
Research Article
Ann Transl Med Epidemiol. 2014;1(1): 1004.
Statins and Finasteride Use Differentially Modify the Impact of Metformin on Prostate Cancer Incidence in Men with Type 2 Diabetes
Wang Chen-Pin1*, Hernandez Javier2, Carlos Lorenzo3, John R Downs3, Ian M Thompson2, Bradley Pollock1 and Donna Lehman3
1Department of Epidemiology and Biostatistics, University of Texas Health Science Center San Antonio, USA
2Department of Urology, University of Texas Health Science Center San Antonio, USA
3Department of Medicine; University of Texas Health Science Center San Antonio, USA
*Corresponding author: Wang, Chen-Pin, Department of Epidemiology and Biostatistics, University of Texas Health Science Center San Antonio, USA
Received: October 06, 2014; Accepted: November 11, 2014; Published: November 12, 2014
Abstract
Background: Metformin has shown promise for cancer prevention. Prior studies suggested that metformin might interact potential prostate cancer (PCa) prevention agents: finasteride and statins. This study assessed if concurrent use of statins or finasteride modified the long-term impact of metformin on PCa risk in men with type 2 diabetes (T2DM).
Materials and Methods: The study cohort consisted of 71,999 men with T2DM seen in the Veteran Administration Health Care System, without prior cancer or liver diseases, nor prescription of thiazolidinediones or insulin between FY2003-FY2013. Cox proportional hazard analyses (adjusting for covariates and propensity scores of metformin use) were conducted to compare the hazard ratio (HR) of PCa associated with metformin use between statins or finasteride users and none users.
Results: Mean follow-up was 6.4±2.8 years; 5.2% (N= 3,756) of the cohort subsequently received a PCa diagnosis. Both statins and finasteride significantly modified the impact of metformin on PCa incidence (p–value<0.001): HR’s of PCa associated with metformin use were 0.89 (p–value=0.02) among non-statin /non-finasteride users, 0.73 (p–value<0.001) among statin users, and 1.42 (p–value<0.001) among finasteride users.
Conclusion: Metformin was associated with reduced PCa risk in men with T2DM. This impact was enhanced by statins but reversed by finasteride. Metformin, statins, and finasteride are potential PCa prevention agents. The interaction of these drugs on PCa risk needs further confirmation in other cohorts. Our finding of differential impacts of metformin, statins, and finasteride (alone or in combination) on PCa risk is informative for treatment management in men at risk for PCa and T2DM.
Keywords: Prostate cancer; Metformin; Statins; Finasteride; Type 2 diabetes
Introduction
Prostate Cancer (PCa) prevention is an attractive strategy in men with type 2 diabetes (T2DM) as this population appears to be at a higher risk for high-grade, potentially-lethal PCa compared to men without diabetes [1,2]. Metformin, a biguanide drug, is commonly prescribed as first-line glucose-lowering therapy for patients with T2DM and endorsed by a recent consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes [3]. Metformin has also showed promise for cancer prevention [4-10]. The potential mechanisms of this antineoplastic effect include a reduction of hyperinsulinemia through activation of the AMP-Activated Protein Kinase (AMPK) pathway, inhibition of the mammalian target of rapamycin pathway, blockade of cell cycle progression, and alteration of anti-inflammatory properties [4,11-16]. The effect of metformin on PCa risk is unclear. While two observational human studies found metformin use to be associated with a decreased PCa risk in the general population [17,18], two studies among diabetic subjects did not find a significant reduction in PCa risk with metformin use [19,20]; a dose-dependent increase was observed in a more recent study [21]. The discrepancy of these findings could be due to the difference in study design, some of which could be subject to time period bias [22].
Our group previously examined 5,042 veterans with T2DM treated with either sulfonylureas or metformin as their sole glucose-lowering agent [21]. In that study that carefully avoided confounding and the time period bias by design [22], we found that the incidence of PCa among concurrent metformin and statin users was significantly lower as compared to the incidence among those with sulfonylureas use but not statins. Paradoxically, the incidence of PCa was significantly higher among metformin users without concurrent statin use [21]. Statins are 3-Hydroxy-3-Methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitors, which are recommended for patients with T2DM primarily for preventing cardiovascular diseases. Statins may also affect PCa tumorigenesis by blocking the mevalonate pathway and reducing cholesterol and/or through multiple pleiotropic effects [23]. The favorable joint effect of metformin and statins on PCa may be due to their joint effects via lipid-lowering or a combination of pleiotropic effects (e.g., via improving inflammatory conditions) [21,24,25]. The interactive effect of metformin and statins on PCa found in our previous analysis is yet to be confirmed in a broader population with T2DM, especially including a comparison between a broader range of metformin users (e.g., not limited to sole metformin users) and non-metformin users (e.g., not limited to sulfonylurea users).
Finasteride, a 5α-reductase inhibitor used for treating benign prostatic hypertrophy (BPH), was initially found to reduce PCa risk by 24.8% in the Prostate Cancer Prevention Trial (PCPT); and longterm follow-up found the risk reduction to be 30% [26,27]. Recently, studies have shown that finasteride attenuated the insulin-like growth factor binding protein (IGFBP2) or C-peptide induced PCa risk in the PCPT [28,29], which suggests that the beneficial effect of finasteride on reduced PCa could be attenuated among individuals who are less hyperinsulinemic, such as those treated with an insulin sensitizer like metformin. To date, no studies have examined whether finasteride modulates the impact of metformin on PCa.
With these questions in mind, we examined an 11-year longitudinal historical population of 71,999 men with T2DM who were insulin naïve in the Veteran Administration Health Care System (VAHCS) to determine if the impact of metformin on PCa risk was modified by concurrent use of statins or finasteride.
Materials and Methods
Study cohort
We drew our study sample from the 268,136 VAHCS enrollees who were ≥40 years of age in 2003, and had at least one primary care visit (defined as any visit to the general medicine, geriatric, or diabetes clinics) as well as a diagnosis of T2DM (diagnosis using ICD-9 CM codes of 250.00 or 250.02) each year during FY2001-FY2002. Our criteria of identifying patients with T2DM could miss at most 3% T2DM who only had diagnoses of diabetes complications [30]. We further narrowed the study cohort to 71,999 men who also met the following criteria: (i) no prescription of any glucoselowering medication in FY2002; (ii) having had prescription(s) of metformin as a glucose-lowering medication for ≥180 days or none during the study period, (iii) having had prescription(s) of statin as a lipid-lowering medication for ≥180 days or none during the study period, (iv) having had prescription(s) of finasteride for ≥180 days or none during the study period, (v) no prescription for insulin or any thiazolidinedione (TZD) during the study period; (vi) no liver or renal diseases during the study period; (vii) no cancer diagnosis nor metformin exposure prior to FY2003; and (viii) no missing data on any baseline covariates (age, race, hemoglobin A1c (HbA1c), body mass index (BMI), and age-adjusted Charlson co-morbidity score [31]). Regarding criterion (v), we excluded patients who were on a TZD and/or insulin to better assess the association between metformin and PCa risk to eliminate potential confounding effects of other glucose-lowering medications which could themselves have an effect on PCa incidence or progression as reported in the literature [32,33]. All study procedures were approved by the Institutional Review Board of the UT Health Science Center San Antonioand Research and Development of the South Texas Audie L. Murphy Veterans Hospital.
Data sources
We used four VAHCS datasets for this study. VAHCS Inpatient and Outpatient Medical SAS Datasets were used to identify the cohort of men with T2DM and their associated characteristics, including demographic variables and comorbidities (based on diagnosis codes). Additional clinical variables were extracted from the VA Decision Support System (medication prescription records, HbA1c, LDL, and prostate specific antigen (PSA) lab results and dates of measurements) and VAHCS Corporate Data Warehouse (height and weight values for deriving BMI) [34].
Outcomes of interest
The outcome of interest in this study is the incidence (rate) of the initial PCa diagnosis during the study period. The dependent variable used in our analyses is the time interval between the starting date (October 1 2002, the starting date of FY2003) for those without use of any oral glucose-lowering medication, the initiation of nonmetformin glucose-lowering medication for those non-metformin glucose-lowering medication users, and the initiation of metformin for metformin users) to the initial PCa diagnosis observed during the study period. A PCa event was defined as having an ICD-9 diagnosis of 185 from either primary or secondary diagnosis code. Those who died prior to the first PCa event observed during the study period, or those who remained alive but never had a PCa event during the study period were treated as censored data. The study termination date for each patient corresponded to either the date of the initial PCa diagnosis, the date of death, or September 30, 2012 (the end of followup), whichever came first.
Predictors and measures
Medication exposure: For this study, the metformin group consisted of patients who had metformin prescription of any dose for 180 days or more but never had any TZD or insulin during the study period. We regarded sufficient metformin exposure as a minimum of 180 days since most clinical trials on metformin were 24 weeks or longer and other studies have used a similar exposure cut-point [20]. Non-metformin users were patients who had no prescription for metformin, and never had any TZD or insulin during the study period. Statin users consisted of patients who had any type of statin prescription at any dose for 180 days or more during the study period. Non-statin users consisted of patients who never had any type of statin prescription during the study period. Finasteride users were patients who had any finasteride prescription for 180 days or more during the study period.
Covariates: Covariates adjusted for in the analyses included demographic and clinical characteristics of the patient. Demographic characteristics included age, and race/ethnicity (Caucasian, African- American, Hispanic, others). Clinical characteristics included ageadjusted Charlson co-morbidity score, and the mean change of low density lipoprotein (LDL) and HbA1c levels from baseline, and the maximum PSA during the study period.
Statistical analyses
The Cox proportional hazard model adjusting for covariates and the propensity scores of metformin use was conducted to assess the hazard ratio of PCa associated with metformin use, where the propensity scores of metformin use were incorporated as the inverse probability weights in the analyses. Each of these propensity scores was the likelihood of being treated with metformin conditioned on each subject’s pretreatment characteristics. Using these scores, individuals were weighted differently to achieve balance in covariates at baseline between the metformin users and non-metformin users, and therefore potential confounding due to pretreatment characteristics was minimized [35]. The propensity scores were calculated using logistic regression analysis, where the dependent variable was the indicator of receiving metformin treatment and the independent variables were patients’ pretreatment characteristics. We included all significant predictors (i.e., the pretreatment/baseline covariates) in the propensity score models to ensure a credible estimation of the main outcome (PCa incidence) model [35]. The propensity score model of metformin use included HbA1c, age, and race/ethnicity, and Charlson comorbidity score.
To assess the interactive effect between metformin and statin and that between metformin and finasteride, we used the model with metformin by statin interaction and metformin by finasteride interaction. Each of these interaction terms corresponded to the product of the associated indicators of medication use, and its coefficient was assessed by the Wald test with p–value <0.05 being considered significant. In the Cox regression model setup, the interactive effect between metformin and statins compared the hazard ratio associated with metformin use between statin users and nonstatin users, and this effect was assessed using the contrast statement. Similarly, the interactive effect between metformin and finasteride compared the hazard ratio associated with metformin use between finasteride users and non-finasteride users. All statistical analyses were conducted using SAS 9.1.
Results
In our final cohort of 71,999 men with T2DM, 28,308 (39.32%) had prescription(s) for metformin. Among 28,308 men who had prescription(s) for metformin, 24,384 (86.14%) were statin users and 3,744 (13.23%) were finasteride users. Among 43,691 men (60.68%) who did not receive prescription(s) for metformin, 31,900 (73.01%) were statin users and 6,107 (13.98%) were finasteride users. Study subject characteristics are shown in Table 1. Mean age at cohort entry was 66.91±10.22 years, and the mean study period was 6.41±2.76 years. The mean HbA1c at baseline was 6.51±0.96%. A total of 3,756 patients (5.2%) were diagnosed with PCa during the study period.
All
Mean
Users
Std
Non-Metformin
Mean
Users
Std
Metformin
Mean
Users
Std
D
p-value
N
71,999
43,691
28,308
Statin Use (%)
78.17
73.01
86.14
<0.001
Finasteride Use (%)
13.68
13.98
13.23
0.02
Study Length (days)
2339.86
1009.37
2468.21
1080.78
2141.78
850.79
<0.001
PCa (%)
5.22
6.32
3.52
<0.001
Age
66.91
10.22
69.28
9.98
63.27
9.48
<0.001
Black (%)
17.74
17.81
17.62
0.257
Charlson
3.91
2.71
4.21
2.86
3.44
2.39
<0.001
Baseline A1c
6.51
0.96
6.25
0.89
6.92
0.93
<0.001
Mean A1c
6.57
0.90
6.31
0.80
6.98
0.89
<0.001
DA1C
0.06
0.83
0.06
0.78
0.06
0.90
0.5
Baseline LDL
106.71
27.09
107.30
27.77
105.81
25.98
0.002
Mean LDL
89.22
25.00
91.15
24.89
86.26
24.87
<0.001
DLDL
-17.49
24.77
-16.15
24.97
-19.56
24.32
0.001
Baseline BMI
30.98
4.98
30.57
4.85
31.62
5.02
<0.001
DBMI
-1.02
3.15
-1.00
3.72
-1.06
1.98
0.002
Maximum PSA
2.64
5.20
2.99
5.06
2.10
5.28
<0.001
Table 1: Cohort Description in Men with Type 2 Diabetes.
As seen in Table 1, we observed imbalance in subjects’ characteristics at baseline between metformin users and non-users, and, some of these variables are associated with PCa outcome. Age, BMI, HbA1c, Charlson comorbidity score, and race/ethnicity were significantly different between the metformin and non-metformin groups based on chi-square tests. Therefore, in order to assess the impact of metformin use on PCa incidence as if they were balanced in terms of pretreatment characteristics, subjects were weighted by the reciprocal of their propensity scores for metformin use.
Using Cox proportional hazard model adjusted for the propensity scores as well as covariates associated with PCa outcome, we observed significantly different hazard ratio (HR) by medication use as shown in Table 2. Statin use significantly modified the impact of metformin use on PCa risk (p<0.001). Among non-statin users, the HR for PCa associated with metformin use was 0.89 (95% CI = (0.80-0.98), p<0.001), In contrast, among statin users, the HR for PCa associated with metformin use was 0.73 (95% CI = (0.62-0.85), p<0.001). Since the 3-way interaction between metformin, statin and finasteride was not significant, the heterogeneity of the metformin effect on PCa incidence due to statin use did not vary by finasteride use.
HR
p-value
Metformin Use
0.89
0.02
Statin use
0.80
<.001
Finasteride Use
0.44
<.001
Metformin*Statin
0.82
.001
Statin*Finasteride
1.28
0.02
Metformin*Finasteride
1.60
<.001
Age
1.03
<.001
Black
1.82
<.001
Maximum PSA
1.003
<.001
Charlson Comorbidity
1.03
<.001
DA1c
1.16
<.001
DLDL
1.002
<.001
DBMI
1.01
<.001
Results adjusted for propensity score of metformin use.
Table 2: HR of PCa incidence by Metformin, Statin, and Finasteride Use Groups.
Finasteride use during the study period significantly modified the impact of metformin on PCa outcome (p<0.001). In the absence of finasteride use, the covariate adjusted HR for metformin use vs. no use was 0.89 (95% CI = (0.80-0.98), p<0.001), while in the presence of finasteride use, the HR for PCa associated with metformin use was 1.42 (95% CI = (1.18-1.70), p-value<0.001).
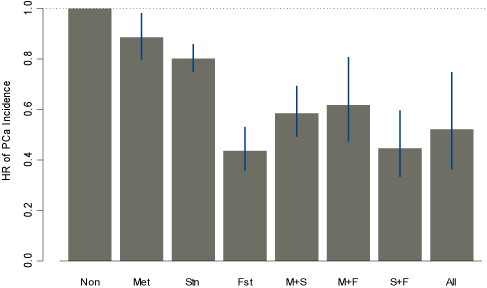
Figure 1: HR of PCa incidence by Metformin, Statin, and Finasteride Use Groups.
Figure 1 summarizes the comparison of PCa risks under different combination use of metformin, statin, and finasteride with those who did not use any of these medications. HR’s for PCa associated with metformin use alone, statin use alone, and finasteride use alone were 0.89 (95% CI = (0.80-0.99), p–value<0.001), 0.80 (95% CI=(0.75-0.86), p–value<0.001), and 0.44 (95% CI=(0.36- 0.53), p–value<0.001), respectively. HR’s for PCa associated with metformin+statin use, metformin+finasteride use, statin+finasteride use, and metformin+statin+finasteride were 0.59 (95% CI=(0.49, 0.69), p–value<0.001), 0.62 (95% CI=(0.47, 0.81), p–value=0.001), 0.45 (95% CI=(0.33, 0.60), p–value<0.001), and 0.52 (95% CI=(0.36,0.75), p–value=0.001), respectively.
As expected, older age (HR=1.03 for 1 year increase in age, 95% CI =(1.028,1.034), p<0.001), black race (HR=1.82, 95% CI =(1.72,1.92), p<0.001), and higher PSA (HR=1.0026 for 1 ng/ml increase in PSA, 95% CI= (1.0024-1.0027), p<0.001) were significantly associated with increased risk of PCa. Greater elevation of HbA1c, LDL, or BMI during the study period was associated with increased risk of PCa (HR= 1.15 for 1% increase in HbA1c, 95% CI=(1.14-1.17), p<0.001; HR= 1.0016 for 1 mg/L increase in LDL, 95% CI= (1.0007-1.0024), p<0.001; HR= 1.01 for 1 kg/m2 increase in BMI, 95% CI =(1.007,1.012), p<0.001).
Discussion
In this 11-year historical study of veterans with T2DM who were insulin naive, we found that compared to other non-TZD oral antihyperglycemic medication or no use of antihyperglycemic medication, the effect of metformin on reduced PCa incidence was significantly enhanced by concurrent use of statins, and the combination use was associated with a greater reduction in PCa than statin alone. This result confirmed the finding of our prior study, which was conducted in men with T2DM who used metformin or sulfonylureas as the sole antihyperglycemia medication [21]. We note that both these two studies obtained cohorts from the veteran populations, and used the same definition of metformin/statin exposure. Also, both studies assessed the impact of metformin on PCa incidence as if they were balanced in terms of baseline covariates (pretreatment characteristics) by weighting subjects by their propensity scores for metformin use group membership. In the present study, metformin use (compared with no metformin use) was associated with decreased PCa risk, while our previous report showed that metformin use alone compared to sulfonylurea use alone was associated with an increased PCa risk [21]. The discrepancy of the metformin effect between our previous study [21] and this study could be due to their differences in the composition of the metformin and non-metformin groups. In the present study, the metformin group consisted of those who used metformin as the sole antihyperglycemia medication only (28.2%), those who used both metformin and sulfonylurea (71.5%), and those who used metformin and other non-sulfonylurea antihyperglycemia medication (0.3 %); while in the non-metformin group, 51.6% used sulfonylurea as the sole antihyperglycemia medication, 48.1% did not use any oral antihyperglycemia medication, and 0.3 % used non-sulfonylurea antihyperglycemia medication with or without sulfonylurea.
Our investigation of the interaction between metformin and finasteride (as shown in Figure 1) suggested that finasteride alone without metformin or statin (HR=0.44, 95% CI=(0.36-0.53)) could be a potential PCa prevention drug for men with T2DM who remained insulin naive, and this PCa prevention benefit was greater compared to that found in PCPT which comprised a cohort with 6.6% of diagnosed diabetic subjects [36]. Although in this study the beneficial impact of finasteride on decreased PCa risk was attenuated in diabetic patients with metformin use (HR=0.70 with 95% CI=(0.55-0.89)), the magnitude of the finasteride impact was similar to that found in PCPT. Our finding that metformin countered the finasteride effect on reduced PCa seems to be consistent with the interaction between finasteride and IGFBP2 reported in the PCPT study, where serum IGFBP2 (a modulator of insulin-like growth factors) and C-peptide (a measure of insulinemia) levels were positively associated with increased PCa risk in non-finasteride users, but not in finasteride users [28,29]. This interaction indicates that finasteride might interfere with some of the effects common to metformin use, such as those on IGFBP2 and C—peptide. Future studies are needed to confirm whether this is directly related to insulinemia or insulin resistance.
We have also examined the interaction between statins and finasteride on PCa incidence. As shown in Table 2, HR associated with statin use in non-finasteride users was 0.80, while this effect was significantly diminished in finasteride users (HR=1.02, p=0.42). These results suggest that while both statins and finasteride could be potential PCa chemo-preventive agents, simultaneous use of both could be less effective than each used alone. This is potentially related to the involvement of Cytochrome P450 Isoenzyme 3A4 (CYP3A4) in the oxidative metabolism of statins (predominantly CYP3A4 and CYP2C9) and in the oxidation of finasteride [37].
Our study found that greater increase in HbA1c, LDL, or BMI was associated with increased risk of PCa. These results are consistent with the literature which reported that insulin resistance, obesity, and high LDL are risks factors for PCa [26,28,29]. Metformin has been shown to improve insulin resistance and lower LDL and BMI, and hence could potentially prevent PCa. These effects could be consequences of the activation of the AMPK pathway and alteration of anti-inflammatory properties [4,11-16]. However, the impact of metformin on these metabolic biomarkers found in our long-term observational clinical cohort did not reach clinical significance (see Table 1). Thus the potential anti-PCa effect of metformin might be attributed to other actions of mechanism, such as its inhibition of cell senescence [38] or its influence on the TWIST/N-cadheri/NFkB signaling pathway [39]. These actions are yet to be confirmed in clinical studies.
Our study has limitations. Confounding by indication could occur if patients with certain characteristics that are associated with the risk of PCa were also related to the use metformin, statins, or finasteride (see Table 1 for the baseline differences between the metformin and non-metformin groups). Nevertheless, we have managed to minimize the bias due to confounding by indication using the propensity score weighting technique in our analyses. We have also employed strategies to avoid time-related biases [22] by using a cohort study design, carefully defining the medication exposure groups, and adjusting for confounding associated with diabetes duration using data available (i.e., age, baseline HbA1c, and comorbidities). In particular, note that in the analyses (not shown) that included those who used glucose-lowering medications prior to FY2003 (i.e., a study design that is likely to subject to time-related bias [22] since the starting dates for those with prior glucose-lowering medication use fell outside the study period), the impact of metformin on PCa was found to be neutral, while the rest of the results remained similar to those shown in Table 2. The contrast of this result and Table 2 further confirms the time-related bias associated with the metformin impact on PCa observed in [22].
Although we could not exclude residual confounding, such as diabetes duration, life style, behaviors, or an increased likelihood of PCa referral/detection/screening due to elevated PSA, the impact of confounding could require a substantial imbalance among exposure groups. In fact, the guidelines for PCa screening by American Urology Association [40] and National Comprehensive Cancer Network [41] are mainly based on patient’s age, race, family history of PCa, prior PSA levels and results from the digital rectal examination. Among these factors, age probably is the only factor that could be associated with metformin use or glucose control in patients with diabetes. Although in general men of older age are more likely to be recommended for PCa screening, and the non metformin group in this study was older than the metformin group, the potential PCa screening bias due to age difference between the metformin and nonmetformin groups is likely to be limited due to the propensity score adjustment used in the analyses. Moreover, our preliminary analyses of PSA data showed that metformin, statins, or the combination of both were significantly associated with a similar increase (1.03 ng/ ml) in PSA; finasteride was significantly associated with 0.87 ng/ml reduction in PSA; and the combination of finasteride and metformin was not associated with the change in PSA. These analyses further confirmed that the medication impacts on PCa found in this study could have been subject to very limited bias associated with PCa referral/detection/screening. Second, the impact of metformin on PCa risks could vary by the metformin dosage [21,38]. However, we were bound to assess this variation given the data available in this study. Third, our results were derived from patients who had no missing covariates, and whether the results reported here can be generalizable to patients with missing covariates remain to be seen. To address this concern, we compared the patients’ characteristics between those without and with any missing covariates. Table 3 suggests that compared to those without missing covariates (or included in the analyses), those with missing covariates (or excluded from the analyses) were relatively old, had less comorbidity, and had shorter follow-up period. We then used the generalizability technique [42], and conducted further Cox regression analyses to assess the medication effects among those with missing covariates based on the likelihood with the covariate distribution for those without missing covariates being weighted to match the observed covariate distribution for those with missing covariates. As shown in Table 4, the medication effects were similar between those with and without missing covariates (with the exception that the statin by finasteride interaction was not significant among those with missing covariates).
No Missing
Mean
Covariates
Std
With Missing
Mean
Covariates
Std
D
p-value
N
71,999
102202
Metformin Use (%)
38.80
14.59
<0.001
Statin Use (%)
78.17
52.23
<0.001
Finasteride Use (%)
13.68
9.56
<0.001
Study Length (days)
2339.86
1009.37
1838.27
1268.99
<0.001
PCa (%)
5.22
5.68
<0.001
Age
66.91
10.22
71.94
10.15
<0.001
Black (%)
17.74
14.87
<0.001
Charlson
3.91
2.71
3.04
2.50
<0.001
Baseline A1c
6.51
0.96
6.65
1.16
<0.001
Mean A1c
6.57
0.90
6.52
1.02
<0.001
DA1C
0.06
0.83
-0.02
0.95
<0.001
Baseline LDL
106.71
27.09
105.06
28.92
<0.001
Mean LDL
89.22
25.00
90.29
26.95
<0.001
DLDL
-17.49
24.77
-14.96
27.35
<0.001
Baseline BMI
30.98
4.98
30.54
7.01
<0.001
DBMI
-1.02
3.15
-0.96
9.88
0.02
Maximum PSA
2.64
5.20
3.60
7.71
<0.001
Table 3: Comparison between Patients without Missing Covariates and Patients With Missing Covariates.
No Missing
HR
Covariates
p-value
With Missing
HR
Covariates
p-value
Metformin Use
0.89
0.02
0.87
<.001
Statin use
0.80
<.001
0.75
<.001
Finasteride Use
0.44
<.001
0.50
<.001
Metformin*Statin
0.82
.001
0.84
<.001
Statin*Finasteride
1.28
0.02
1.09
0.10
Metformin*Finasteride
1.60
<.001
1.48
<.001
Age
1.03
<.001
1.03
<.001
Black
1.82
<.001
1.83
<.001
Maximum PSA
1.003
<.001
1.003
<.001
Charlson Comorbidity
1.03
<.001
1.02
<.001
DA1c
1.16
<.001
1.17
<.001
DLDL
1.002
<.001
1.003
<.001
DBMI
1.01
<.001
1.01
<.001
Results adjusted for propensity scores of metformin use and propensity scores of having missing covariates.
Table 4: Multivariate Cox Proportional Analysis of Metformin, Statin, and Finasteride Effects on PCa Incidence Rate in Men with Type 2 Diabetes: Comparison between Patients Without Missing Covariates and Patients With Missing Covariates.
The results of our study need to be confirmed in other nonveteran cohorts of men with T2DM. However, our results may help manage the treatments of men with T2DM. Metformin, statins, and finasteride used alone or in combination could lead to differential reduction in the risk of PCa: according to Figure 1, (i) the combination use metformin and statins could be more effective for PCa prevention compared to metformin or statin alone; (ii) the combination use metformin and finasteride could be more effective compared to metformin alone but not as effective as finasteride use alone; (iii&) the combination use statins and finasteride or finasteride alone could be more effective compared to statin use alone. Inaddition the consideration of PCa risk, clinical decisions regarding prescribing metformin, statins and finasteride in men with diabetes also requires careful evaluation of patients’ glucose/lipid control and cardiovascular diseases.
Conclusion
Furthermore, while men with T2DM are at a greater risk for highgrade PCa [1,2], they are commonly prescribed with metformin and statins that can potentially reduce the risk factors associated with high grade PCa such as metabolic syndromes and high LDL [43-46]. Future studies are needed to examine the impact of these drugs on high grade PCa prevention.
Acknowledgement
This work is sponsored in part by NCI grants R21CA161180 and P30CA054174.
Guarantor Statement
Dr. Wang is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
References
- Abdollah F, Briganti A, Suardi N, Gallina A, Capitanio U, Salonia A, et al. Does diabetes mellitus increase the risk of high-grade prostate cancer in patients undergoing radical prostatectomy? Prostate Cancer Prostatic Dis. 2011; 14: 74-78.
- Mitin T, Chen M, Moran BJ, Dosoretz DE, Katin MJ, Braccioforte MH, et al. Diabetes mellitus, race, and the odds of high-grade prostate cancer in men diagnosed with prostate cancer in the United States. J Clin Oncol 2011; 29.
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009; 32: 193-203.
- Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010; 70: 2465-2475.
- Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2010; 3: 1451-1461.
- Farwell WR, D'Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011; 103: 885-892.
- Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004; 22: 2388-2394.
- Hamilton RJ, Freedland SJ. Review of recent evidence in support of a role for statins in the prevention of prostate cancer. Curr Opin Urol. 2008; 18: 333-339.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009; 32: 1620-1625.
- Goldstraw MA, Amoroso P, Kirby RS. Do statins protect against prostate cancer? BJU Int. 2006; 97: 1147-1148.
- Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008; 27: 3576-3586.
- Flavin R, Zadra G, Loda M. Metabolic alterations and targeted therapies in prostate cancer. J Pathol. 2011; 223: 283-294.
- Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006; 66: 10269-10273.
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009; 325: 201-204.
- Spindler SR, Mote PL. Screening candidate longevity therapeutics using gene-expression arrays. Gerontology. 2007; 53: 306-321.
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, et al. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006; 5: 97-108.
- Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol. 2008; 168: 925-931.
- Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009; 20: 1617-1622.
- Azoulay L, Dell'Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev. 2011; 20: 337-344.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009; 52: 1766-1777.
- Lehman DM, Lorenzo C, Hernandez J, Wang CP. Statin use as a moderator of metformin effect on risk for prostate cancer among type 2 diabetic patients. Diabetes Care. 2012; 35: 1002-1007.
- Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012; 35: 2665-2673.
- Papadopoulos G, Delakas D, Nakopoulou L, Kassimatis T. Statins and prostate cancer: molecular and clinical aspects. Eur J Cancer. 2011; 47: 819-830.
- Matafome P, Louro T, Rodrigues L, Crisóstomo J, Nunes E, Amaral C, et al. Metformin and atorvastatin combination further protect the liver in type 2 diabetes with hyperlipidaemia. Diabetes Metab Res Rev. 2011; 27: 54-62.
- Woodard J, Platanias LC. AMP-activated kinase (AMPK)-generated signals in malignant melanoma cell growth and survival. Biochem Biophys Res Commun. 2010; 398: 135-139.
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003; 349: 215-224.
- Thompson IM Jr, Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013; 369: 603-610.
- Neuhouser ML, Platz EA, Till C, Tangen CM, Goodman PJ, Kristal A, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins and prostate cancer risk: results from the prostate cancer prevention trial. Cancer Prev Res (Phila). 2013; 6: 91-99.
- Neuhouser ML, Till C, Kristal A, Goodman P, Hoque A, Platz EA, et al. Finasteride modifies the relation between serum C-peptide and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer Prev Res (Phila). 2010; 3: 279-289.
- Parchman ML, Wang CP. Initiation of insulin among veterans with type 2 diabetes and sustained elevation of A1c. Prim Care Diabetes. 2012; 6: 19-25.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992; 45: 613-619.
- Mansour M, Schwartz D, Judd R, Akingbemi B, Braden T, Morrison E, et al. Thiazolidinediones/PPAR? agonists and fatty acid synthase inhibitors as an experimental combination therapy for prostate cancer. Int J Oncol. 2011; 38: 537-546.
- Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007; 25: 1476-1481.
- VA Information Resource center.
- Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007; 26: 20-36.
- Moinpour CM, Lovato LC, Thompson IM Jr, Ware JE Jr, Ganz PA, Patrick DL, et al. Profile of men randomized to the prostate cancer prevention trial: baseline health-related quality of life, urinary and sexual functioning, and health behaviors. J Clin Oncol. 2000; 18: 1942-1953.
- Huskey SW, Dean DC, Miller RR, Rasmusson GH, Chiu SH. Identification of human cytochrome P450 isozymes responsible for the in vitro oxidative metabolism of finasteride. Drug Metab Dispos. 1995; 23: 1126-1135.
- Moiseeva O, Deschênes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, Bourdeau V. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-?B activation. Aging Cell. 2013; 12: 489-498.
- Ge R, Wang Z, Olumi AF. Metformin Has an Anti-Cancer Effect by Repressing TWIST/N-Cadherin Signaling. American Urologic Association Meeting. 2013.
- Lawrentschuk N, Abouassaly R, Hackett N, Groll R, Fleshner NE. Health information quality on the internet in urological oncology: a multilingual longitudinal evaluation. Urology. 2009; 74: 1058-1063.
- National Comprehensive Cancer Network guidelines@ and Derivative information products: user guide.
- Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: The ACTG 320 trial. Am J Epidemiol. 2010; 172: 107-115.
- Morote J, Celma A, Planas J, Placer J, Konstantinidis C, Iztueta I, et al. Sedentarism and overweight as risk factors for the detection of prostate cancer and its aggressivenes. Actas Urol Esp. 2014; 38: 232-237.
- Xiang YZ, Xiong H, Cui ZL, Jiang SB, Xia QH, Zhao Y, et al. The association between metabolic syndrome and the risk of prostate cancer, high-grade prostate cancer, advanced prostate cancer, prostate cancer-specific mortality and biochemical recurrence. J ExpClin Cancer Res. 2013; 32: 9.
- Morote J, Ropero J, Planas J, Bastarós JM, Delgado G, Placer J, et al. Metabolic syndrome increases the risk of aggressive prostate cancer detection. BJU Int. 2013; 111: 1031-1036.
- Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, et al. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009; 18: 2807-2813.