharmaceutical Research International. 2022; 34: 42–47.
- Ababio GK, Acquaye J, Ekem I, Aleksenko L, Quaye IK. Variation in Pain and Clinical Indices among Patients with Sickle Cell Disease in Ghana. J Blood Disord. 2016; 3: 1037.
- Antwi-Baffour S, Kyeremeh R, Annison L. Severity of anaemia has corresponding effects on coagulation parameters of sickle cell disease patients. Diseases. 2019; 7: 59.
- Al-Saidi DN. The impact of bone pain crises on platelet parameters in sample of Iraqi sickle cell anemia patients. Journal of Applied Hematology. 2024; 15: 50–54.
- Adam G, Kocak E, Özkan A, Resorlu M, Çinar C, Bozkaya H, et al. Evaluation of platelet distribution width and mean platelet volume in patients with carotid artery stenosis. Angiology. 2014; 66: 375–378.