
Special Article - Hypothyroidism
Annals Thyroid Res. 2017; 3(1): 95-101.
Primary Care Provider Management of Congenital Hypothyroidism Identified Through Newborn Screening
Rosenthal NA1, Bezar E2, Mann S3, Bachrach LK4, Banerjee S5, Geffner ME6, Gottschalk M7, Shapira SK8, Hasegawa L3 and Feuchtbaum L1*
1California Department of Public Health, Genetic Disease Screening Program, USA
2Public Health Foundation Enterprises, USA
3Hawai’i Department of Health Genetics Program, USA
4Lucile Salter Packard Children’s Hospital, Stanford School of Medicine, USA
5Valley Children’s Hospital, USA
6Children’s Hospital Los Angeles, USA
7UCSD School of Medicine, USA
8Centers for Disease Control and Prevention, USA
*Corresponding author: Lisa Feuchtbaum, California Department of Public Health, Genetic Disease Screening Program, Richmond CA 94804, Dr. PH, MPH. 850 Marina Bay Parkway, F-175, Richmond, CA 94804, USA
Received: March 17, 2017; Accepted: April 03, 2017; Published: April 18, 2017
Abstract
Objective: To assess Primary Congenital Hypothyroidism (CH) management patterns and feasibility of providing long-term care for patients with CH identified through newborn screening by Primary Care Providers (PCPs) in California and Hawaii.
Study Design: A survey was mailed to all physicians (N=823) listed as the referral doctor for confirmed patients with CH identified through newborn screening programs in both states between 01/01/2009–12/31/2013. Information was collected on CH management patterns, barriers to providing care, and knowledge on CH treatment. Descriptive statistics and bivariate logistic regression results were reported.
Results: 206 PCPs completed the survey. Among these, 78% currently have patients with CH and 91% indicated willingness to provide long-term care to new patients with CH. Among PCPs currently caring for patients with CH, 17% managed CH by themselves with limited assistance from endocrinologists; 63% were involved in managing CH but endocrinologists played a larger role than PCPs; 19% were not involved in CH care. Only 49% of PCPs correctly answered questions regarding recommended follow-up frequencies and 23% knew the correct age for a trial off levothyroxine for suspected transient CH. Top two perceived barriers to providing long-term care included “need guidance or support from endocrinologists” (61%) and “not familiar with CH treatment guidelines” (28%).
Conclusion: The majority of PCPs surveyed are willing to provide long-term care to patients with CH, but need support from endocrinologists and increased knowledge about current treatment guidelines.
Keywords: Congenital hypothyroidism; Primary care; Management; Newborn screening
Abbreviations
CH: Primary Congenital Hypothyroidism; NBS: Newborn Screening; LTFU: Long-Term Care and Follow-Up; PCP: Primary Care Providers; AAP: American Academy of Pediatrics; CME: Continuing Medical Education
Introduction
Primary Congenital Hypothyroidism (CH) is the most common preventable cause of neurocognitive disability (mental retardation) [1] and is the most common disorder diagnosed through Newborn Screening (NBS) [2]. CH affects about 1 in 2000 California newborns and 1 in 3000 Hawaii newborns, with over 245 new cases diagnosed in both states each year [2,3] CH refers to thyroid hormone deficiency due to dysfunction of the thyroid gland. About 80%?85% of permanent CH cases are caused by developmental failure or maldevelopment of the thyroid gland (i.e., aplasia, hypoplasia, or ectopia). Other permanent cases are caused by an inherited enzyme deficiency affecting synthesis of thyroid hormone (dyshormonogenesis) or a thyrotropin receptor defect [1,4]. Although early detection and initiation of treatment within the newborn period have nearly eradicated severe mental disabilities among patients with CH in countries with NBS, unfavorable clinical outcomes are still present in a subset of patients [5]. CH severity and treatment inadequacy have been associated with a lower Intellectual Quotient (IQ) [6-9], lower educational attainment [10-12], and sensorineural hearing loss [10,13,14]. Patients with severe or inadequately treated CH also have increased risk of congenital malformations (e.g., anomalies of cardiac and nervous system and eyes), obesity [10,15-18], and impaired growth, puberty, and fecundity [19-22]. These findings highlight the importance of adequate treatment and monitoring to maintain optimal thyroid function throughout life to ensure the best neuro developmental, physical, and social outcomes in patients with CH.
Specific diagnostic, treatment, and follow-up guidelines for children with CH have been published by both the American Academy of Pediatrics (AAP) and the European Society for Paediatric Endocrinology [23,24]. Both guidelines recommend frequent followups during childhood. However, a U.S. study using both public and private health insurance data found that over one-third of patients with CH discontinued treatment by three years of age [25]. Although the precise incidence of transient hypothyroidism is unknown (recent estimates range from 13.5%to 54.5% [26-28]), the authors of the analysis suggested that most cases of treatment discontinuation were likely not transient, but parent-initiated without physician supervision [23,25,27]. A follow-up study of patients with CH detected by the Michigan NBS Program showed that 25% of the patients were no longer being treated by three years of age, among which 83% had stopped treatment without medical supervision [29]. Of those who underwent a medically-supervised trial off medication, treatment was resumed in 87% of the cases [29]. These data suggest that discontinuation of treatment without medical supervision is a problem that could result in suboptimal outcomes for many patients. Due to the paucity of long-term follow-up data from the United States, it is unclear how patients with CH are being managed as they age and the potential adverse effects of inadequate treatment or premature discontinuation of levothyroxine treatment.
In both California and Hawaii, patients who screen positive for CH in state NBS programs are usually referred by their Primary Care Providers (PCPs) to endocrinologists for further testing and confirmatory diagnosis. In California, a large percent of screen positive patients are referred to state-contracted endocrine centers to provide follow-up care. However, feedback from endocrinologists indicated that these centers usually do not have the resources needed to follow every patient with CH as frequently as recommended by the AAP guidelines, especially during the first three years of life, when treatment adherence is most critical. Furthermore, it is often more practical and convenient for patients with CH to receive care from their PCPs who are usually in closer proximity and more readily available, especially in rural areas where fewer endocrinologists are available [30]. Parks et al found that in Texas during 2004-2006, 64% of patients with CH were followed and reported by endocrinologists in the first year of life and the proportion decreased to 26% in the second year of life. Underreporting from endocrinologists might exist, but these findings indicated that some patients might be followed by primary care pediatricians [31]. The AAP-recommended CH follow-up visits can be completed during routine well-baby visits. Endocrinologists can serve as consultants after the initial evaluation of the case is completed and provide guidance about modifying treatment when questions arise. The consensus is that PCPs should take more responsibility for providing long-term care and encouraging adherence to treatment for patients with CH with the support and consultation from pediatric endocrinologists.
These recommendations are consistent with the patient-centered medical home model for providing primary care that has been widely endorsed by physicians’ associations, including the AAP and the American Academy of Family Physicians [32]. Studies have shown that, for children and youth with special health-care needs, care provided through a medical home is associated with better care coordination, higher satisfaction with care, and fewer emergency room visits or hospitalizations [33-36]. Compared to metabolic disorders screened for by NBS, CH is easier to manage since it primarily involves the administration of a single drug. Thus, with proper training, the locus of the medical home can be with the PCP when that provider is supported by apediatric endocrinologist for challenging and more nuanced cases. Increased PCP involvement and “ownership” of care for patients with CH would likely improve quality of care, disease management and health outcomes. Understanding the hurdles impeding management of children with CH by pediatricians and whether it is feasible to shift the Long-Term Follow-Up (LTFU) responsibility to PCPs with support from pediatric endocrinologists will strengthen the medical home for these patients.
The purpose of this study was four-fold: 1) to evaluate the current CH case management patterns; 2) to assess the willingness and capability of PCPs to provide LTFU for patients with CH; 3) to identify potential barriers for PCPs to provide LTFU to patients with CH; and 4) to assess PCPs’ willingness to obtain informed consent and provide data to an existing database to evaluate quality of care and patient outcomes for possible research endeavors in the future.
Methods
A cross-sectional survey of PCPs was conducted from February through June of 2014. All PCPs who were listed as the referral doctors for at least one patient with CH born between 2009-2013 were selected from the California and Hawaii NBS databases. A total of 801 physicians from California and 22 physicians from Hawaii were invited to participate in the study.
The survey included five key components relevant to PCPs: 1) current practice in managing patients with CH; 2) barriers and resources needed to conduct CH LTFU; 3) willingness and capability to conduct CH LTFU; 4) willingness and capability to obtain informed consent from patients and provide data to a LTFU database; and 5) clinical outcomes of patients with CH being seen by the PCPs. Results of the fifth component will be reported in a future paper. To maximize response rate, we included a personalized cover letter in the surveymailing packet that described the significance and objectives of the study. To better understand the reasons for non-response, we asked each doctor who was unable to complete the questionnaire to indicate reasons for non-response on a separate “Non-Response Card” enclosed with the initial mailing. A gift card incentive was offered to PCPs who completed either the full survey or the non-response card. An on-line version of the same survey was also made available at the same time. One hundred and thirteen mailing packets were returned as undeliverable after the initial mailing. We were able to obtain their most up-to-date addresses through multiple modes (e.g., internet search and calling the listed offices) for 87 doctors and resent the packets. We failed to find usable addresses for 26 doctors and they were excluded from the response rate calculation. Additionally, we sent out two reminder letters to non-responding PCPs, one and two months after the initial mailing, to boost the response rate.
All data were analyzed using SAS version 9.3 for Windows (SAS Institute, Cary, NC). Continuous variables were expressed as medians with range and categorical variables were presented as numbers and percentages. Descriptive statistics were calculated and the differences between the two states were assessed using Chi-square and Fisher’s exact tests for categorical variables and Wilcoxon Signed Rank Test for continuous variables to compare the difference in medians. Bivariate logistic regression modeling was used to assess the association between selected covariates and key outcome variables. Crude Odds Ratios (ORs) and their 95% Confidence Intervals (CIs) were reported. This study was approved by the State of California, Health and Human Services Agency, Committee for the Protection of Human Subjects, Project Number 13-08-1317.
Results
Response rates and demographics
A total of 238 doctors in California and 10 doctors in Hawaii responded to the mailing, among whom 226 completed the survey (128 paper surveys and 98 on-line surveys) and 19 returned the Non- Response Card (Figure 1). Among doctors who completed the survey, 20 were not PCPs and their responses were excluded from the final analysis. The total response rate was 28% for California doctors and 45% for Hawaii doctors. Among the 19 doctors/offices that returned the Non-Response Card and indicated a reason for not completing the survey, four did not have time to do the survey, seven were specialists instead of PCPs, six did not have patients with CH, one doctor was deceased, and one had security concerns.
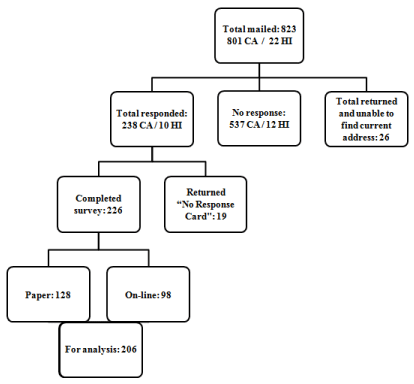
Figure 1: Response rate of the Primary Care Providers (PCPs) surveyed in
California (CA) and Hawaii (HI) in 2014.
Among the 206 PCPs who completed the survey, 51% were male, 46% were White, 39% were Asian, 7% were of Hispanic origins and 8% were of other race or ethnicity (Table 1). The majority of respondents were primary care pediatricians and 8% identified themselves as family physicians. Nearly 50% were in private practice, 33% were in group practice, 13% were in community health centers, and only 4% were in a hospital-based practice or HMO. Respondents had been in medical practice for a median of 18 years (range 2–43 years). There was no statistically significant difference in these variables between respondents from California and Hawaii.
Characteristics
CA (%, n=196)
HI (%, n=10)
All (%, n=206)
Male sex
51
60
51
Race/ethnicity
Hispanic/Latino
7
0
7
White
47
20
46
Asian
38
60
39
Black
3
0
3
Mixed or Other
5
20
5
Medical specialty
Family practice
9
0
8
Pediatrics
91
100
92
Type of practice
Private practice
45
50
45
Group practice
33
30
33
Hospital-based practice
5
0
4
HMO
4
0
4
Community health center
13
20
13
Years in medical practice
Median (range)
18 (2, 43)
19 (8, 29)
18 (2, 43)
Note: There was no statistically significant difference between CA and HI for any of the above variables.
Table 1: Characteristics of the responding primary care providers in California (CA) and Hawaii (HI).
Case management patterns and long-term care feasibility
When California PCPs were asked who usually manages CH for their patients, 17% indicated that their patients’ CH was mainly managed by themselves, but endocrinologists were involved; 63% were involved, but patients’ CH was mainly managed by endocrinologists; and 19% indicated that their patients’ CH was solely managed by endocrinologists. Among Hawaii PCPs, 75% indicated that their patients’ CH was mainly managed by endocrinologists, but they were also involved and the rest indicated that their patients’ CH was solely managed by endocrinologists. Among all respondents, “need guidance or support from endocrinologists” and “not familiar with the CH treatment guidelines” were listed as the two most commonly perceived barriers for PCPs to provide long-term care for patients with CH.
Over 90% of responding PCPs in both states indicated willingness to provide long-term care for new patients with CH. Compared to PCPs who mainly manage patients’ CH by themselves, those who were not involved in their patients’ CH management had 85% lower odds of being willing to provide long-term care for new patients with CH (OR=0.15, 95% CI: 0.03, 0.77). Type of practice and currently having patients with CH were not associated with willingness to provide long-term care for new patients with CH (Table 2 and 4).
Variables
CA (%)
HI (%)
All (%)
Who manages patients’ CH conditions
Solely by PCPs
1
0
1
Mainly by PCPs, but endocrinologists are involved
17
0
16
Mainly by endocrinologists, but PCPs are involved
63
75
64
Solely by endocrinologists
19
25
19
Willing to provide long-term care for new patients with CH
Yes
66
80
67
Maybe
25
20
24
No
6
0
6
Don't know
3
0
3
Perceived barriers for providing long-term care
Need guidance or support from endocrinologists
60
80
61
Not familiar with the CH treatment guidelines
28
40
28
Need more staff time to coordinate care
15
10
15
Patients are not compliant with care
14
10
14
Don’t have enough time
12
10
12
CH long-term care is too complicated
11
0
10
Don’t get enough reimbursement
11
0
10
Anticipate no barriers
20
10
20
Willingness to obtain informed consent
75
100
76
Difficulties in obtaining informed consent
Very difficult
4
0
4
Difficult, but doable
35
20
34
Not difficult at all
39
70
40
Don't know
22
10
21
Willingness to provide LTFU data
Yes
67
90
68
Maybe
19
10
19
No
3
0
3
Don't know
11
0
10
Reasons for not willing to provide LTFU data
Don’t have enough staff or time to enter data
37
0
37
Do not provide long-term care for patients’ CH
37
0
37
Have concerns over patient’s privacy
19
0
19
The LTFU data is not relevant to clinical practice
7
0
7
It is not important to collect LTFU data for CH
0
0
0
Compensation required to provide LTFU data
$50/patient/year
5
20
6
$100/patient/year
18
20
18
$150/patient/year
14
0
13
$200/patient/year
18
0
17
No compensation is needed
18
40
19
Don't know/Other
27
20
27
Note: There was no statistically significant difference between California and Hawaii for any of the above variables. CA: California; HI: Hawaii.
Table 2: Case management patterns, perceived barriers, and willingness to providing Long-Term care and Follow-Up (LTFU) for patients with primary Congenital Hypothyroidism (CH).
Over two-thirds of PCPs in both states expressed willingness to obtain informed consent from patients to record and share LTFU data on their patients with CH. Nearly 75% of PCPs consider obtaining informed consent doable with little or no difficulties. About 87% of PCPs from both states indicated willingness to report LTFU data if given a reasonable compensation. PCPs currently caring for patients with CH were more likely to indicate willingness to provide LTFU data than those who had no patient. Among the 27 California PCPs who were not willing to collect LTFU data, over one-third indicated “do not have enough staff or time to enter data” or “do not provide long-term care for patients’ CH condition” as the main reasons for their unwillingness to do so. In terms of compensation required to provide LTFU data, 73% of PCPs would be satisfied with a compensation less than $200 per patient per year (Tables 2 and 4).
Knowledge about CH-related management
When asked about the recommended frequency of blood tests for patients with CH in three different age groups (<6 months, 6 months to 3 years, and >3 years), the proportion of PCPs who correctly answered each question was72%, 60%, and 73% for each age group, respectively. Only 49% of PCPs correctly answered all three questions about the recommended follow-up frequencies (Table 3). There was no statistical difference in the prevalence of knowing the recommended follow-up frequencies between PCPs who reported different management patterns, type of practice, or number of patients (Table 4).
Variables
CA (%)
HI (%)
All (%)
Know the recommended frequency of blood tests
In the first six months of age
72
70
72
Between six months and three years of age
59
70
60
Over three years of age
74
50
73
Know all frequencies
49
50
49
Familiarity with indications for trial off levothyroxine
Very familiar
3
0
2
Somewhat familiar
29
20
28
Not familiar
54
70
55
Do not know
15
10
14
Know at what age for trial off levothyroxine
23
30
23
Note: There was no statistically significant difference between California and Hawaii for any of the above variables. CA: California; HI: Hawaii.
Table 3: Knowledge of current treatment guidelines for primary congenital hypothyroidism.
Covariates
Know recommended follow-up frequencies
OR (95% CI)*
Willing to provide care for new patients with CH^ (Yes and Maybe)
OR (95% CI)*
Willing to provide LTFU data (Yes and Maybe)
OR (95% CI)*
Likelihood to participate in CME (Very likely and Likely)
OR (95% CI)*
Currently having =1 patients with CH^
Yes
1.1 (0.5, 2.1)
1.8 (0.6, 5.4)
3.1 (1.3, 7.6)
3.0 (1.1, 8.5)
No
Reference
Reference
Reference
Reference
Management patterns
Mainly by PCP$, but endocrinologists are involved
Reference
Reference
Reference
Reference
Mainly by endocrinologists, but PCP is involved
0.9 (0.4, 2.0)
4.1 (0.5, 30.6)
1.3 (0.3, 5.2)
3.0 (0.8, 11.4)
Solely by endocrinologists
1.2 (0.4, 3.2)
0.15 (0.03, 0.77)
0.6 (0.1, 3.0)
1.2 (0.3, 5.3)
Type of practice
Private practice
Reference
Reference
Reference
Reference
Group practice
1.1 (0.6, 2.0)
1.1 (0.4, 3.0)
1.6 (0.5, 4.8)
0.4 (0.1, 1.2)
Hospital-based practice
0.12 (0.01, 1.04)
1.0 (0.1, 8.7)
0.25 (0.05, 1.14)
0.22 (0.04, 1.35)
HMO$
1.7 (0.4, 7.4)
N/A
0.12 (0.03, 0.57)
0.22 (0.04, 1.35)
Community health center
1.7 (0.7, 4.1)
3.2 (0.4, 26.3)
0.5 (0.2, 1.7)
1.8 (0.2, 15.5)
*OR: Odds Ratio; CI: Confidence Interval; ^CH: Primary Congenital Hypothyroidism
Significant odds ratios are shown in bold. $PCP: Primary Care Providers; HMO: Health Maintenance Organization.
Table 4: Association between selected covariates and key outcome variables.
When assessing PCPs’ familiarity with indications for a trial off levothyroxine therapy to assess the transient status of CH, only 2% were very familiar, 28% were somewhat familiar, 55% were not familiar, and 14% did not know. Only 23% of PCPs in both states knew the recommended age for such a trial (Table 3).
Preference for CH-related continuing medical education
Over 80% of responding PCPs in both states indicated being “likely” or “very likely” to participate in Continuing Medical Education (CME) about CH if such courses are available. PCPs who reported currently having patients with CH were more likely to participate in CH-related CME than those without patients (Table 4). With regard to the preferred format for CME, over 60% of respondents chose webinars, about one-third chose in-person classes, and one-third chose grand rounds presentations.
Discussion
This study of current management patterns of patients with CH and feasibility of PCP involvement in long-term care provides important insights on how to assure high quality primary care and optimal clinical outcomes for patients with CH. Although the majority of PCPs who completed the survey were willing to provide long-term care to their patients with CH, endocrinologists were identified as assuming the primary management responsibilities in most of the cases in both states. The two most commonly perceived barriers by PCPs to providing long-term care for patients with CH were: needing guidance from endocrinologists and lack of familiarity with the current CH treatment guidelines. We identified a general lack of knowledge about CH treatment and management among responding PCPs. Nonetheless, the majority of respondents were willing to provide long-term care for new patients with CH. Specifically, they considered it feasible to obtain informed consent from patients with CH or their guardians, and would be willing to provide LTFU data to an existing database for a compensation of $200 or less per patient per year.
Long-term follow-up data for patients identified through NBS can help evaluate the clinical outcomes and health service utilization patterns of patients with diagnosed disorders in diverse populations. Multiple national agencies have released initiatives to establish a comprehensive, sustainable, and feasible long-term follow-up data collection system to improve the ultimate health outcomes of patients with screened disorders [37-40]. For patients with CH, optimal treatment throughout life is critical for achieving the best neurological, physical, economic, and social outcomes [24,40]. How to effectively deliver the needed care to all patients with CH and ensure adequate treatment for everyone remains a challenge in both California and Hawaii. National data showed that there is a shortage of pediatric endocrinologists nationwide; the average time that patients have to wait to see a pediatric endocrinologist is 10.3 weeks and the average distance to pediatric endocrinology care is over 26 miles [30]. Findings from this study indicate the possibility of integrating such care into primary care practices. The majority of PCPs in both states were willing to take the responsibility of caring for patients with CH with support and guidance from endocrinologists. Future efforts should be focused on how to improve the communication between PCPs and endocrinologists and to ensure that each patient receives appropriate and comprehensive care.
To our knowledge, this is the first survey of PCPs to identify a knowledge gap in CH management. To increase PCPs’ capability for providing long-term care for patients with CH, one avenue would be to provide CME opportunities for PCPs to learn about current guidelines for CH treatment and management. In response to the documented strong interests in CME training from responding PCPs, we have since developed a CH management curriculum entitled, “Congenital Hypothyroidism: What Every Pediatrician Needs to Know.” This course has been offered through Pediatric Grand Rounds throughout California and Hawaii. Building off of this CME course for PCH providers, Stanford University recently developed an online CME course on CH that is applicable more broadly to pediatric care providers (https://med.stanford.edu/cme/courses/ online/hypothyroidism.html). These teaching modules review how to confirm diagnosis, initiate treatment if required, monitor thyroid function, adjust levothyroxine dosing for infants, children and adolescents, and underscore the need for providers to educate families about the importance of adherence to treatment. We hope that such efforts will increase the confidence and competence of PCPs to manage patients with CH and improve their health outcomes.
We recognize a key limitation in this study. The overall response rate of completed surveys was 28.4%, despite the substantial efforts to improve the response rate as outlined in methods section [41]. The top three reported reasons for not completing the survey among those who returned the Non-Response Card were: being specialists themselves, having no time, and not currently having any patient with CH. The first two reasons may not cause any response bias. The third reason, not currently caring for any patient with CH, could result in an overestimation of several outcome variables including the percentage of doctors currently having patients with CH and knowledge about CH management. It is possible that PCPs currently caring for patients with CH would be more likely to participate in the study and have better knowledge than those without any patient with CH. Prior studies have shown that provider surveys are resistant to non-response bias and our response rate was better than other provider surveys of similar length [41-44]. We believe that the findings may be reasonably representative of PCPs in both states.
Conclusion
PCPs in both California and Hawaii expressed willingness to provide long-term care for patients with CH, but to fulfill the responsibility they need additional training on up-to-date CH-related treatment and management guidelines, as well as strong support from endocrinologists who can provide consultation for dealing with difficult cases. In addition, PCPs stated willingness to provide longterm follow-up data for patients with CH to existing databases. In future efforts to improve the clinical outcomes for patients with CH or other disorders identified by NBS and ensure that they receive high quality primary care services we should try to better engage PCPs in the care process and improve the collaboration between PCPs and specialists. More research is needed to assess the effectiveness of educational efforts for PCPs and the feasibility of conducting longterm follow-up for rare diseases in primary care settings.
Acknowledgement
We would like to thank the Human Resources and Services Administration for providing funding for this study. We also thank all doctors who participated in the survey, and colleagues at the California Genetic Disease Screening Laboratories and Genetic Disease Screening Program, including, Tracey Bishop, Shellye Lessing, Norah Ojeda, Bob Currier, Sona Saha and Hari Haran,for their help with the survey contents and administration. Drs. Ning Rosenthal and Lisa Feuchtbaum had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This project was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant D93MC26187-02-00: Demonstration Projects for Integrating Newborn Screening Long Term Follow-Up into Primary Care Practice. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, the Centers for Disease Control and Prevention, HHS or the U.S. Government. None of the coauthors has conflicts of interest relevant to this article to disclose.
Funding Source
This project is supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant D93MC26187-02-00: Demonstration Projects for Integrating Newborn Screening Long Term Follow- Up into Primary Care Practice. The funding agency does not have a conflict of interest with this research or this manuscript and did not influence (1) the study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication.
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, the Centers for Disease Control and Prevention, HHS or the U.S. Government.
References
- Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010; 5: 17.
- Feuchtbaum L, Carter J, Dowray S, Currier RJ, Lorey F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet Med. 2012; 14: 937-945.
- Palmer G, Kong J, Harding C, LaFranchi SL, Thomas G, Wall M, et al. Hawai'i Practitioner's Manual. 2011.
- Hinton CF, Harris KB, Borgfeld L, Drummond-Borg M, Eaton R, Lorey F, et al. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010; 125: 37-47.
- Leger J. Congenital hypothyroidism: a clinical update of long-term outcome in young adults. Eur J Endocrinol. 2015; 172: 67-77.
- Heyerdahl S, Kase BF, Lie SO. Intellectual development in children with congenital hypothyroidism in relation to recommended thyroxine treatment. J Pediatr. 1991; 118: 850-857.
- New England Congenital Hypothyroidism Collaborative. Characteristics of infantile hypothyroidism discovered on neonatal screening. J Pediatr. 1984; 104: 539-544.
- New England Congenital Hypothyroidism Collaborative. Correlation of cognitive test scores and adequacy of treatment in adolescents with congenital hypothyroidism. J Pediatr. 1994; 124: 383-387.
- Ordooei M, Mottaghipisheh H, Fallah R, Rabiee A. Cognitive outcomes for congenital hypothyroid and healthy children: a comparative study. Iran J Child Neurol. 2014; 8: 28-32.
- Leger J, Ecosse E, Roussey M, Lanoe JL, Larroque B. Subtle health impairment and socioeducational attainment in young adult patients with congenital hypothyroidism diagnosed by neonatal screening: a longitudinal population-based cohort study. J Clin Endocrinol Metab. 2011; 96: 1771-1782.
- Leger J, Larroque B, Norton J. Influence of severity of congenital hypothyroidism and adequacy of treatment on school achievement in young adolescents: a population-based cohort study. Acta Paediatr. 2001; 90: 1249-1256.
- Oerbeck B, Sundet K, Kase BF, Heyerdahl S. Congenital hypothyroidism: influence of disease severity and L-thyroxine treatment on intellectual, motor, and school-associated outcomes in young adults. Pediatrics. 2003; 112: 923-930.
- Lichtenberger-Geslin L, Dos Santos S, Hassani Y, Ecosse E, Van Den Abbeele T, Leger J. Factors associated with hearing impairment in patients with congenital hypothyroidism treated since the neonatal period: a national population-based study. J Clin Endocrinol Metab. 2013; 98: 3644-3652.
- Rovet J, Walker W, Bliss B, Buchanan L, Ehrlich R. Long-term sequelae of hearing impairment in congenital hypothyroidism. J Pediatr. 1996; 128: 776-783.
- Grandolfo ME, Sagliocca L, Stazi MA, Medda E, Olivieri A, Sorcini M. The evaluation of the risk factors for congenital hypothyroidism: the outlook of a case-control study. Ann Ist Super Sanita. 1994; 30: 295-298.
- Olivieri A. Epidemiology of congenital hypothyroidism: what can be deduced from the Italian registry of infants with congenital hypothyroidism. J Matern Fetal Neonatal Med. 2012; 25: 7-9.
- Olivieri A, Stazi MA, Mastroiacovo P, Fazzini C, Medda E, Spagnolo A, et al. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for Congenital Hypothyroidism (1991-1998). J Clin Endocrinol Metab. 2002; 87: 557-562.
- Salerno M, Oliviero U, Lettiero T, Guardasole V, Mattiacci DM, Saldamarco L, et al. Long-term cardiovascular effects of levothyroxine therapy in young adults with congenital hypothyroidism. J Clin Endocrinol Metab. 2008; 93: 2486-2491.
- Hassani Y, Larroque B, Dos Santos S, Ecosse E, Bouyer J, Leger J. Fecundity in young adults treated early for congenital hypothyroidism is related to the initial severity of the disease: a longitudinal population-based cohort study. J Clin Endocrinol Metab. 2012; 97: 1897-1904.
- Leger J, Czernichow P. Congenital hypothyroidism: decreased growth velocity in the first weeks of life. Biol Neonate. 1989; 55: 218-223.
- Ng SM, Wong SC, Didi M. Head circumference and linear growth during the first 3 years in treated congenital hypothyroidism in relation to aetiology and initial biochemical severity. Clin Endocrinol (Oxf). 2004; 61: 155-159.
- Salerno M, Micillo M, Di Maio S, Capalbo D, Ferri P, Lettiero T, et al. Longitudinal growth, sexual maturation and final height in patients with congenital hypothyroidism detected by neonatal screening. Eur J Endocrinol. 2001; 145: 377-383.
- American Academy of Pediatrics, Rose SR, Section on Endocrine, Committee on Genetics ATA, Brown RS, Public Health Committee LWPES, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006; 117: 2290-2303.
- Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European society for paediatric endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014; 99: 363-384.
- Kemper AR, Ouyang L, Grosse SD. Discontinuation of thyroid hormone treatment among children in the United States with congenital hypothyroidism: findings from health insurance claims data. BMC Pediatr. 2010; 10: 9.
- Ordooei M, A RA, Soleimanizad R, Mirjalili F. Prevalence of Permanent Congenital Hypothyroidism in Children in Yazd, Central Iran. Iranian journal of public health. 2013; 42: 1016-1020.
- Eugster EA, LeMay D, Zerin JM, Pescovitz OH. Definitive diagnosis in children with congenital hypothyroidism. J Pediatr. 2004; 144: 643-647.
- Matos DM, Ramalho RJ, Carvalho BM, Almeida MA, Passos LF, Vasconcelos TT, et al. Evolution to permanent or transient conditions in children with positive neonatal TSH screening tests in Sergipe, Brazil. Archives of endocrinology and metabolism. 2016; 60: 450-456.
- Korzeniewski SJ, Grigorescu V, Kleyn M, Young WI, Birbeck G, Todem D, et al. Transient hypothyroidism at 3-year follow-up among cases of congenital hypothyroidism detected by newborn screening. J Pediatr. 2013; 162: 177-182.
- Rimsza ME. Pediatric Workforce Shortages: Policy and Advocacy Challenges. 2015.
- Parks JS, Lin M, Grosse SD, Hinton CF, Drummond-Borg M, Borgfeld L, et al. The impact of transient hypothyroidism on the increasing rate of congenital hypothyroidism in the United States. Pediatrics. 2010; 125: 54-63.
- AAFP, AAP, ACP. Guidelines for Patient-Centered Medical Home (PCMH) Recognition and Accreditation Programs. 2011.
- Cooley WC, McAllister JW, Sherrieb K, Kuhlthau K. Improved outcomes associated with medical home implementation in pediatric primary care. Pediatrics. 2009; 124: 358-364.
- Stape J. The patient-centered medical home: how to advance patient care through technology. J Med Pract Manage. 2010; 26: 178-181.
- Strickland BB, Jones JR, Ghandour RM, Kogan MD, Newacheck PW. The medical home: health care access and impact for children and youth in the United States. Pediatrics. 2011; 127: 604-611.
- Hamilton LJ, Lerner CF, Presson AP, Klitzner TS. Effects of a medical home program for children with special health care needs on parental perceptions of care in an ethnically diverse patient population. Matern Child Health J. 2013; 17: 463-469.
- Berry SA, Lloyd-Puryear MA, Watson MS. Long-term follow-up of newborn screening patients. Genetics in medicine: official journal of the American College of Medical Genetics. 2010; 12: 267-268.
- Hinton CF, Feuchtbaum L, Kus CA, Kemper AR, Berry SA, Levy-Fisch J, et al. What questions should newborn screening long-term follow-up be able to answer? A statement of the US Secretary for Health and Human Services' Advisory Committee on Heritable Disorders in Newborns and Children. Genetics in medicine: official journal of the American College of Medical Genetics. 2011; 13: 861-865.
- Hinton CF, Mai CT, Nabukera SK, Botto LD, Feuchtbaum L, Romitti PA, et al. Developing a public health-tracking system for follow-up of newborn screening metabolic conditions: a four-state pilot project structure and initial findings. Genetics in medicine: official journal of the American College of Medical Genetics. 2014; 16: 484-490.
- Lloyd-Puryear MA, Brower A. Long-term follow-up in newborn screening: A systems approach for improving health outcomes. Genet Med. 2010; 12: 256-260.
- VanGeest JB, Johnson TP, Welch VL. Methodologies for improving response rates in surveys of physicians: a systematic review. Eval Health Prof. 2007; 30: 303-321.
- Bjertnaes OA, Garratt A, Botten G. Nonresponse bias and cost-effectiveness in a Norwegian survey of family physicians. Eval Health Prof. 2008; 31: 65-80.
- Kellerman SE, Herold J. Physician response to surveys. A review of the literature. Am J Prev Med. 2001; 20: 61-67.
- McFarlane E, Olmsted MG, Murphy J, Hill CA. Nonresponse bias in a mail survey of physicians. Eval Health Prof. 2007; 30: 170-185.