
Research Article
Annals Thyroid Res. 2014;1(1): 5-10.
Development of More Sensitive Bioassay of TSAb Due to the Modification of Conventional Assay and its Measurement in M22-TRAb-Seronegative Graves' Patients
Keiichi Kamijo1* and Kazuyoshi Togashi2
1Kamijo Thyroid Clinic and Kamijo Thyroid Research Institute, Japan
2Yamasa Corporations, Japan
*Corresponding author: Keiichi Kamijo, Kamijo Thyroid Clinic and Kamijo Thyroid Research Institute, Sapporo, Hokkaido, Japan
Received: August 18, 2014; Accepted: Sep 11, 2014; Published: Sep 15, 2014
Abstract
Background: Graves’ disease (GD) is caused by thyrotropin (TSH) receptor antibodies (TRAb) that bind to TSHR and activate thyrocytes. The measurement of TRAb therefore has been used to assist in the diagnosis of GD, although approximately 3 % of GD patients were negative for M22-TRAb. This study evaluated a newly modified TSAb (referred as TSAb) assay using porcine thyroid cells which is characterized by more sensitive and rapid assay system compared with conventional TSAb assay.
Methods: First, we compared TSAb and conventional TSAb assay using porcine cells. In TSAb assay, sera were treated with dextran coated charcoal to remove intrinsic AMP and the incubation buffer contained 6% of polyethylene glycol to intensify the cAMP production of porcine cells due to TSAb stimulation. After cell lysis with TritonX-100, cAMP was measured with EIA.
Next, TSAb were measured using a modified TSAb assay in 17 M22-TRAbseronegative patients with untreated GD to investigate whether these cases have autoantibodies against the TSH receptor.
Results: The sensitivity and specificity of TSAb for GD and Painless Thyroiditis (PT) were 99.1% and 94.7%, respectively, at the optimal cut-off value of 120%. The positive predictive value of TSAb for GD was significantly higher than that of conventional TSAb (99.1% vs. 94.5%, p<0.05) and the negative predictive rate of PT by TSAb was also significantly higher than that of conventional TSAb (94.7% vs. 86.2%, p<0.01). TSAb was detected in all 17 M22-seronegative patients with untreated GD. In addition, in 8 of 10 M22- seronegative GD patients M22-TRAb became positive for 6-month of follow-up.
Conclusion: This study demonstrates that a newly modified TSAb assay is more useful in the differential diagnosis of GD from PT than conventional TSAb assay and highlights that a modified TSAb assay could demonstrate the existence of autoantibodies against TSHR in M22^TRAb-seronegative GD patients. Furthermore, in 80% of M22-seronegative GD patients, M22-TRAb appeared during follow-up.
Introduction
Graves’ disease (GD) is an autoimmune thyroid diseases caused by autoantibodies directed to the thyroid-stimulating hormone receptor (TSHR) [1-6]. The detection of autoantibodies against the TSHR (TRAb) has been clinically used as an aid in the differential diagnosis of hyperthyroidism or in monitoring of GD patients during treatment with antithyroid drug [7]. Until recently, TRAb measurements were performed using competitive inhibition of labeled TSH binding to the TSH receptor [6,8]. TRAb assays have improved over the years and finally as a third generation, a rapid and fully automated electrochemiluminescence immunoassay for TRAb called M22-TRAb assay which was used M22, a labeled thyroid-stimulating human monoclonal TSHR autoantibody [9], was developed. TRAb was detected by their ability to competitively inhibit M22-binding to solubilized porcine TSHR [10-13]. On the other hand, it has been reported that thyroid stimulating Ab (TSAb) , which is measured with conventional TSAb assay using porcine cells, was detected in only 89.3 % of GD patients and further 13 % of patients with Painless Thyroiditis (PT), thus making the differential diagnosis between GD and PT difficult [14]. In the present study, after a newly modified bioassay named TSAb kit ⌈Yamasa⌋ EIA (referred as TSAb) was developed, we report what are the modifications of the new assay that might improve the analytical performance at first. Next, we compared the analytical performance and clinical utility between conventional bioassay and TSAb assay that employs porcine cells. Furthermore, TSAb were measured using a modified TSAb assay in 17 M22-seronegative patients with untreated GD to investigate whether these cases have autoantibodies against the TSH receptor.
Materials and Methods
Subjects
Thirty-nine (3.6%) of 1.085 consecutive untreated GD patients were negative for M22-TRAb. Of them, 17 M22-TRAb-seronegative patients with untreated GD (39±17 years (M±SD); 1 man and 16 women) were included in this study.
In addition, 109 patients with newly diagnosed GD (39±13 years; 15 men and 94 women) and 94 patients with painless thyroiditis (PT) (35±12 years; 5 men and 89 women) were also selected for comparison between conventional TSAb assay and newly modified TSAb assay.
The diagnosis of GD was made on the basis of clinical and laboratory evidence of hyperthyroidism, the presence of a diffusely enlarged thyroid gland and increased diffuse thyroid uptake 20 minutes after Tc-99m injection (>2.0%) and/or increased vascularity index (>90%) in power color Doppler ultrasonography, as previously reported [8]. Inclusion criteria for M22-TRAb-seronegative Graves’ disease were undetected M22-TRAb (≤2.0IU/L) in addition to diagnostic standard of GD described above. The diagnosis of PT was based on laboratory evidence of thyrotoxicosis and decreased diffuse thyroid uptake of 20 minutes after Tc-99m injection (<0.5%) and/ or decreased vascularity (<50%) by power Doppler ultrasonography with negative M22-TRAb, as previously reported [8]. Finally, PT diagnosis is confirmed by spontaneous normalization of thyroid function during follow-up of a few months. Included also in this study were 110 normal controls (34±14 years; 17 men and 93 women) who were euthyroid with negative thyroid autoantibody and had normal thyroid volumes measured by thyroid ultrasonography. This study was approved by the Committee for Medical Research Ethics of the Kamijo Thyroid Research Institute and the Kamijo Thyroid Clinic, and all participants signed an informed consent form.
TSAb in patient sera was measured with TSAb kit ?Yamasa? EIA bioassay according to the manufacturer’s instruction (Yamasa Corporation, Chiba, Japan). This bioassay is specific to stimulating TSAb activation due to wild type receptor expressed on cultured porcine thyroid cells. Binding of TSAb in the patient to TSHR on porcine thyroid cells leads to activation of adenylate cyclase by interaction with the regulatory guanidine nucleotide binding (G) protein (Figure 1). Subsequently, detachment of the a-subunits from βγ complex activates adenylate cyclase through the effector to increase intracellular cAMP and release into culture medium.
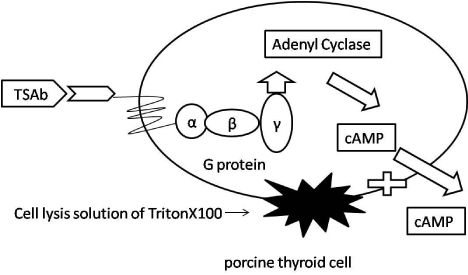
Figure 1: The principle of a modified TSAb assay.
TSAb binding to the wild type TSHR on porcine thyroid cell induced a signaling
cascade resulting in increased of cAMP. Incubation buffer contained Anti-
TSH Ab and 6% PEG. TSAb level in patient sera is determined by measuring
cAMP existed inside porcine thyroid cell plus culture medium.
A modified TSAb assay
TSAb in patient sera was measured with TSAb kit ?Yamasa? EIA bioassay according to the manufacturer’s instruction (Yamasa Corporation, Chiba, Japan). This bioassay is specific to stimulating TSAb activation due to wild type receptor expressed on cultured porcine thyroid cells. Binding of TSAb in the patient to TSHR on porcine thyroid cells leads to activation of adenylate cyclase by interaction with the regulatory guanidine nucleotide binding (G) protein (Figure 1). Subsequently, detachment of the a-subunits from βγ complex activates adenylate cyclase through the effector to increase intracellular cAMP and release into culture medium.
TSAb was assayed as follows. One hundred μL of serum sample was treated with Dextran Coated Charcoal (DCC) to absorb endogenous cAMP and after centrifugation; 25μLaliquot was added to a well of 96-well microplate, which is confluent with cultured porcine thyroid cells expressing wild type TSHR, together with 50μL of reaction buffer. The reaction buffer is containing 20μL of anti-TSH antibody for the purpose of neutralizing endogenous TSH and 6% of Polyethylene Glycol (PEG) to intensify cAMP production by TSAb. The plate was incubated at 37°C, 5%CO2 for 4 hours. One-hundred μL of the cell lysis solution TritonX100. 100μL were added to each well. The determination of cAMP which is inside the porcine thyroid cells plus culture medium was measured by solid phase enzyme immunoassay and the total assay time was 1 hour. All assays were performed in duplicate. The TSAb level of each patient serum was expressed sample(S) as the percentage corrected by normal control (N, TSAb, 100%) and positive control (P, TSAb, 750%) described below.
TSAb(%)=100+ (S-N)/(P-N) x 650
Conventional TSAb assay
Conventional TSAb was assayed by measuring cAMP production in cultured medium according to the previously reported method [15]. The TSAb level of each patient serum was expressed as percentage of cAMP production compared to that of normal control serum. The cut-off value of TSAb was 180%.
M22-TRAb assay
The M22-TRAb levels in the patients’ sera were measured by an inhibition assay kit-Elecsys anti-TSH receptor assay (Roche Diagnostics GmbH, Penzberg, Germany) according to its instruction manual [8]. This assay detects TRAb by using inhibition of a monoclonal antibody (M22) binding the extracellular domain of porcine TSHR. The estimated optimal M22-TRAb cutoff value was 2.0IU/L [10,12,13].
Free thyroxine, free triiodothyronine, TPOAb, TgAb and TSH assay
Free thyroxine (FT4), free triiodothyronine (FT3), TPOAb, TgAb and TSH levels were determined with Elecsys Free T4 assay, Elecsys FT3 assay, Elecsys anti-TgAb and Elecsys TSH assay (Roche Diagnostics GmbH), respectively.
Statistical analysis
All data are presented as mean±standard deviations. Statistical analysis was performed using Stat Flex version 6.0 (Artec corporations, Osaka, Japan). The optimal cut-off point was obtained by Receiver Operating Characteristic (ROC) analysis and the Area under Curve (AUC) was also calculated. The AUCs of the two different assay as well as proportion were evaluated by the delta test. Statistical significance was accepted when p was <0.05. Statically sufficient data of intraassay and interassay were judged when Coefficient Variation (CV) was less than 10%.
Results
Fundamental data on TSAb
1) Effect of DCC on serum cAMP concentrations
Preliminary experiment showed that each basal cAMP level in 3 normal controls and 3 GD patients was decreased after treatment with DCC, respectively, as shown in Table 1.
DCC treatment clearly decreased endogenous cAMP in sera obtained from 3 normal controls and 3 GD patients, respectively.
Sample List
Serum cAMP levels (pmol/L)
DCC-treated serum cAMP levesl (pmol/L)
normal subject 1
3.95
1.71
normal subject 2
2.35
1.75
normal subject 3
2.13
1.72
Mean±SD
2.81±0.99
1.73±0.02
Graves' disease 1
2.09
1.33
Graves' disease 2
2.22
1.49
Graves' disease 3
2.72
1.4
Mean±SD
2.34±0.33
1.41±0.08
Table 1: Effect of DCC treatment on serum cAMP levels in normal controls and GD patients.
DCC treatment clearly decreased endogenous cAMP in sera obtained from 3 normal controls and 3 GD patients, respectively.
2) Inhibitory effect of anti-TSH Ab on cAMP productions in incubation medium stimulated by highly elevated TSH concentration
The influence of 4 μg of anti-TSH Ab was examined in the TSH concentrations ranging from 1.2 to 292.0 μU/mL. As shown in Table 2, anti-TSH Ab almost always inhibits the TSH-stimulated elevations of cAMP.
TSH induced the dose-response elevations of cAMP in porcine thyroid cells, but anti-TSH Ab made it possible to measure TSAb even in case of sera containing high levels of TSH by excluding TSH-induced cAMP increment.
TSH .values.
(μU/mL)cAMP concentration (pmol/mL)
Anti-TSH Ab (+)
TSH Ab (-)
1.2
4.6
5.4
1.7
4.7
5.6
2.8
4.5
5.6
3.2
4.9
5.2
3.4
4.8
5.1
4
4.8
5.1
8.1
4.6
5.4
50.5
4.7
8.6
73.6
5.1
9.6
216.1
5.3
22.3
292
6.1
37.5
Table 2: Inhibitory effect of anti-TSH Ab in the incubation buffer on the elevation of cAMP stimulated by high concentration of TSH.
TSH induced the dose-response elevations of cAMP in porcine thyroid cells, but anti-TSH Ab made it possible to measure TSAb even in case of sera containing high levels of TSH by excluding TSH-induced cAMP increment.
3) Interrelationship between concentrations of polyethylene glycol in incubation buffer and cAMP production in porcine thyroid cells due to the stimulation by TSAb
The cAMP production of porcine thyroid cells was investigated after addition of DCC-treated sera from normal controls and GD patients, under the condition of incubation buffer containing 2.5%, 2.7%, 4.0%, 5.3%, 6.0%, 6.7% and 8.0 % of PEG , respectively, Table 3 disclosed that PEG has the positive stimulatory effects on cAMP levels which was produced by GD patient’s sera, reaching the maximum effect at 6% PEG concentration, although there was no significant effect of PEG on cAMP production after addition of normal serum.
PEG
(%)cAMP concentration (pmol/mL)
Normal subjects
Graves' disease
1
2
3
1
2
3
4
5
2.1
6.37
5.79
6.24
12.14
12.97
10.38
18.49
12.11
2.7
6.37
5.8
6.05
21.9
20.67
18.83
37.79
27.68
4
6.09
5.45
6.01
37.54
26.67
28.83
59.57
38.88
5.3
5.37
4.65
5.1
81.51
45.93
60.14
99.54
78.46
6
5.64
4.85
5.31
75.39
57.49
81.89
120.6
126.8
6.7
5.04
4.44
4.89
72.5
56.95
79.01
112.38
137.28
8
4.8
4.29
4.33
41.71
41.21
67.03
82.13
142.06
Table 3: Interrelationship between concentrations of Polyethylene Glycol (PEG) in incubation buffer and TSAb-stimulated cAMP production of porcine thyroid cell after addition of sera obtained from normal controls and the GD patients. The maximum augmentation effect of PEG on cAMP production by GD patient’s sera was observed in 6 % of PEG, although PEG failed to stimulate cAMP production in sera of normal controls.
4) Intra- and interassay variability of the modified TSAb assay
To estimate intra- and interassay variability of the TSAb assay, we measured the TSAb levels of three different serum samples. Intraassay coefficient of variation (CV) were 5.7%, 3.2% and 5.9%, respectively (Table 4a). Interassay of CV of each assay were 4.5%, 3.7% and 5.6%, respectively (Table 4b).
Sample 1
Sample 2
Sample 3
a) Intraassay variation
1
120
285
688
2
123
283
678
3
136
264
606
4
136
270
661
5
130
274
710
Average
129
275
669
SD
7
9
39
CV
5.7
3.2
5.9
b) Interassay variation
1
116
285
688
2
125
283
678
3
121
264
606
4
129
270
661
Average
123
276
658
SD
6
10
37
CV
4.5
3.7
5.6
Table 4: Intra- and interassay variation of the TSAb assay. Intra-and interassay coefficient of variations showed less than 10%.
5) Estimation of the cut-off value of TSAb by the ROC curve analysis
To evaluate the optimal TSAb cutoff-value, we performed ROC analysis of TSAb levels with untreated GD and normal controls. The ROC analysis suggested 120% of TSAb as the optimal cut-off value (Figure 2). The sensitivity and specificity at the optimal cut-off value were 99.1% and 100%, respectively.
The ROC analysis suggested 120% of TSAb as the optimal cut-off value. The sensitivity and specificity at the optimal cut-off value were 99.1% and 100%, respectively. Using the ROC analysis with untreated GD as positive and controls as negative references, we compared the diagnostic performance of conventional TSAb and TSAb. TSAb showed superior diagnostic performance, p<0.05.
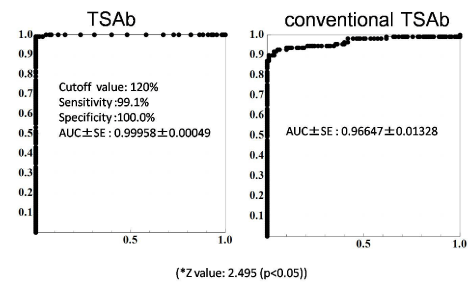
Figure 2: ROC analysis for estimation of TSAb and conventional TSAb
cutoff values obtained from the ROC analysis with untreated GD (n=109) and
normal controls (n=110).
The ROC analysis suggested 120% of TSAb as the optimal cut-off value. The
sensitivity and specificity at the optimal cut-off value were 99.1% and 100%,
respectively. Using the ROC analysis with untreated GD as positive and
controls as negative references, we compared the diagnostic performance
of conventional TSAb and TSAb. TSAb showed superior diagnostic
performance, p<0.05.
Clinical studies
1) Comparison of the diagnostic performance of conventional TSAb assay and a modified TSAb assay in GD and PT
Using the ROC analysis with untreated GD as positive and controls as negative references, we compared the diagnostic performance of conventional TSAb and TSAb. TSAb showed superior diagnostic performance, p<0.05 (Figure 3). The positive rate 99.1% of TSAb in patients with untreated GD was significantly higher than 94.5% for conventional TSAb (p<0.05). Moreover, the negative rate 94.7% of TSAb in patients with PT was significantly higher than 86.2% for conventional TSAb. As a result, TSAb showed higher diagnostic accuracy (p<0.01) in differentiating GD and PT (Delta test).
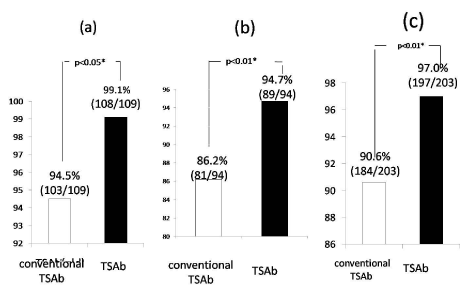
Figure 3: Comparison of positive rates (a) in untreated GD and negative
rates (b) in PT between conventional TSAb and a modified TSAb assay and
(c)each diagnostic performance rates.
The positive rate 99.1% of TSAb in patients with untreated GD was
significantly higher than 94.5% for conventional TSAb (p<0.05). Moreover,
the negative rate 94.7% of TSAb in patients with PT was significantly higher
than 86.2% for conventional TSAb.
2) TSAb concentrations in M22-TRAb-seronegative patients with newly diagnosed GD
Table 5 summarized the data on 17 M22-seronegative patients with untreated GD. The case 2 did not performed Tc-99m uptake because of breastfeeding, although she was diagnosed with GD based on remarkably elevated vascularity index, 99%, as shown in Figure 4. At initial visit, she was negative for M22-TRAb which was detected after treatment with methimazol (MMI), as shown in Table 6. In contrast, her TSAb values were remarkably elevated before and after treatment (Table 6). Of them, 16(94%) were positive for TSAb. Only the case 5 was negative for both M22-TRAb and TSAb at initial visit, but approximately 1 month later both of them became positive spontaneously. At that time the diagnosis of GD was confirmed by elevated Tc-99m uptake, 3.73% and an increased vascularity index, 97.5%, as shown in Table 7. Overall, 17 M22-TRAb-seronegative GD were all positive for serum TSAb before treatment. Next, the changes in M22-TRAb were also investigated for 6 months of follow-up after initial visit, as shown in Figure 5. In 8 out of 10 patients M22-TRAb became positive, in 5 cases after treatment with MMI, 1 case after KI therapy and 2 cases during follow-up because of suspicion for painless thyroiditis.
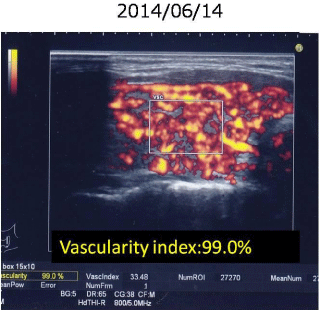
Figure 4: Vascularity index in a breastfeeding GD woman (case 2).
Instead of Tc-99m uptake, vascularity index, 99.0% can establish the
diagnosis of Graves’ disease.
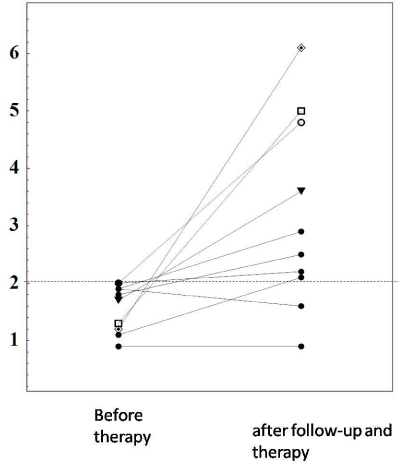
Figure 5: Changes in M-22-TRAb levels before and after follow-up and within
6 months of treatment with MMI in 10 M22-negative untreated GD patients.
In 8 of 10 patients with M22-TRAb-seronegative GD, M22-TRAb showed
positive changes during follow-up in 2 cases without therapy and within 6
months after initiation of MMI in 6 case.
Case No
Age
Gender
Tc-99m uptake (1.0-2.0%)
TSAb (?120%)
M22-TRAb (?2.0 IU/L)
TPOAb (?45IU/mL)
TgAb (?30IU/mL)
FT3 (2.00-4.40 pg/mL)
FT4 (0.80-1.90 ng/dL)
1
40
F
9.5
233
1.9
367.6
329.4
7.54
3.3
2
35
F
ne*
728
1.9
45.5
455.6
16.55
6.04
3
20
F
2.07
332
1.9
45.5
2116
14.54
5.92
4
15
F
4.72
162
1.7
>600
>4000
9.72
4.33
5
56
F
3.73
118
1.8
5
45.5
7,24
6.19
6
28
M
5.6
358
1.5
303.9
10.9
18.12
4.75
7
27
F
7.74
606
1.9
235.6
3273
11.67
4.37
8
47
F
13.1
143
1.9
208.4
4000
8.99
2.89
9
44
F
2.82
127
0.9
407.8
495.9
10.54
4.34
10
40
F
2.13
152
1.1
7.1
42.5
10.22
3.31
11
60
F
1.97
166
0.9
24.3
326
7.44
2.41
12
33
F
3.4
137
1.7
207.5
1154
12.98
3.53
13
69
F
3.86
253
1.3
284.2
192.1
10.24
3.68
14
13
F
3.45
345
2
16.5
258.9
11.43
3.52
15
36
F
5.43
357
1.3
5
1352
18.72
7.2
16
65
F
2.54
140
1.1
ne
11.3
5.88
2.17
17
29
F
3.7
321
1.2
503
103.4
7.23
2.21
Table 5: Changes in M-22-TRAb levels before and after follow-up and within 6 months of treatment with MMI in 10 M22-negative untreated GD patients. In 8 of 10 patients with M22-TRAb-seronegative GD, M22-TRAb showed positive changes during follow-up in 2 cases without therapy and within 6 months after initiation of MMI in 6 case.
Only case 2 was unable to perform Tc-99m uptake because of breastfeeding. TSAb is remarkably elevated, although M22-TRAb is 2IU/L or less. After treatment with MMI, M22-TRAb became positive.
Date (yr/mo/day)
Comment
Vascularity Index(%)
FT3 (2.00-4.40 pg/mL)
FT4 (0.80-1.9 0ng/dL)
TSH (0.45-4.50 μU/mL)
M22-TRAb(ECLIA) (?2.0IU/LP)
TSAb (<120%)
12/17/2013
childbirth
5/22/2014
Breast feeding
74.40%
16.55
6.04
<0.01
2
728%
5/30/2014
15.15
4.58
<0.01
1.7
785%
6/14/2014
MMI 10mg/d
99%
16.17
4.91
<0.01
1.5
880%
6/26/2014
MMI 10mg/d
6.27
2.23
<0.01
2.2
1163%
7/12/2014
MMI
5mg/d and 10mg/d on alternative days4.1
1.44
<0.01
2.1
>1,700%
8/2/2014
MMI 1omg/d
4.38
1.73
<0.01
2.7
Table 6: Changes in thyroid function tests before and after treatment with MMI 10 mg daily during breastfeeding in a 35-year-old female (case 2) with negative M22- TRAb and strongly positive TSAb Graves’ disease.
Only case 2 was unable to perform Tc-99m uptake because of breastfeeding. TSAb is remarkably elevated, although M22-TRAb is 2IU/L or less. After treatment with MMI, M22-TRAb became positive.
Date (yr/mo/day)
Comment
Vascularity Index(%) (<60%)
Tc-99m uptake (1-2%)
FT3 (2.00-4.40 pg/mL)
FT4 (0.80-1.9 0ng/dL)
TSH (0.45-4.50 μU/mL)
M22-TRAb(ECLIA) (?2.0IU/L)
TSAb(EIA) (<120%)
6/11/2014
7.24
2.72
<0.01
1.8
118
7/14/2014
6.19
2.26
<0.01
2.5
157
7/25/2014
MMI 15mg/d started
97.5
3.73*
8/9/2014
MMI 1mg/d
6.22
2.35
<0.01
3.4
166
Table 7: A 56-year-old female Graves’ disease patient (case 5) whose M22-TRAb and TSAb appears just follow-up. Only case 5 of M22-seronegative GD at initial visit was negative for TSAb, followed by positive changes just during follow-up without therapy because of suspicion of PT.
Discussion
In order to improve sensitivity and specificity, Yamasa corporations made a modified TSAb assay whose characteristics in comparison with conventional TSAb assay are presented in Table 8. In a modified TSAb assay, serum was treated with DCC which decreased the concentrations of cAMP in the sera and cell strainer procedure made porcine thyroid cell clusters a similar size. Furthermore, 6% of PEG in the incubation buffer intensified the cAMP production by TSAb in DCC-treated sera from GD patients, not found in control sera, although its mechanism remained unclear. The use of cell lysis solution is expected to increase the performance by measurement of cAMP which was not only secreted into the incubation medium but also remained inside porcine thyroid cells. Moreover, total assay time of cAMP by EIA in a modified TSAb assay was one hour, whereas by RIA in conventional TSAb assay it was two days.
Conventional TSAb assay
A modified TSAb assay
Benefit of new assay
Serum sample Smaller amount serum
400μL
100μL
Serum treated with PEG Simplified
Yes
No
Serum treated with DCC Removal of intrinsic cAMP
No
Yes
Cell strainer procedure
No
Yes
Uniformity of cell cluster Incubation medium buffer
1+PEG of TASb-stimulated
No
Yes
Enhancement cAMP production
1+Anti-TSH Ab
No
Yes
measurable even if incubation time
Serum TSH is
4 hours
4 hours
High
Cell lysis solution TritonX100
No
Yes
An increase in sensitivity by measurement of cAMP in the cell and incubation medium together
Assay of cAMP
RIA
EIA
Shortening of assay time
Total assay time
(2 days)
(1 hour)
Abbreviations: PEG: Polyethylene Glycol; DCC: Dextran-Coated Charcoal; RIA: Radio Immunoassay; EIA: Enzyme Immunoassay
Table 8: Comparison of characteristic between conventional TSAb and a modified TSAb assay.
Finally, it should be emphasized that various modifications of TSAb assay seemed to be associated with higher diagnostic accuracy in differentiating GD and PT, compared with conventional TSAb assay
The diagnosis of hyperthyroidism is based on clinical manifestation, serum examination including thyroid function tests and TRAb, Tc-99m uptake scintigraphy and thyroid ultrasonography. Graves’ disease is the most common cause of hyperthyroidism in Japan where iodine is richly ingested. Thyrotoxicosis in Kamijo Thyroid Clinic is consisted of 67% of GD, 18% of PT, 9 % of subacute thyroiditis, 2% of toxic nodular goiter and 4% of gestational transient hyperthyroidism (data unshown). The presence of TRAb is critical for the diagnosis of GD, although M22-TRAb was not detected in 39 (3.6%) of 1.085 consecutive untreated GD patients in our series. So we next investigateda newly modified TSAb in M22-TRAb-seronegative GD patients to clarify whether their diagnosis is autoimmune hyperthyroidism or non-autoimmune hyperthyroidism. Also Vos et al [18] reported that the prevalence of TRAb-seronegativity in 259 untreated patients with GD is 5.4% using a second generation assay. On the other hand, Nishihara et al [18] showed germline mutations of the TSH receptor occurred in 4 of the 89 patients (4.5%), including 3 definitive constitutively activating mutations (L512Q, E575K, and D617Y). Finally, as would be expected, a modified TSAb assay could detect TRAb in all 17 M22-seronegative GD patients, indicating autoimmune hyperthyroidism. Of interest, 8 of 10 patients with M22- negative Graves’ patients became positive for 6 months of follow-up.
Conclusion
In conclusion, the current study demonstrated that a modified TSAb assay can have a superior diagnostic performance to conventional TSAb assay and this sensitive bioassay is remarkably useful for the demonstration of the existence of autoantibody directed to TSHR even in GD patients with M22-seronegativity.
References
- Rees Smith B, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988; 9: 106-121.
- Kohn LD, Kosugi S, Ban T, Saji M, Ikuyama S, Giuliani C, et al. Molecular basis for the autoreactivity against thyroid stimulating hormone receptor. Int Rev Immunol. 1992; 9: 135-165.
- Weetman AP, McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev. 1994; 15: 788-830.
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998; 19: 673-716.
- Akamizu T. Antithyrotropin receptor antibody: an update. Thyroid. 2001; 11: 1123-1134.
- Smith BR, Sanders J, Furmaniak J. TSH receptor antibodies. Thyroid. 2007; 17: 923-938.
- Cho BY. Clinical applications of TSH receptor antibodies in thyroid diseases. J Korean Med Sci. 2002; 17: 293-301.
- Kamijo K. TSH-receptor antibody measurement in patients with various thyrotoxicosis and Hashimoto's thyroiditis: a comparison of two two-step assays, coated plate ELISA using porcine TSH-receptor and coated tube radioassay using human recombinant TSH-receptor. Endocr J. 2003; 50: 113-116.
- Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, et al. Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid. 2004; 14: 560-570.
- Gassner D, Stock W, Golla R, Roth HJ. First automated assay for thyrotropin receptor autoantibodies. Clin Chem Lab Med. 2009; 47: 1091-1095.
- Kamijo K, Ishikawa K, Tanaka M. Clinical evaluation of 3rd generation assay for thyrotropin receptor antibodies: the M22-biotin-based ELISA initiated by Smith. Endocr J. 2005; 52: 525-529.
- Yoshimura Noh J, Miyazaki N, Ito Koichi K, Takeda K, Hiramatsu S, Morita S, et al. Evaluation of a new rapid and fully automated electrochemiluminescence immunoassay for thyrotropin receptor autoantibodies. Thyroid. 2008; 18: 1157-1164.
- Kamijo K. Study on cutoff value setting for differential diagnosis between Graves' disease and painless thyroiditis using the TRAb (Elecsys TRAb) measurement via the fully automated electrochemiluminescence immunoassay system. Endocr J. 2010; 57: 895-902.
- Kamijo K, Murayama H, Uzu T, Togashi K, Kahaly GJ. A novel bioreporter assay for thyrotropin receptor antibodies using a chimeric thyrotropin receptor (mc4) is more useful in differentiation of Graves' disease from painless thyroiditis than conventional thyrotropin-stimulating antibody assay using porcine thyroid cells. Thyroid. 2010; 20: 851-856.
- Kamijo K, Nagata A, Sato Y. Clinical significance of a sensitive assay for thyroid-stimulating antibodies in Graves' disease using polyethylene glycol at high concentrations and porcine thyroid cells. Endocr J. 1999; 46: 397-403.
- Kamijo K, Togashi K. ECLIA-TRAB and Mc4-TSAb. J Jap Thyroid Association. 2001; 2: 106-110.
- Vos XG, Smit N, Endert E, Tijssen JG, Wiersinga WM. Frequency and characteristics of TBII-seronegative patients in a population with untreated Graves' hyperthyroidism: a prospective study. Clin Endocrinol (Oxf). 2008; 69: 311-317.
- Nishihara E, Fukata S, Hishinuma A, Amino N, Miyauchi A. Prevalence of thyrotropin receptor germline mutations and clinical courses in 89 hyperthyroid patients with diffuse goiter and negative anti-thyrotropin receptor antibodies. Thyroid. 2014; 24: 789-795.