
Review Article
Annals Thyroid Res. 2014;1(2): 17-22.
Thyroid Function Testing – A Review
Huang HL1 and Aw TC2*
1Department of Nuclear Medicine and PET, Singapore General Hospital, Singapore
2Department of Lab Medicine, Changi General Hospital, Singapore
*Corresponding author: Aw TC, Department of Lab Medicine, Changi General Hospital, 2 Simei Street 3, Singapore 529889, Singapore
Received: November 01, 2014; Accepted: November 11, 2014; Published: November 12, 2014
Abstract
Thyroid function testing is common and the indications for their use are increasing. This review explores the indications and components of thyroid testing. A panel consisting of thyroid stimulating hormone and free thyroxine should form the first line of diagnostic tests. Further differentiation of various pathologies can be made with thyroid antibodies or radio uptake scanning. For the follow up of differentiated thyroid cancer serum thyroglobulin serves as a biomarker. The sensible use of thyroid function tests and their interpretation requires sufficient understanding of clinical medicine and laboratory science.
Keywords: Thyroid function testing; TSH; Free thyroxine
Abbreviations
TFT: Thyroid Function Tests; DTC: Differentiated Thyroid Cancer; TSH: Thyroid Stimulating Hormone; TRH: Thyrotropin Releasing Hormone; T4: Thyroxine; fT4: Free Thyroxine; T3: Triiodothyronine; fT3: Free T3; TPO-Ab: Thyroid Peroxidise Antibodies; Tg-Ab: Thyroglobulin Antibodies; Tg: Thyroglobulin; TSHoma: TSH Secreting Pituitary Adenoma; RTH: Resistance to Thyroid Hormone; NTI: Non-Thyroidal Illness; HPT: Hypothalamic- Pituitary-Thyroid; TRH: Thyrotropin Releasing Hormone
Introduction
Thyroid function testing constitutes the most frequent endocrine tests requested of a clinical laboratory [1,2]. An understanding of the components of thyroid testing and the indications for each is essential in aiding clinical diagnosis and influencing the management of a diverse range of thyroid related conditions. This review aims to provide a brief scientific update for practicing doctors and a small dose of clinical medicine for scientists. It will not include testing for perinatal thyroid disorders, thyroid nodules and thyroid cancer as these subjects merit more detailed consideration separately. The rarer causes of thyroid dysfunction (TSH secreting pituitary adenomas – TSHomas, thyroid hormone resistance syndromes – RTH) and Familial Dysalbuminemic Hyperthyroxinemia (FDH) will be mentioned briefly as these conditions may confound the results of and the interpretation of Thyroid Function Tests (TFT).
Background
Thyroid hormone excess and deficiency can present in a diverse manner, thus adequate and accurate laboratory assessment is vital. Thyroid dysfunction is associated with adverse cardiovascular and skeletal outcomes [3]. Thyroid testing can provide an objective diagnosis in most instances of sometimes subtle or uncommon clinical manifestations of disease. Treatment may include ablative therapy (surgery or radio-iodine) and long term (anti-thyroid drugs) or life-long (thyroid hormone replacement) medications. Frequent monitoring of these patients is required. Thyroid disorders are common. In the US national health survey 4.6% of the population had hypothyroidism (0.3% overt and 4.3% subclinical) and 1.3% had hyperthyroidism (0.5% overt and 0.7% subclinical) [4]. Thyroid cancer is also becoming more common through increased detection via imaging. Differentiated Thyroid Cancer (DTC) has a relatively favourable prognosis if treated and monitored properly. The clinical use of thyroid testing in a general practice has increased by 50% over the last decade [5]. Radionuclide thyroid uptake testing may occasionally be needed to evaluate the functional status of thyroid tissue [6].
Thyroid hormones (T4 and T3) are regulated by pituitary TSH. In turn, TSH is controlled by the interaction between feedback inhibition by thyroid hormones and hypothalamic Thyrotropin Releasing Hormone (TRH) [7]. The relationship between TSH and fT4 is inversely loglinear such that a small change in fT4 is accompanied by a large reciprocal change in TSH [8]. Each individual has a genetically determined unique set point for the TSH/free T4 relationship [9]. In early thyroid dysfunction, TSH responds to this set-point wherein the fT4 is normal while TSH is depressed (subclinical hyperthyroidism) and vice versa in subclinical hypothyroidism (elevated TSH and normal fT4) [10-12]. The concentration of thyroid analytes varies widely within individuals as well as between individuals.
Indications
Thyroid Function Tests (TFT) are used as screening tools, to confirm clinical diagnoses, to assess adequacy of therapy, and to monitor treatment of DTC. Thyroid function is best assessed by measuring TSH when pituitary or hypothalamic disease is absent. In addition, patients should not be on drugs which can affect the thyroid [13]. Metformin (commonly prescribed for type 2 diabetes mellitus) can cause a significant reduction in TSH levels [14,15].
Screening
Routine TFT screen in an asymptomatic subject is controversial [16] though this was proposed by the American Thyroid Association in 2000 [17]. However, a new systematic review has found no evidence on benefits and harms of screening versus not screening [18]. However, screening can identify subjects with subclinical thyroid dysfunction as well as undiagnosed overt thyroid disease. TFT may be indicated for subjects at risk for thyroid disease such as women over 50 years, the elderly, those with significant family (thyroid disease) and personal medical history (palpitations, neck irradiation, autoimmune disease), and medications (amiodarone, lithium). For screening, TSH alone will usually suffice. Laboratories can proactively provide reflex fT4 testing when TSH is abnormal instead of a repeat test on another occasion. A normal TSH indicates euthyroidism and no further testing is needed. Thyroid antibodies (thyroid peroxidase and thyroglobulin) may be employed in at-risk subjects to stratify for future thyroid dysfunction.
Screening in hospitalized or ill patients is not recommended unless thyroid disease is strongly suspected since changes in thyroid hormones, binding proteins, and TSH concentrations occur in severe Non-Thyroidal Illness (NTI) [19]. Also known as euthyroid sick syndrome, NTI is a syndrome of abnormal TFT (notably low T3 or fT3), deranged Hypothalamic-Pituitary-Thyroid (HPT) axis, abnormal thyroid hormone binding proteins and impaired thyroid hormone action in the absence of any intrinsic disease of the HPT axis [20]. In NTI, patients are ill or hospitalized and can have dynamic changes in TFT results as illness progresses or recover. Very different fT4-TSH combinations may be encountered underscoring the need to avoid using TFT in hospitalized or ill subjects. In NTI, T4 and TSH levels are usually normal but in severe or prolonged illness low T4 (and fT4) and an inappropriately low normal or decreased TSH (0.1-0.4 mIU/L) may be seen. TSH levels below <0.01 mIU/L are very unusual except in very severe illnesses in the intensive care environment. Early in the course of NTI fT4 may increase followed by progressive decline with disease progression. T3 (and fT3) decline from the onset. Uncommonly, a low TSH and normal fT4 may be seen in NTI and can be distinguished from hyperthyroidism by remembering the clinical milieu and if necessary finding a low T3 (or fT3). Thus the use of both TSH and fT4 is necessary to accurately assess thyroid dysfunction in ill and hospitalized patients.
Diagnosis of thyroid conditions
One of the more common indications for thyroid testing is in the evaluation of clinically suspected thyroid dysfunction. A basic initial screen can include TSH alone but the use of TSH in conjunction with fT4 is preferred [16,21]. Further testing such as for thyroid antibodies, thyroglobulin or even radionuclide uptake studies should follow based on initial test results.
Suspected hyperthyroidism: Hyperthyroidism refers to the excessive production and secretion of thyroid hormones by the thyroid gland. The resulting clinical condition (fatigue, nervousness, weight loss despite normal or enhanced appetite, heat intolerance, palpitations) is termed thyrotoxicosis [21]. While the two terms are often used interchangeably subjects who become thyrotoxic from exogenous thyroid hormones (e.g. surreptitious consumption) are strictly not hyperthyroid and their thyroid hormone production may be suppressed. Older subjects (>60 years) often present with a paucity of symptoms other than weight loss and breathlessness [22].
An algorithm for evaluating suspected hyperthyroidism is provided (Figure 1). A thyroid panel comprising TSH and fT4 will usually suffice. In overt hyperthyroidism TSH is often <0.01 mIU/L (or undetectable) with elevations in both fT4 and fT3. The most common causes of primary hyperthyroidism is Graves disease (an autoimmune disease consequent to stimulatory TSH-receptor antibodies - TRAb), followed by toxic nodular goiter (multinodular or adenoma) and thyroiditis. In thyroiditis preformed thyroid hormones stored in thyroid follicles are released. Thyroiditis of the subacute variety (i.e. de Quervain’s thyroiditis) is characterised by pain and elevated inflammatory markers (sedimentation rate and C-reactive protein) whereas they are normal in painless and druginduced thyroiditis [23]. Most patients with Graves’ disease have obvious presentation (symptoms of thyrotoxicosis, eye signs and a smooth goiter on physical examination). Together with the classical TFT results no further investigations are needed. When presentation is atypical (e.g. euthyroid ophthalmopathy) measurement of TRAb may be helpful [24]. Over 90% of Graves’ disease has positive TRAbs. Where evaluation is unclear a radionuclide thyroid scan may be needed to show homogenous increased uptake in Graves’ disease versus a focal increase in toxic nodules (multi-nodular goiter or adenoma). In thyroiditis and iatrogenic hyperthyroidism (exogenous consumption) the uptake is low or absent [6]. Serum thyroglobulin (Tg) can be used to distinguish thyroiditis (elevated Tg) from iatrogenic hyperthyroidism (normal Tg).
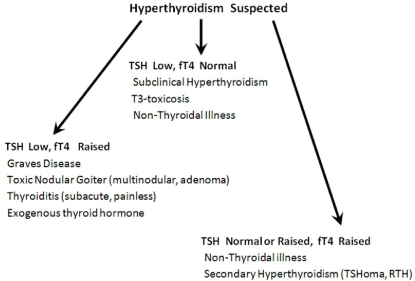
Figure 1: Algorithm for suspected Hyperthyroidism.
A normal fT4 and a low TSH (<0.01 mIU/L) is seen in subclinical hyperthyroidism. In early or mild hyperthyroidism TSH is undetectable while fT4 may still be normal and only fT3 is elevated - a state termed T3-toxicosis [21]. This condition may represent very early disease, autonomous thyroid nodules or hyperthyroidism in iodine-deficient areas. Aside from T3-toxicosis there is no need for including fT3 in a thyroid test panel.
In suspected hyperthyroidism with elevated fT4 but nonsuppressed TSH (normal or raised) some rare conditions must be considered. Pituitary TSH secreting adenomas (TSHoma) cause hyperthyroidism, goiter (often nodular), visual problems, headache and detectable imaging (CT scan or MRI) lesions [25]. The characteristic biochemical abnormalities are normal or elevated TSH in the presence of high T4 (and fT4). Accounting for less than 1% of all pituitary adenomas, TSHoma may sometimes (<30%) cosecrete growth hormone and prolactin. Another rare condition that may be indistinguishable from TSHoma is the syndrome of Resistance to Thyroid Hormone (RTH) [26]. In RTH there is reduced responsiveness of target tissues to thyroid hormone due to genetic mutations in the thyroid hormone receptor TRß. More recently other genetic defects which decrease the efficacy of thyroid hormones through altered cell membrane transport and metabolism have also been included as part of the RTH syndromes. The characteristic features of RTH include goiter, raised fT4, non-suppressed TSH, and responsiveness to Thyrotropin Releasing Hormone (TRH). Despite the elevated levels of thyroid hormones there is paucity of the usual hyperthyroid symptoms other than tachycardia and hyperkinetic behaviour. RTH is familial in 75% of cases in an autosomal dominant fashion. In contrast TSHoma has hypersecretion of the hormone α-subunit, exhibits a blunted TSH response to TRH, does not occur in families, and is associated with elevated levels of Sex- Hormone Binding Globulin (SHBG). From a laboratory standpoint a normal or raised TSH and elevated fT4 may be seen in some cases of NTI as well as in the rare condition of Familial Dysalbuminemic Hyperthyroxinemia (FDH). In FDH there is an abnormal albumin with increased affinity for T4 resulting in elevated thyroid hormones but subjects are euthyroid [27].
Suspected hypothyroidism: An algorithm is provided (Figure 2). A thyroid panel comprising TSH and fT4 are the tests of choice for suspected hypothyroidism. There is no role for fT3 in the evaluation of hypothyroidism since fT3 only declines much later than fT4, may even be normal in less severe forms of hypothyroidism and is decreased in NTI. In overt primary hypothyroidism the TSH levels are often > 15.0 mIU/L with low fT4 levels. Autoimmune thyroid disease of the Hashimoto variety is the most frequent cause and is associated with the presence of thyroid antibodies (anti-thyroid peroxidise and anti-thyroglobulin). Careful attention to history may uncover iatrogenic causes (current treatment with anti-thyroid drugs, previous thyroidectomy, neck irradiation) or side-effects of medications (lithium, amiodarone, interferon-α, sunitinib) [13].
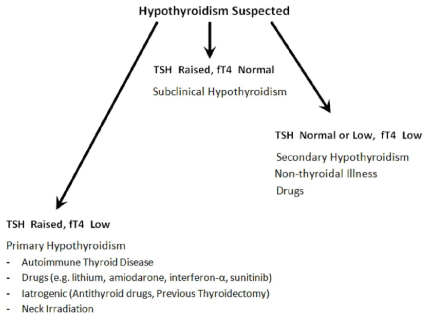
Figure 2: Algorithm for suspected Hypothyroidism.
In subclinical hypothyroidism TSH levels are often between 4.0- 10.0 mIU/L with normal fT4 levels. Thyroid antibody tests will help identify higher risk patients for thyroxine therapy. If TSH is normal or low and the fT4 is low consider secondary or central hypothyroidism. This pattern may also be found during the recovery phase of NTI. If central hypothyroidism is suspected such patients will need a full pituitary function assessment (measurement of growth hormone, FSH, LH, prolactin, estrogen, testosterone, ACTH and cortisol) and pituitary imaging to look for pituitary or hypothalamic tumors. A careful history might reveal a drug cause (e.g. aspirin, steroids, nonsteroidal anti-inflammatory drugs, phenytoin) [13], side-effect from previous head and neck irradiation, and possible NTI. RTH might also enter the differential diagnosis if all other causes have been ruled out.
Monitoring therapy
Treatment with thyroxine: Patients with primary hypothyroidism on thyroxine can be monitored by assessing serum TSH [16]. If TSH is high, the dose needs to be increased; if it is low, the dose needs to be reduced. Excessive suppression of serum TSH can increase the risk of both atrial fibrillation and bone disease. When commencing thyroxine replacement, fT4 is used to monitor therapy as there is a lag in the pituitary TSH response in adjusting to a new homeostatic state. fT4 should also be used to titrate the thyroid hormone dose in patients with secondary hypothyroidism due to pituitary or hypothalamic disease since TSH release is impaired. Serum TSH is only accurate if steady state conditions are present, which takes 1 to 3 months after starting or adjusting the thyroxine dose. As T3 is generally not used for treating hypothyroidism [28] there is no role for evaluating T3 (and fT3). However, when patients are on T3 therapy, assessment of therapy then requires measurement of serum T3 (and fT3) and TSH.
Treatment of hyperthyroidism: During the early treatment of hyperthyroidism, TSH may remain subnormal for several weeks and rarely for several months. One must therefore rely upon serum fT4 when assessing the efficacy of anti-thyroid drugs, radioiodine, or surgery. Once steady-state conditions are achieved TSH can be used to assess the efficacy of therapy.
Treatment of differentiated thyroid cancer (DTC): In thyroid cancer, patients are placed on thyroid hormone suppression to minimise growth of thyroid cancer cells. The target for TSH suppression is <0.1mU/L for high risk patients while low-risk patients can afford less vigorous TSH suppression (0.1-0.5 mIU/L) [29]. Serum thyroglobulin (Tg) can also be used as a sensitive and specific tumour marker of DTC [29]. In patients who are disease-free (negative neck ultrasound and undetectable Tg), TSH levels of 0.3–2.0 mIU/L is permissible. In low-risk disease (Stage I or II DTC), an increase in Tg of <1.5 ng/mL in the presence of a normal TSH is allowed.
Commonly Included Tests
Thyroid tests commonly used include TSH, free hormones, thyroid antibodies (TPO-Ab, Tg-Ab), TSH receptor antibodies (TRAb), and Thyroglobulin (Tg). Serum is used for testing. The blood sample should be collected in a plain tube and sent to the lab immediately for processing. Thyroid medications (thyroid hormones and antithyroid drugs) should be omitted prior to blood taking. To reduce the variability of test results specimens should be obtained at the same time of day and prandial state. Early morning fasting values are higher than late morning non-fasting TSH by an average of 25% [30]. TSH also exhibits diurnal rhythmicity with a nadir in the late afternoon and peak at midnight [31].
Thyrotropin or Thyroid Stimulating Hormone (TSH)
Most of the current TSH assays in use can measure TSH levels of 0.01 mIU/L or lower with an imprecision of 20%. This level of TSH is also known as the functional sensitivity of the assay. Serum TSH in untreated hyperthyroidism is often <0.01mIU/L. Thus we can distinguish mild hyperthyroidism from euthyroid subjects. However, some sick euthyroid subjects in intensive care units may have TSH values at or near 0.01mIU/L and require TSH assays with better detection limits (e.g. 0.004 mIU/L) for clearer diagnostic delineation [32]. The normal range for serum TSH is around 0.4–4.0 mIU/L. There is some debate over the appropriate upper reference limit [33]. The National Academy of Clinical Biochemistry maintains that the upper TSH limit should be 2.5 mIU/L [34] since 95% of rigorously screened euthyroid volunteers have values between 0.4–2.5 mIU/L. Applying a lower upper reference limit for TSH will substantially increase the diagnosis of subclinical hypothyroidism [35]. Agebased TSH normal ranges have also been proposed. In the United States NHANES III population of 13,444 subjects, the TSH reference range was 0.4–4.5 mIU/L after excluding those with a positive family history, drugs, goitre, nodules, or positive TPO-Ab [36]. Moreover, a close examination of the NHANES III data reveals that the reference limit for TSH only begins to move up (>7.5 mIU/L) in subjects above 70 years old.
TSH in conjunction with fT4 has been identified as the best screening tool for those suspected of having thyroid disorders [37]. In early thyroid dysfunction the minor changes in T4 and T3 amplifies serum TSH response. The entity of subclinical thyroid disease is being increasingly recognised consequent to the ability of TSH to reflect the minor biochemical alterations of thyroid hormones. A consistently abnormal TSH in the face of thyroid hormone levels within the laboratory reference range probably indicates that these thyroid hormone levels are not normal for the individual. While there is no firm consensus on the management of subclinical thyroid disease, support for treatment of subclinical hyperthyroidism is growing [38,39] especially in those who are older, with greater suppression of TSH (<0.1 mIU/L) and with other co-morbidities [40,41]. The case for treating subclinical hypothyroidism is less clear. A recent re-analysis of the Wickham survey data noted the association of subclinical hypothyroidism with coronary events and mortality as well as attenuation of cardiac morbidity and mortality with thyroxine therapy [42]. In a 13-year community-based cohort of women with thyroid antibodies and TSH between 2.5–4.0 mIU/L, they progressed to subclinical and overt hypothyroidism [43] suggesting the need for targeted screening and follow-up. This trend can only impact laboratory thyroid testing.
Free T4 (fT4) and Free T3 (fT3)
FT4 and fT3 assays have largely replaced total T4 and total T3 measurements but most modern methods are using an estimate of the true free hormone levels [44]. While accurate fT4 concentrations can be determined using liquid chromatography-tandem mass spectrometry [45] this method remains of research interest only and for manufacturers. For direct free hormone assays, the use of one-step hormone-labelled analog methods is discouraged. For one-step labelled antibody free T4 methods, the assay should extract only 1–2% of total hormone concentration off the binding proteins. Samples containing high hormone labels should exhibit parallelism when diluted and show <10% deviation from expected values.
A raised free T4 should be interpreted in the context of TSH levels to arrive at an accurate assessment. The use of fT3 should be limited to the evaluation of T3-toxicosis, monitoring treatment of thyroid storm, and in the rapid pre-operative preparation of the acutely thyrotoxic patient. A low fT4 in the clinical context suspicious of hypothyroidism should be interpreted with accompanying TSH levels. In both raised and low fT4, care must be taken to consider NTI causing derangement of free hormone levels when TSH remains normal.
Thyroid antibodies
An antibody panel consisting of Anti-Thyroid Peroxidise (TPOAb) and Anti-Thyroglobulin (Tg-Ab) has been used to aid in the differential diagnosis of thyroid disorders. TPO-Ab and Tg-Ab are present in over 90% of patients with autoimmune thyroid disease [46]. In the US NHANES III study TgAb were positive in 10.4% and TPOAb, in 11.3% of thyroid disease-free subjects [4]. Positive antibodies were more prevalent in women than men and increased with age, and TPO-Ab was less prevalent in blacks (4.5%) than in whites (12.3%). TPO-Ab was significantly associated with hypo or hyperthyroidism, but TgAb were not. Thus, TPO-Ab has sufficient sensitivity and specificity to be used singly for diagnosis of autoimmune thyroditis [47]. As Tg-Ab is less specific, its use is confined to the follow-up of thyroid cancer [46]. In subclinical hypothyroidism TPO-Ab predicts progression to overt hypothyroidism - 4.3% per year versus 2.6% per year in TPO-Ab negative subjects [48].
TSH receptor antibodies (TRAb)
TRAbs may be either stimulating or blocking in nature, but most assays cannot distinguish between the two and measure binding to the TSH receptor. A rapid (27 minute), automated electrochemiluminescent TRAb assay is now available [49]. Using this assay TRAbs were detected in 97% of Graves’ disease. It is helpful in the evaluation of possible Graves’ disease without extra thyroidal features, unilateral exophthalmos or amiodarone thyrotoxicosis [24]. It has also been used to predict relapse after anti-thyroid therapy [49].
Thyroglobulin (Tg)
Tg is produced in normal states and in Differentiated Thyroid Cancer (DTC). After thyroidectomy for DTC, Tg is commonly measured following radioiodine ablation of the remnant normal thyroid tissue to detect any tumor recurrence [50]. However, the presence of Tg-Abs in serum will result in under-estimation of the true Tg levels due to assay interference [51]. Thus it is important that TgAb is measured at the same time as Tg [52]. If a rising Tg is observed, careful ultrasonography of the neck should be performed [53].
Radio nuclide uptake studies
Thyroid scans are functional studies to assess thyroid activity [6]. Technetium (99mTc) pertechnetate has largely replaced radioiodine as the cost and radiation dose is lower. The main application of thyroid scan is to differentiate the cause of hyperthyroidism when conventional evaluation is unclear. The pattern of tracer uptake will indicate the likely diagnosis. The increased uptake in Graves’ disease is diffusely homogenous while that in toxic nodular goiter is focal. Thyroid uptake is reduced in thyroiditis and factitious hyperthyroidism. There is no role for thyroid scans in the evaluation of hypothyroidism.
Conclusion
Thyroid function tests have gained wide clinical utility. However caution must be exercised whenever test results are at variance with the clinical picture or form an unusual pattern. In such instances re-evaluate the clinical history and consider the patient’s age, medications (including thyroxine or anti-thyroid drugs) and possible non-thyroidal illness. Then re-assess the thyroid status and exclude assay artefacts. Finally consider the possibility of rare disorders. It is only with a firm understanding of the laboratory science underpinning thyroid function tests and an appreciation of their medical context will users successfully interpret the broad array of thyroid function tests.
References
- Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013; 27: 745-762.
- Aw TC, Yap YFC. Thyroid function tests. Proc Singapore Healthcare 2011; 20: 132-137.
- Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006; 295: 1033-1041.
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002; 87: 489-499.
- Bayram C, Valenti L, Britt H. Orders for thyroid function tests - changes over 10 years. Aust Fam Physician. 2012; 41: 555.
- Lee JC, Khafagi FA. Thyroid scans. Aust Fam Physician. 2012; 41: 584-586.
- Martínez-Sánchez N, Alvarez CV, Fernø J, Nogueiras R, Diéguez C, López M. Hypothalamic effects of thyroid hormones on metabolism. Best Pract Res Clin Endocrinol Metab. 2014; 28: 703-712.
- van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clin Chem. 2011; 57: 122-127.
- Benhadi N, Fliers E, Visser TJ, Reitsma JB, Wiersinga WM. Pilot study on the assessment of the setpoint of the hypothalamus-pituitary-thyroid axis in healthy volunteers. Eur J Endocrinol. 2010; 162: 323-329.
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012; 379: 1142-1154.
- Franklyn JA. The thyroid--too much and too little across the ages. The consequences of subclinical thyroid dysfunction. Clin Endocrinol (Oxf). 2013; 78: 1-8.
- Fatourechi MM, Fatourechi V. An update on subclinical hypothyroidism and subclinical hyperthyroidism. Expert Rev Endocrinol Metab 2014; 9: 137-151.
- Barbesino G. Drugs affecting thyroid function. Thyroid. 2010; 20: 763-770.
- Fournier JP, Yin H, Yu OH, Azoulay L. Metformin and low levels of thyroid-stimulating hormone in patients with type 2 diabetes mellitus. CMAJ. 2014; 186: 1138-1145.
- Lupoli R, Di Minno A, Tortora A, Ambrosino P, Lupoli GA, Di Minno MN. Effects of treatment with metformin on TSH levels: a meta-analysis of literature studies. J Clin Endocrinol Metab. 2014; 99: E143-148.
- Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. American Association of Clinical Endocrinologists and the American Thyroid Association Clinical Practice Guidelines for hypothyroidism in adults. Thyroid. 2012; 22: 1200-1235.
- Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000; 160: 1573-1575.
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2014.
- Van den Berghe G. Non-Thyroidal Illness in the ICU: A Syndrome with Different Faces. Thyroid. 2014; 24: 1456-1465.
- Pappa TA, Vagenakis AG, Alevizaki M. The nonthyroidal illness syndrome in the non-critically ill patient. Eur J Clin Invest. 2011; 41: 212-220.
- Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: Management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011; 21: 593-646.
- Boelaert K, Torlinska B, Holder RL, Franklyn JA. Older subjects with hyperthyroidism present with a paucity of symptoms and signs: a large cross-sectional study. J Clin Endocrinol Metab. 2010; 95: 2715-2726.
- Pearce EN, Bogazzi F, Martino E, Brogioni S, Pardini E, Pellegrini G, et al. The prevalence of elevated serum C-reactive protein levels in inflammatory and noninflammatory thyroid disease. Thyroid. 2003; 13: 643-648.
- Matthews DC, Syed AA. The role of TSH receptor antibodies in the management of Graves' disease. Eur J Intern Med. 2011; 22: 213-216.
- Beck-Peccoz P, Persani L. Thyrotropin-secreting pituitary adenomas. Chapter 13b. In ‘Thyroid Disease’ at Thyroid Manager. 2013.
- Refetoff S, Dumitrescu AM. Reduced sensitivity to thyroid hormone: defects of transport, metabolism and action. Chapter 16. In ‘Thyroid Disease’ at Thyroid Manager. 2012.
- Refetoff S. Abnormal thyroid hormone transport. In ‘Thyroid Disease’ at Thyroid Manager. 2012.
- Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism. Thyroid. 2014.
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association Management Guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19: 1167-1214.
- Plumelle D, Lombard E, Nicolay A, Portugal H. Influence of diet and sample collection time on 77 laboratory tests on healthy adults. Clin Biochem. 2014; 47: 31-37.
- Roelfsema F, Veldhuis JD. Thyrotropin secretion patterns in health and disease. Endocr Rev. 2013; 34: 619-657.
- Boonen E, Van den Berghe G. Endocrine responses to critical illness: novel insights and therapeutic implications. J Clin Endocrinol Metab. 2014; 99: 1569-1582.
- Laurberg P, Andersen S, Carlé A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol. 2011; 7: 232-239.
- Biondi B. The normal TSH reference range: what has changed in the last decade? J Clin Endocrinol Metab. 2013; 98: 3584-3587.
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab. 2008; 93: 1224-1230.
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and anti-thyroid antibodies in the U.S. population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007; 92: 4575–4582.
- Estrada JM, Soldin D, Buckey TM, Burman KD, Soldin OP. Thyrotropin isoforms: implications for thyrotropin analysis and clinical practice. Thyroid. 2014; 24: 411-423.
- Mitchell AL, Pearce SH. How should we treat patients with low serum thyrotropin concentrations? Clin Endocrinol (Oxf). 2010; 72: 292-296.
- Mai VQ, Burch HB. A stepwise approach to the evaluation and treatment of subclinical hyperthyroidism. Endocr Pract. 2012; 18: 772-780.
- Wirth CD, Blum MR, da Costa BR, Baumgartner C, Collet TH, Medici M, et al. Subclinical thyroid dysfunction and the risk for fractures: a systematic review and meta-analysis. Ann Intern Med. 2014; 161: 189-199.
- Garin MC, Arnold AM, Lee JS, Robbins J, Cappola AR. Subclinical thyroid dysfunction and hip fracture and bone mineral density in older adults: the cardiovascular health study. J Clin Endocrinol Metab. 2014; 99: 2657-2664.
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham Survey cohort. J Clin Endocrinol Metab. 2010; 95: 1734-1740.
- Walsh JP, Bremner AP, Feddema P, Leedman PJ, Brown SJ, O'Leary P. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab. 2010; 95: 1095-1104.
- Thienpont LM, Van Uytfanghe K, Poppe K, Velkeniers B. Determination of free thyroid hormones. Best Pract Res Clin Endocrinol Metab. 2013; 27: 689-700.
- Soldin OP, Soldin SJ. Thyroid hormone testing by tandem mass spectrometry. Clin Biochem. 2011; 44: 89-94.
- So M, MacIsaac RJ, Grossmann M. Hypothyroidism. Aust Fam Physician. 2012; 41: 556-562.
- Topliss DJ, Eastman CJ. 5: Diagnosis and management of hyperthyroidism and hypothyroidism. Med J Aust. 2004; 180: 186-193.
- Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002; 87: 3221-3226.
- Schott M, Hermsen D, Broecker-Preuss M, Casati M, Mas JC, Eckstein A, et al. Clinical value of the first automated TSH receptor autoantibody assay for the diagnosis of Graves' disease (GD): an international multicentre trial. Clin Endocrinol (Oxf). 2009; 71: 566-573.
- Cox AE, LeBeau SO. Diagnosis and treatment of differentiated thyroid carcinoma. Radiol Clin North Am. 2011; 49: 453-462, vi.
- Spencer CA, Petrovic I, Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab 2011; 96:1283-1291.
- Spencer CA. Clinical review: Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. 2011; 96: 3615-3627.
- Elisei R, Agate L, Viola D, Matrone A, Biagini A, Molinaro E. How to manage patients with differentiated thyroid cancer and a rising serum thyroglobulin level. Endocrinol Metab Clin North Am. 2014; 43: 331-344.