
Research Article
Annals Thyroid Res. 2019; 5(2): 198-201.
Measured Negative Pressure in Syringes Used for Aspiration Biopsy: Volume and Pressure Relationship
Giordano NJ1, Rosen JE2 and Lee SL3*
1Department of Surgery, Boston Medical Center, USA
2MedStar Washington Hospital Center, USA
3Department of Medicine, Boston Medical Center, USA
*Corresponding author: Lee SL, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center, 720 Harrison Avenue, Doctor’s Office Building 8117, Boston, MA 02118, USA
Received: April 25, 2019; Accepted: May 20, 2019; Published: May 27, 2019
Abstract
Background: Fine-Needle Aspiration Biopsy (FNAB) is the diagnostic test of choice for the evaluation of thyroid nodules. This study evaluates the pressurevolume relationship with 3mL and 10mL syringes used for FNAB in an effort to optimize the mechanics of performing a clinical gold standard procedure.
Methods: A gas pressure sensor was attached to 3 or 10mL syringes via a Luer lock connection. Static pressure measurements were analyzed, and compared against theoretical calculations of pressure and volume.
Results: Pressure measurements demonstrated a reduction in pressure with increasing volumes tested. There was no difference in pressure achieved using a 3 and 10mL syringe at the volumetric change of 1 and 2mL. A change of 3 mL produced 79% of the maximum negative pressure attained by fully withdrawing the plunger of a 10mL syringe. Measured negative pressure highly correlated with the theoretical calculations based on Boyle’s gas law.
Conclusion: To optimize the FNAB technique, clinicians should understand the negative pressure-volume relationship that occurs with aspiration with a syringe during a needle biopsy. The negative pressure obtained for each volume was comparable for 3 and 10mL syringes. A volume change of 3mL attains nearly 80% of the maximum negative pressure obtained compared to a 10mL aspiration and is adequate to perform FNAB. This study documents a physical property of FNAB based on Boyle’s gas law that provides insight, and establishes a standard to establish effective methods to achieve an optimal thyroid FNAB.
Keywords: Aspiration; Negative pressure; Needle biopsy; Fine needle aspiration biopsy; Pressure-volume relationship
Abbreviations
FNAB: Fine-Needle Aspiration Biopsy; US: Ultrasound; mL: Milliliter; atm: atmosphere of pressure
Introduction
Thyroid cancer is the most common endocrine malignancy in the United States. Approximately 53,990 new cases are expected to be diagnosed in 2018 [1]. Thyroid nodules are a common clinical finding especially after the increased use of high resolution imaging such us Ultrasound (US) technology, computed tomography and magnetic resonance imaging but thyroid nodules have a low risk of malignancy of between 5 to 10% [2-7]. Clinical evaluation to diagnose malignancy includes a careful clinical evaluation, TSH evaluation, a thyroid US exam and a Fine-Needle Aspiration Biopsy (FNAB) of nodules [8,9]. Thyroid FNAB is the most accurate test for determining malignancy and is the recommended diagnostic test in the initial evaluation of thyroid nodules [3,8-10] by the American Thyroid Association and the American Association of Clinical Endocrinologists [8,9]. The biopsy technique often uses aspiration to obtain cells or fluid from the thyroid nodule using a needle attached to a syringe [2,3,11-14]. The conceptual basis of a fine needle aspiration biopsy is pulling back on the syringe plunger creates a suction (negative pressure) to aspirate cells from a thyroid nodule into the needle for cytological examination. A nonscientific survey of the 10 endocrinologists at Boston Medical Center who perform thyroid fine needle biopsies showed 1 physician use 0-1 milliliter (mL), 4 physicians use 2-3mL, 3 physicians use 4-6mL and 2 physicians use > 6mL of aspiration during a FNAB. When asked why they used this volume, there was no scientific basis or knowledge of expert guidelines to support their clinical habit. The optimal negative pressure for FNAB is not known. Excess negative pressure during a biopsy increases the probability of an blood-contaminated specimen interfering with the cytological analysis that can result in a biopsy that contains inadequate numbers of thyroid follicular cells for analysis (Bethesda I classification) [15] and cause complications of local pain and hematoma [16,17]. Clinicians use many different techniques to perform fine needle biopsy but there have been no studies to date that determine the aspiration volume and the generated vacuum necessary to optimal negative pressure or syringe size to use during FNAB of thyroid nodules. Understanding the pressure-volume relationship when generating negative pressure in the needle and syringe during a FNAB will allow clinicians to optimize their technique for needle biopsies. This study performed pressure measurements generated using 3mL and 10mL syringes at different volumes of aspiration and compared the measured negative pressures with those predicted by Boyles’ law of gases. Boyle’s law is an experimental gas law that describes how the pressure of gas decreases as the volume of the container increases if the temperature and amount of gas remains unchanged within a closed system.
Materials and Methods
Theoretical calculations
Boyle’s law was used to calculate the theoretical difference in pressures generated from incremental 1mL changes in volume in 3mL and 10mL Luer lock syringes. The theoretical calculations assumed an initial pressure of 1 atmosphere of pressure (atm; 101.3 kPa) in Boston, MA (5.8 m above sea level) at a constant room temperature (23oC). In our calculations, the syringe plunger seal started at a resting position of 1mL. The new Pressure (P2) with pulling out the syringe plunger can be calculated knowing the initial pressure (P1 = 101.3 kPa) after a change in volume from V1 to V2. Mathematically this can be described as P2=P1xV1/V2. The equation shows that, as volume in the syringe increases there is a proportional decrease in the pressure of the in the closed system of the syringe at a constant temperature.
Gas pressure sensor system
A gas pressure system (Vierner Software & Technology, Beaverton, OR) was used to measure the changes in gas pressure with a pressure transducer (Honeywell, Morristown, NJ) designed to measure absolute pressure. A membrane inside of the transducer flexes as pressure changes causing a relative alteration in output voltage that is measured by the gas pressure system. The device takes 100 microseconds to generate a response time, and is accurate within 0.25% of the full scale span best fit straight line. The pressure sensor was fitted directly to the syringe with a Luer lock to minimize dead space and insure a proper seal (Figure 1). 10ml syringe and 3ml syringes, the most common size syringes used for the application of a FNAB, were used in these experiments. The syringes were individually sealed in sterile packaging ((Becton Dickinson, Franklin Lakes, NJ). Three different syringes of each size were used to insure reproducibility of the data. Pressures were measured three times at each volume selected. The device was re-calibrated to zero every time the syringe was connected to the sensor. All measurements for both the 10mL and the 3mL syringes were taken within a half hour in an effort to maintain the same barometric pressure and temperature throughout each cycle of testing. Initially the plunger was set in the syringe at 1mL, attached to the gas pressure sensor device by the Luer lock and then the volume in the syringe was increased in 1mL increments by manually pulling back on the plunger. At each volume, static pressure measurement was obtained at least 3-4 seconds after aligning the plunger seal at the desire volume to ensure stabilization of the pressure reading. Between each measurement, the plunger was released, and observed to spontaneously return to the initial plunger position at 1mL to ensure a proper seal between the sensor and the syringe was maintained.
Figure 1: Diagram of gas pressure sensor attached to Luer lock syringe
during pressure measurements.
Results
Initially the plunger was set in the syringe at 1mL. The syringe was attached by a Luer lock to the gas pressure sensor device (Figure 1). The volume in the syringe was increased in 1 mL increments by manually pulling back on the plunger and the measurement of the pressure within the syringe was measured in triplicate. The process was repeated with three 3mL and three 10mL syringes. In agreement with Boyle’s law, the measured pressue in kPa (average of 9 measurements ± standard deviation) decreased in a curvilinear inverse relatiopnship with increasing the volume of the syringe in a closed system (Figure 2). The results from the empirical measurements for both the 3mL and 10mL syringes closely overlapped and are not statistically different at 1 (P=0.14) and 2mL (P=0.08) of displaced volume (Figure 2). The theorectical calculation of pressure was found to be significantly larger than observed for both the 3mL and 10mL syringes at each volume tested ( P=0.0003). This variation was found to be more pronounced at the intitial volume changes, and was minimized at the larger volume changes (Figure 2). We hypothesized that the difference was due to the small constant contribution from the dead space within the syringe. The dead space is the volume in the syringe between the plunger and the Luer tip after the plunger is depressed completely into the syringe. The measured volume of the “dead space” was obtained from the literature to be 0.202 ± 0.019mL in a 10mL syringe [18]. After the application of this correction factor, there was no signiciant difference between the theorectical and average observed pressure of the 10 mL syringe at all volumes (Figure 3). When the syringe plunger is withdrawn by 3mL, approximately 79.0% of the maxium negative pressure is achieved while additional suction from 4mL, 5mL or 8mL shows a relatively small increase to 86%, 90% and 98% of the maximum negative pressure achieved at 9mL, respectively (Table 1).
Change in Volume (mL)
Measured % Maximum Pressure*
Theoretical % Maximum
Pressure*,¥
0
0
0
1
48.65%
55.56%
2
68.16%
74.07%
3
78.80%
83.33%
4
85.64%
88.89%
5
90.36%
92.59%
6
93.67%
95.24%
7
96.34%
97.22%
8
98.37%
98.77%
9
100.00%
100.00%
*Results shown in the percentage of the maximum negative pressure generated when the plunger is moved to 9mL or theoretically calculated using Boyle’s gas law at 9mL.¥ Corrected for dead space, 0.202mL.
Table 1: Measured and theoretical % maximal negative pressure at different aspiration volumes in the 10mL syringe.
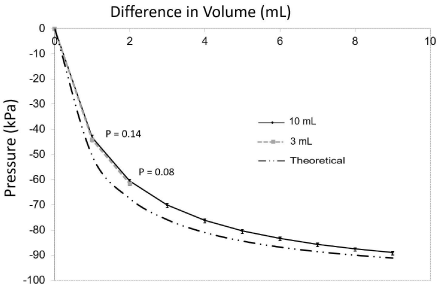
Figure 2: Results from the pressure measurements (average ± standard
deviation) with the 10mL, and 3mL syringes compared to the theoretical
measurements calculated with Boyle’s law.
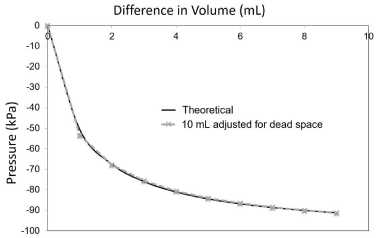
Figure 3: Results from the 10mL pressure measurements adjusted for dead
space of the syringe attachment to the sensor (0.202mL). The results are
compared against the theoretical values calculated from Boyle’s law.
Discussion
The conceptual basis of a fine needle aspiration biopsy is pulling back on the plunger of a syringe attached to a needle creates a suction (negative pressure) to aspirate cells from a thyroid nodule into the needle for cytological examination. Aspirated volumes that are too small may not allow optimal retrieval of cells while aspirated volumes that are too large are difficult to accomplish with a single hand without a use of an aspiration device and may cause excessive blood in the sample making interpretation difficult or impossible or increased tissue injury at the site of the biopsy. This purpose of this study is to determine scientifically negative pressure obtained in a syringe during aspiration to optimize the FNAB procedure.
As predicted by Boyle’s law P2=P1xV1/V2, the measured pressue decreases in a hyperbolic shape of an inverse relationship with the increasing the volume of the syringe in a closed system. The discrepancy between the theoretical and empirical measurements decreased with an increase in the change of volume (Figure 1). This suggests there is a constant difference between the theoretical and empirical data. We theorized it was due to dead space between the sensor and the syringe body within the area of the Luer lock. The dead space is defined is the volume left in a syringe after the plunger has been push down into the syringe completely. Dead space has been measured by Kûme [18] in a 1mL to 10mL disposable syringes and was found to vary between 0.075 to 0.202mL. We selected the dead space in a 10mL syringe to be 0.202mL. When this small constant volume was included in the calculations, there was a strong agreement between the empirical measurements and ”corrected” theoretical calculations confirming Boyle’s law is applicable in this clinical system (Figure 2). These data show that approximately 80% of the maximum negative pressure can be generated from a volume change of 3mL (Table 1). The size of the syringe is found to have no impact on the pressure generated from a syringe. A 3mL syringe, with a narrower syringe body will generate the same pressures as a wider, 10mL syringe because the only dependent variable for pressure change is the change in volume. Therefore, 3mL and 10mL syringes are suitable for a FNAB although maximum negative pressure at 2mL of aspiration obtains only ~70% of the maximum vacuum obtained with a 10mL syringe.
The results of a FNAB have been found to be operator dependent [19] and in an effort to optimize the FNAB technique, clinicians should understand the negative pressure-volume relationship that occurs with aspiration. This data suggests that during aspiration, a change of 3mL will yield approximately 80% of the maximum suction capable of being generated from a 10mL syringe. Because of the curvilinear hyperbolic inverse volume-pressure relationship, increasing the volume of aspiration above 3mL becomes increasingly inefficient at changing the negative pressure and more difficult to perform with a single hand biopsy technique.
There are limitations on the study since these experiments were only conducted at approximately sea level, and the results of this study can’t be applied to other altitudes. Further the pressure changes are not measured during a FNB within the biopsy needle. The volume of a needle is small compared to the dead space (0.075 – 0.202ml) measured by Kume [18] in 1mL to 10mL disposable syringes and should not significantly alter the negative-pressure curve reported here. Specific implications cannot be drawn to every circumstance in which a FNAB is performed; however this study documents a physical property of FNAB equipment based on Boyle’s gas law that provides insight, and establishes a standard to establish effective methods to achieve an optimal thyroid FNAB.
Future directions of this study might determine what the optimal pressure for collecting cells during a thyroid biopsy. Although this study, informs us of the negative pressures obtained during aspiration, it does not answer the larger question whether no negative pressure fine needle biopsy techniques such as capillary or Fine Needle Non-Aspiration Cytology (FNNAC) is a superior method of performing a thyroid nodule fine needle biopsy. Since FNNAC of the thyroid was first reported by Santos [20], there has been controversy over which method is superior. A meta-analysis examining 16 studies composed of 1,842 patients and 2,221 cytologies showed no difference with respect to inadequate results, superior smears, diagnostic performance (accuracy, sensitivity, specificity, negative predictive value, and positive predictive value), and average score of other parameters (background blood or clot, amount of cellular material, degree of cellular degeneration, degree of cellular trauma, and retention of appropriate architecture) [21]. There does not appearance a clear advantage when FNAB is compared to FNNAC [15]. Many clinicians continue to use FNAB, and this study provides knowledge of negative pressures generated at various volumes that clinicians need to discover their optimal condition to collect tissue for cytological evaluation of thyroid nodules.
Acknowledgement
We would like to thank Drs. Alexander Golger, John E. Straub and Valentin Voroshilov for their guidance and support with this study.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin. 2018; 68: 7-30.
- Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, et al. The National Cancer Institute thyroid fine needle aspiration state of the science conference: a summation. Cytojournal. 2008; 5: 6.
- Dean DS, Gharib H. Fine-needle aspiration biopsy of the thyroid gland. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext. South Dartmouth (MA). 2000.
- Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993; 118: 282-289.
- Gharib H, Goellner JR, Johnson DA. Fine-needle aspiration cytology of the thyroid: a 12-year experience with 11,000 biopsies. Clinics in Laboratory Medicine. 1993; 13: 699-709.
- Burman KD, Wartofsky L. Thyroid nodules. N Engl J Med. 2016; 374: 1294- 1295.
- Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004; 351: 1764-1771.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26: 1-133.
- Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical guidelines for clinical practice for thediagnosis and management of thyroid nodules--2016 update. Endocr Pract. 2016; 22: 622-639.
- Oertel YC. Fine-needle aspiration and the diagnosis of thyroid cancer. Endocrinol Metab Clin North Am. 1996; 25: 69-91.
- Tublin ME, Martin JA, Rollin LJ, Pealer K, Kurs-Lasky M, Ohori NP. Ultrasoundguided fine-needle aspiration versus fine-needle capillary sampling biopsy of thyroid nodules: does technique matter? Journal of Ultrasound in Medicine: official journal of the American Institute of Ultrasound in Medicine. 2007; 26: 1697-1701.
- de Carvalho GA, Paz-Filho G, Cavalcanti TC, Graf H. Adequacy and diagnostic accuracy of aspiration vs. capillary fine needle thyroid biopsies. Endocr Pathol. 2009; 20: 204-208.
- Layfield LJ, Cibas ES, Baloch Z. Thyroid fine needle aspiration cytology: a review of the National Cancer Institute state of the science symposium. Cytopathology. 2010; 21: 75-85.
- Layfield LJ, Cibas ES, Gharib H, Mandel SJ. Thyroid aspiration cytology: current status. CA Cancer J Clin. 2009; 59: 99-110.
- Pothier DD, Narula AA. Should we apply suction during fine needle cytology of thyroid lesions? A systematic review and meta-analysis. Ann R Coll Surg Engl. 2006; 88: 643-645.
- Polyzos SA, Anastasilakis AD. Clinical complications following thyroid fineneedle biopsy: a systematic review. Clin Endocrinol (Oxf). 2009; 71: 157-165.
- Polyzos SA, Anastasilakis AD. Systematic review of cases reporting blood extravasation-related complications after thyroid fine-needle biopsy. Journal of Otolaryngology -Head & Neck Surgery. 2010; 39: 532-541.
- Küme T, Sisman AR, Solak A, Tuglu B, Çinkooglu B, Coker C. The effects of different syringe volume, needle size and sample volume on blood gas analysis in syringes washed with heparin. Biochem Med (Zagreb). 2012; 22: 189-201.
- De Fiori E, Rampinelli C, Turco F, Bonello L, Bellomi M. Role of operator experience in ultrasound-guided fine-needle aspiration biopsy of the thyroid. La Radiologia Medica. 2010; 115: 612-618.
- Santos JE, Leiman G. Nonaspiration fine needle cytology. Application of a new technique to nodular thyroid disease. Acta Cytol. 1988; 32: 353-356.
- Song H, Wei C, Li D, Hua K, Song J, Maskey N, et al. Comparison of fine needle aspiration and fine needle nonaspiration cytology of Thyroid Nodules: A meta-Analysis. Biomed Res Int. 2015; 796120.