
Research Article
Ann Transl Med Epidemiol. 2023; 8(1): 1018.
Impact of Various Covid -19 Vaccines on General Health and Different Age Groups in Pakistan
Anwaar Iftikhar*; Nazim Hussain*; Mubeen Akhtar; Sana Hussain; Rida Farooq; Ruhma Maqsood; Mehvish Mumtaz; Muhammad Hamza Ashraf; Muhammad Asif Muneer
Centre for Applied Molecular Biology (CAMB), University of the Punjab, Quaid-e-Azam Campus, Lahore, Pakistan
*Corresponding author: Anwaar Iftikhar & Nazim Hussain Centre for Applied Molecular Biology (CAMB), University of the Punjab, Quaid-e-Azam Campus, Lahore, Pakistan. Email: anwaariftikhar33@gmail.com; nazim.camb@pu.edu.pk
Received: August 17, 2023 Accepted: September 27, 2023 Published: October 04, 2023
Abstract
The virus that causes Covid-19, known as SARS-CoV-2 [Severe Acute Respiratory Syndrome Coronavirus 2], has affected various regions of Pakistan. It is already known on a global level and has an impact on many aspects of human life. Vaccination is considered one of the most effective methods of eliminating COVID-19. The main objective of this research is to assess both the positive and negative impacts of COVID-19 vaccines among Pakistanis. Countries have approved numerous vaccines, including Pfizer-Biotech and Moderna, Sinopharm, Sputnik V, Sinovac, CanSino, and Vaxzevria. Therefore, Pakistan officially used Pfizer, AstraZeneca, Moderna, Sinopharm, Sinovac, and CanSino. The approach involves a cross-sectional evaluation of the effects of COVID-19 vaccinations among Pakistanis. There have also been reports of people in various regions of the country refusing vaccinations. The analysis also focuses on age factors, such as middle, upper, and old age. Gender analysis was also included in the study. The vaccinations assessment comprises 4000 people who received vaccine doses in Pakistan. The Chi-Square approach is used to assess the long-term effects of COVID-19 vaccinations, and a sample t-test is used to determine age-related adverse effects. People usually reported mild pain at the injection sites after the first dose of the COVID-19 vaccine, with fatigue and headache being the most prevalent side effects. The age range of the participants was >50, >35 to 50, and >18 to 35 years. Soreness was the most prevalent side effect in all age groups after the second dose. Individuals over the age of 50 are more likely to experience negative effects following the COVID-19 vaccination. Females preferred less severe side effects over men. Furthermore, positive results include strong immunity against COVID-19, decreased hospitalization compared to unvaccinated patients, and more rapid recovery among those who have been vaccinated. The first and second COVID-19 vaccine doses were predictable. The most prevalent adverse effects were headache and fever, although comorbidities and prior diseases were also linked. Several states have begun the vaccination procedure, providing them with a significant advantage against COVID-19 with about 90% accuracy.
Keywords: Covid-19 vaccination; Adverse effects; SARS CoV-2; WHO; Health diseases
Introduction
COVID-19 has not only affected people's lifestyles but has also made their lives chaotic. It has severely damaged Pakistan's economy. COVID-19 has impacted society, increasing the emergency worldwide and causing an extensive public health crisis. COVID-19 variations have been found worldwide [24]. SARS-CoV is a member of the Coronavirus family, with the genre beta-coronavirus. It is 30 kilobytes and expresses 29 distinct types of protein [2]. COVID-19 originated in China and was declared a pandemic in March 2020 [6]. The pandemic effects list will be relevant until the vaccine is complete and administered [15].
On November 12, 2020, WHO issued data containing 212 vaccines, including attenuated, inactivated, vector-based, and DNA-based vaccines [29]. As of July, there were 2,022,598 cases, with 6.46 million fatalities. Vaccination against COVID-19 was a potential technique for dealing with the issue. Many states have been striving to improve their healthcare and economic strategies in order to keep the current epidemic under control as much as possible. Most developing countries are currently grappling with the challenge of adapting to and using their limited financial and medical resources in the face of extreme challenges. These countries' governments are making significant efforts to control the virus's global spread [1].
The planet has shrunk to the size of a town, with regular surveillance of the emergence and reappearance of localized breakouts or global epidemics that swiftly spread contagious illnesses [19]. According to data, the total death rate for those suffering from chronic disease (COVID-19) ranges from 2.0% to 3.0% globally, which is somewhat higher than the H1NI fatality rate? This viral infection (Covid-19) is still a common and dangerous illness in comparison to severe acute respiratory syndrome [16]. Covid-19 has distinct traits that set it apart from other viruses. Individuals infected with COVID-19 may develop mild to severe symptoms, with a significant percentage of persons remaining symptom-free. The most commonly reported coronavirus symptoms are coughing and breathing problems [4].
For unknown reasons, the spread of COVID-19 disease in children is frequently mild or moderate as compared to that in adults. However, serious and fatal effects on children have also been reported [28]. Our globe divides itself into pre-COVID-19 and post-COVID-19 zones, just like before and after World War II. Economic growth began to decline in the second quarter of 2019, but it appeared that the situation would improve in 2020, led by the large emerging countries, and return to sustainable development by 2021 [14]. Several vaccinations are available, and around 13 different vaccines are presently in use. Because the virus is still relatively new, the side effects of COVID-19 vaccines are unknown. Sinovac Biotech China's Corona Vac, a pure attenuated coronavirus vaccine, has been reported to provide active protection against Covid-19.
It is critical to develop an effective vaccine against the infectious virus SARS-CoV-2, which is the cause of the coronavirus disease 2019 (COVID-19) outbreak. The COVID-19 vaccines employ several methods to provide disease defense; nevertheless, the process by which the immune system might produce symptoms Outstanding work soon after the discovery of the COVID-19 genome has resulted in more than 300 vaccine programs [12]. As a result, Pakistan has changed physically and emotionally due to comparing good and bad influences [3,27].
In Pakistan, various vaccines have been administered to people in two doses with a two-month gap. On May 8, 2020, Pakistan received the first delivery of Oxford-AstraZeneca COVID-19 vaccines from the COVAX Facility. Recently, Sinopharm, Sinovac, CanSino-BiO, and Sputnik doses have been administered in Pakistan [19]. Additionally, it is well-acknowledged that COVID-19 vaccines could have serious adverse effects. Trial studies of the Sinopharm Covid-19 vaccine's phases 1 and 2 were conducted in China through one survey for each stage [20]. COVID-19-neutralizing antibody response was elicited by the vaccination with minimal risk of side effects, according to data from 4000 individuals [21].
Pfizer and Moderna RNA-based vaccines have drawn the greatest attention in terms of vaccination adverse responses due to their quick development and manufacturing [19a. By March 10, 2022, a total of 101,881,176 people in Pakistan had received the vaccination, with 128.074,138 receiving at least one dose and 4,869,245 receiving a booster dose. [20a]. Immunization against COVID-19 could lower the risk of developing and transmitting the virus that causes COVID-19. Vaccines can also help prevent major diseases and death. So far, all available data on the adverse effects of the COVID-19 vaccination have been published through manufacturer-funded trials that follow drug authority guidelines and are overseen by third parties.
As a result, this research aimed to assess the prevalence of COVID-19 vaccines' beneficial and harmful effects among Pakistanis [22]. As a result, this investigation sought to gather information about the adverse impact of COVID-19 immunization following the first and successive doses, as suggested by the government of Pakistan.
Methodology
The diagram illustrates the flow chart of the strategy employed in the research to examine the impact of vaccination for COVID-19, as represented in Figure 1.
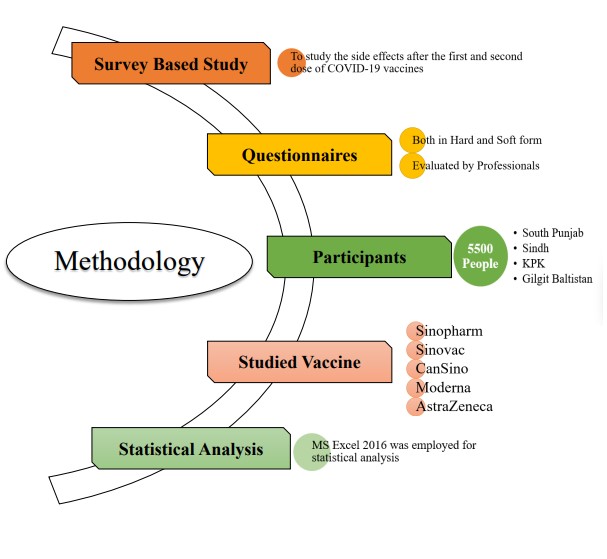
Figure 1: Schematic representation depicting the methodology for the conducted study.
Survey Study
This retrospective survey-based study was conducted to examine the effects of COVID-19 vaccination among individuals in Pakistan. The analysis used a questionnaire in hard copy and an online Google form, which were randomly distributed to people using social media platforms and physical questions at various locations such as educational institutions, private offices, and government buildings. Prospective respondents were given access to a webpage with a summary of the survey's objectives and instructions for completing the questionnaire [23].
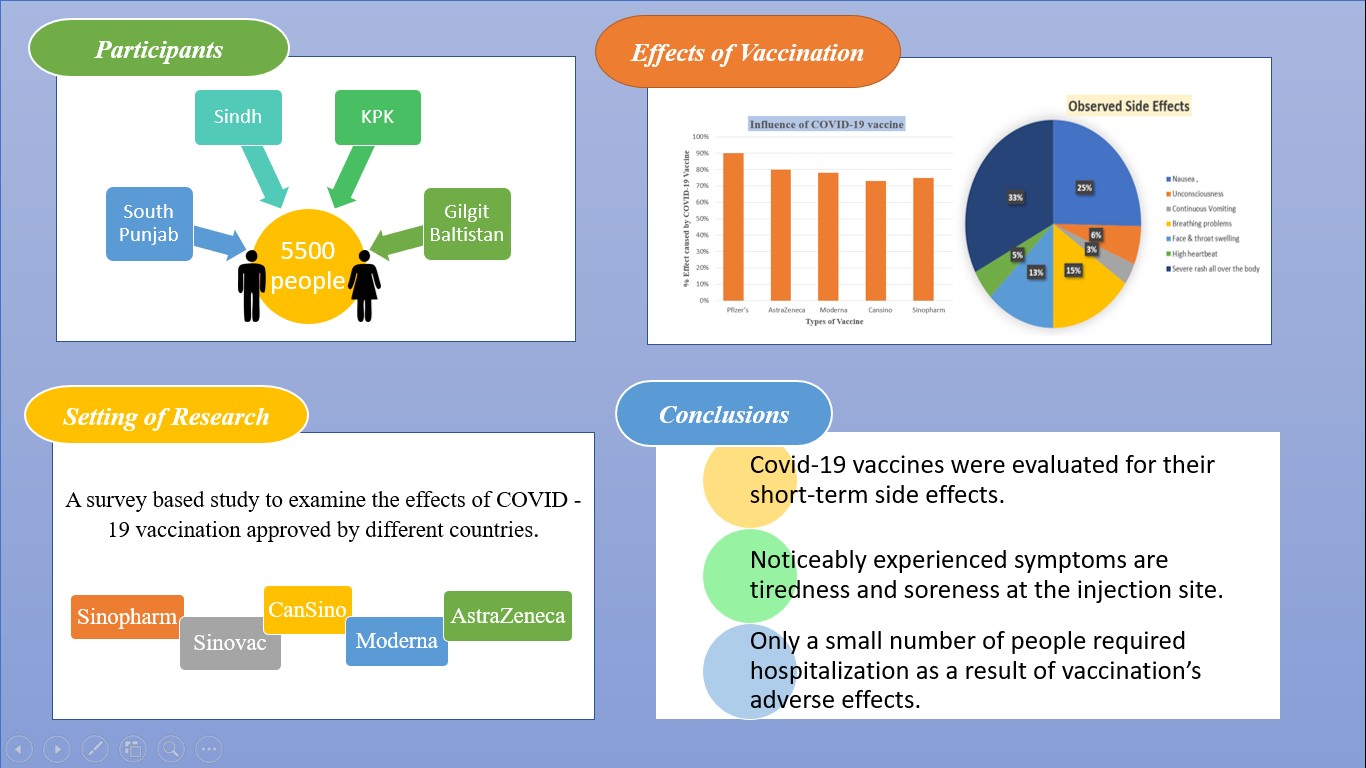
Participants unwilling to respond to the survey could not open the online form. The physical component of the study involves people giving their consent to answer the questionnaire. About 90% of the respondents responded clearly, including the proper sign after the COVID-19 vaccination. 5500 people from South Punjab, Sindh, KPK, and Gilgit-Baltistan participated in this study, ranging in age from >20 to 65 years. About 70% of people filled out the online Google form through social media like WhatsApp, Facebook, and Telegram. 30% of records were filled out during in-field trips at various locations in different regions of Pakistan.
About 10% of forms had a lot of mistakes, were rejected, and were refilled by the same person. The responses of the majority of the people regarding this study were very positive, and 10% of the people hesitated to answer because of their busy routine work [24,25]. Based on the objectives of the research, those who had received a COVID-19 vaccination from Pfizer, Sinopharm, Sinovac, CanSino-BiO, and Sputnik were included in the study. Each respondent could provide their opinions on the most effective ways to treat the sickness and any unusual symptoms they had encountered.
Data Collection
The study was designed based on comprehensive literature research and recommendations from the WHO, NCOC, and NIH in Pakistan on the predicted responses to the COVID-19 vaccines, such as those from Pfizer, Sinopharm, Sinovac, CanSino-BiO, and Moderna. The questionnaire survey had multiple-choice questions and was offered in both English and Urdu. The National Institute of Health (NIH), in collaboration with the Ministry of Health, NCOC, and WHO, initiated a COVID-19 vaccination program for older people in Islamabad, then covered rural and urban regions in Pakistan [26]. A panel of professionals evaluated the assessment by providing opinions on various topical questions. The questionnaire was divided into four pieces.
Initially, an investigation is conducted regarding age and gender, marital status, educational attainment, employment, ethnic background, and geographical location. Furthermore, inquiries were made into the individuals' pre-existing long-term illnesses or morbidities before long-term-long-term long-term infections or morbidities before the individuals before vaccination. These conditions included malignancy, autoimmune diseases, persistent bronchial ailments, high blood sugar levels, being overweight, arterial disease, and allergic responses associated with vaccinations. Third, the inquiries concerned the appearance of indications following the first dose of COVID-19 vaccination for people with chronic conditions and healthy people. The last section includes questions about the first and second doses of the vaccine, which were asked individually based on the adverse effects of each dosage.
Data was collected from those who had received COVID-19 vaccines for the study. Mild to severe soreness at the injection site, a high temperature, migraine, weariness, bloating, loose stool, coughing, allergies, discomfort in the muscles, indigestion, back pain, and lethargy are just a few of the symptoms that participants reported experiencing.
Covid-19 Vaccination Status in Pakistan
The total population of Pakistan is 220.9 million; according to NCOC COVID-19 vaccination status, the total doses given are 292 million for COVID-19 vaccination, of which 131 million people have been fully vaccinated, with a percentage of 59.3% [5].
Biostatistics Analysis
A statistical analysis of the study based on groups was performed using MS Excel 2016, and group charts were created using this program.
Results
5500 respondents participated in the survey. 1850 individuals received the vaccination from Pfizer, whereas 1100 people were vaccinated with the AstraZeneca vaccine. 900 respondents were vaccinated with Moderna. CanSino is administered to 550 people. 550 received the Sinovac. 250 participants received the Sinopharm vaccine. Each COVID-19 vaccination was administered twice to each subject. Both males and females were part of this research. The age factor ranges from 20 to 65 years.
Some side complications include discomfort at the point of the injection, limb discomfort, stiffness in the back, thirst, excessive perspiration, eye pain, and earache. These symptoms were more common in those people aged 40 to 65 who had already been affected by other eye and ear diseases after the COVID-19 vaccination. Graph 1 compares the overall effects of COVID-19 vaccines in Pakistan.
This graph shows the overall cause of Covid-19 vaccine side effects faced by individuals vaccinated in Pakistan and appeared side symptoms. About 90% of post-vaccination reactions occurred to Pfizer's Covid-19 vaccine among individuals in Pakistan. People vaccinated with the AstraZeneca vaccine were reported to have 80% of their symptoms disappear after this COVID-19 vaccine. Respondents with the Moderna COVID-19 vaccine had 78% reactions. Participants received COVID-19 vaccines like CanSino at approximately 73%, Sinovac at 81%, and Sinopharm at 75%, all experiencing adverse effects after their doses.
GRAPHICAL ABSTRACT:
Covid-19 Vaccines in Pakistan; Overall Occurrence of Mild Side Effects
The COVID-19 vaccines like Pfizer, AstraZeneca, Moderna, CanSino, Sinovac, and Sinopharm in Pakistan had a wide range of mild side effects like pain and soreness, redness, weakness, loss of appetite, feeling thirsty, sweating at the injection site, stomach pain, abdominal pain, coughing, diarrhea, allergic reactions likely at the skin, eye, and ear pain, muscle fatigue, and tiredness. Graph 2 indicates that the mild side effects of COVID-19 vaccines from Pfizer, AstraZeneca, Moderna, CanSino, Sinovac, and Sinopharm were common among people in Pakistan. Post-reaction after two doses of COVID-19 vaccines shows mild symptoms like pain at the injection site 95%, tiredness 90%, muscle fatigue 75%, weakness 98%, the soreness 83%, headache 75%, fever 55%, abdominal pain 34%, allergic reactions 43%, stomach pain 74%, extreme sweating 65%, eye pain 25 arrhea, allergic reactions likely at the skin, eye, and ear pain, muscle fatigue, and tiredness. Graph 2 indicates that the mild side effects of COVID-19 vaccines from Pfizer, AstraZeneca, Moderna, CanSino, Sinovac, and Sinopharm were common among people in Pakistan. Graph 2.2: Representation of the percentage of mild side effects of Covid-19 vaccines on general health in Pakistan. Post-reaction after two doses of COVID-19 vaccines shows mild symptoms like pain at the injection site 95%, tiredness 90%, muscle fatigue 75%, weakness 98%, soreness 83%, headache 75%, fever 55%, abdominal pain 34%, allergic reactions 43%, stomach pain 74%, extreme sweating 65%, eye pain 25%, and ear pain 12%.
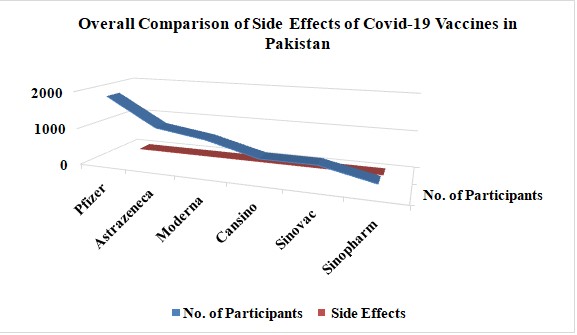
Graph 2.1: Overall Comparison of Covid-19 Vaccines Side Effects in Pakistan.
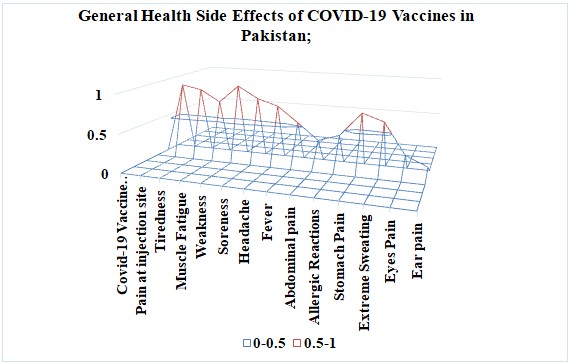
Graph 2.2: Representation of the percentage of mild side effects of Covid-19 vaccines on general health in Pakistan.
Covid-19 Vaccines in Pakistan; Overall Severe Post Vaccination Reactions
Pfizer, AstraZeneca, Moderna, CanSino, Sinovac, and Sinopharm's COVID-19 vaccines caused severe reactions in 5500 people in Pakistan, including nausea (38%), unconsciousness (9%), continuous vomiting (5%), breathing problems (23%), face and throat swelling (19%), a high heartbeat (7%), a severe rash all over the body (49%), a high fever (34%), and hospitalization (34%). Graph 3 depicts the COVID-19 vaccines administered in Pakistan, as well as the overall severe post-vaccination reactions among study participants.
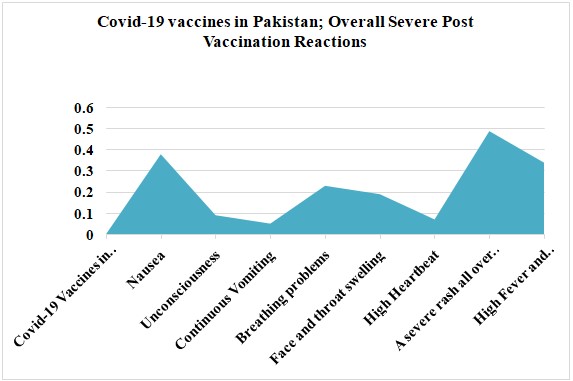
Graph 3: Covid-19 vaccines in Pakistan; Overall Severe Post Vaccination Reactions.
Duration of Mild versus Severe COVID-19 Vaccine Post-Reactions in Pakistan Based on the Age Factor
Covid-19 post-vaccination reactions among research participants last between 12 and 48 hours for mild side effects and 48 to 72 hours for severe side effects. Table 1 compares duration between mild and severe side effects of the COVID-19 vaccine in Pakistan in different age groups.
Sr.no
Age Factor
Mild Effects Duration
Severe Effects Duration
1
>20 to 35 years
12 to 18 hours
2 to 3 days
2
>35 to 50 years
18 to 24 hours
4 to 5 days
3
>50 to 65 years
24 to 36 hours
6 to 7 days
Table 1: Duration of Mild versus Severe Covid-19 Vaccines Post Reactions in Pakistan Based on Age Factor.
The existence of mild COVID-19 vaccine effects stimulates approximately differently among different age groups. However, severe side effects took longer compared to the favorable impact of COVID-19 vaccines in Pakistan. Older people, aged >50 to 65 years, have more symptoms shown by COVID-19 vaccines. Middle-aged people (>35 to 50 years) have been less affected by the COVID-19 vaccine administration in Pakistan. Younger age groups have shown very low efficacy of COVID-19 effects in Pakistan.
Comparative Analysis of Covid-19 Vaccines Administered in Pakistan
In Pakistan, data was collected from various geographic locations, including residents of every province. The study's focus, however, is on how the COVID-19 vaccines in Pakistan affected people in both moderate and severe ways. The people of Pakistan have seen both positive and negative effects from the COVID-19 vaccine. Graph 4 illustrates the total number of COVID-19 vaccinations administered in Pakistan, their two dosages, and the proportion of recipients.
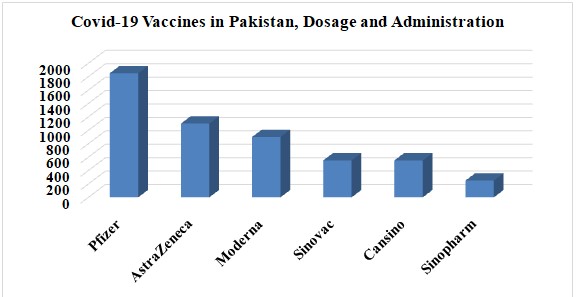
Graph 4: Illustration of overall Covid-1 vaccines in Pakistan, Dosage and Administration.
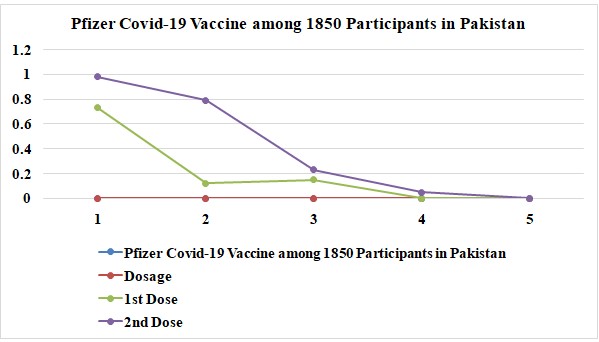
Graph 5: Pfizer Covid-19 vaccine acute or mild side effects and severe effects among 1850 participants in Pakistan.
In this survey, Pfizer COVID-19 recipients have the most significant number of participants, with AstraZeneca coming in second. In third place was Moderna. The CanSino COVID-19 vaccine is third, followed by Sinovac. Sinopharm is listed at position five.
Pfizer covid-19 Vaccination
In this study, 5500 people were examined, and 1850 received Covid-19 Pfizer vaccination injections. Out of the 1850,73% of individuals who received the Pfizer vaccine's initial dosage, some experienced acute reactions. 12% of people who received the COVID-19 vaccine from Pfizer's first dosage had severe side effects. 15% said there were no side effects. After the shot, participants who received the second Pfizer dose experienced negative side effects. After receiving a double amount of Pfizer, 67% reported experiencing adverse side effects. After receiving the Pfizer COVID-19 vaccination, 25% reported mild side effects. 8% of people without harmful side effects from the first dose also showed side effects. The graph below depicts the acute, soft, and severe adverse effects of the Pfizer COVID-19 vaccination among 1850 participants in Pakistan.
About 5% showed mild effects after the 2nd dose of the COVID-19 Pfizer vaccine among these participants in Pakistan. The Pfizer vaccine has significantly affected the people of Pakistan.
Astra Zeneca
AstraZeneca was used to immunize 1100 participants. Of these participants, 80% of those who received the vaccine's initial AstraZeneca dosage reported side effects. 15% of those polled claimed there were no side effects. 5% of people who received the AstraZeneca vaccine experienced the mild side effects listed in the table below. The AstraZeneca vaccine's second dose resulted in severe post-vaccinal reactions in 68% of recipients. 1% reported no impact, while 31% reported just minor ones. Among 1100 participants in Pakistan, Graph 6 shows the effects of AstraZeneca COVID-19.

Graph 6: Illustration of AstraZeneca Covid-19 effects among 1100 Participants in Pakistan.
Moderna
The modern vaccine was administered to 900 respondents. In the first dosage of the Moderna COVID-19 vaccine, 3% of recipients suffered severe side effects. Around 78% of those who took the initial dose experienced mild adverse effects, while 19% had no side effects. 62% of those who received the second dosage of the Moderna vaccine experienced severe side effects, and 15% of those who received the double dose had just minor side effects. According to recent reports, 23% of people made claims about minor impacts. The results of Moderna COVID-19 on 900 participants in Pakistan are shown in Graph 7.
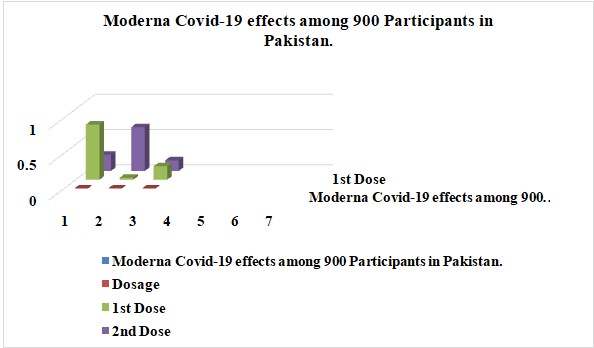
Graph 7: Moderna Covid-19 effects among 900 Participants in Pakistan.
CanSino
The CanSino vaccine doses were administered to 550 participants in this study. The CanSino vaccine's post-vaccination effects in Pakistani participants have shown to be effective, with hardly any adverse side effects. 90% of those who received the first dose of 550 said they only had minor negative impacts. After receiving the CanSino COVID-19 vaccine for the first time, 10% of Pakistanis were thought to be unaffected. Participants did not experience serious adverse effects. Only 10% of people got severe symptoms in the second dose, while 75% experienced mild ones. After the CanSino Covid-19 vaccination was administered in Pakistan, 5% of people had no mild or severe reactions. 550 subjects in Pakistan are shown in Graph 8 to show how the CanSino Covid-19 vaccine affected them.

Graph 8: CanSino Covid-19 effects among 550 Participants in Pakistan.
Sinovac
Two doses of the Covid-19 Sinovac vaccine were administered to 550 research participants in Pakistan. Out of 550, 65% of participants in the Sinovac COVID-19 vaccination, reports indicated minor side effects, 15% reported more serious ones, and 10% reported none. After the second dose of the Sinovac Covid-19 vaccine, 75% of people reported minor side effects, 13% revealed severe adverse effects, and 12% reported no side effects. In Pakistan, the Sinovac Covid-19 vaccination was superior to other Covid-19 vaccines. Graph 9 depicts Sinovac COVID-19's effects on 550 participants in Pakistan.

Graph 9: Sinovac Covid-19 effects among 550 Participants in Pakistan.
Sinopharm
250 participants in the research study received the Sinopharm Covid-19 vaccine in Pakistan. Of 250, 72% had mild side effects, 21% reported none, and 7% reported severe side effects. Out of 250 persons who received the second dosage of this vaccination in Pakistan, 73% reported minor adverse effects. In contrast, 18% said they had severe side effects and already suffered from other health issues. 9% of individuals reported no side effects. Among 250 participants in Pakistan, Graph 10 shows the outcomes of the Sinopharm Covid-19 vaccination.
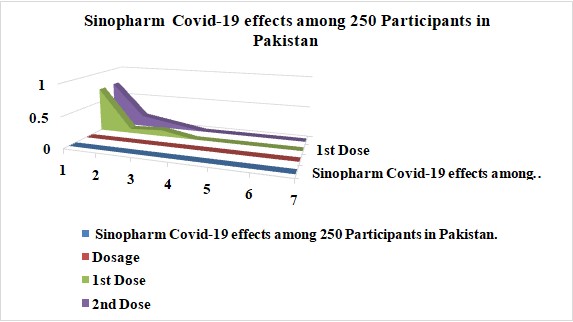
Graph 10: Sinopharm Covid-19 effects among 250 Participants in Pakistan.
The survey form had a section where people with other diseases were asked about their post-reaction to COVID-19 vaccines. This group of people was more significant than the group of people who did not have any pre-existing conditions. Both groups received COVID-19 vaccines and experienced post-reactions. However, those with pre-existing disorders had more severe symptoms than the other group–people without pre-existing conditions experienced either mild or severe post-reactions to COVID-19 vaccines.
Discussion
Although the post-reaction following the COVID-19 vaccine is uncommon, it is regarded as evidence that the vaccine works and the immune system responds strongly [5]. The COVID-19 immunization campaign in Pakistan got underway on May 8, 2021. The COVID-19 vaccine performed outstandingly in halting the spread of the coronavirus among Pakistanis. Officially used in Pakistan were vaccines from Pfizer, AstraZeneca, Moderna, Sinopharm, Sinovac, and CanSino, which produced a neutralizing antibody response with fewer side effects [5]. This study demonstrates that each Covidien-19 vaccine's single dose can have mild to severe side effects. People with other conditions had more severe adverse effects after receiving the second dose of the COVID-19 vaccination, whereas others displayed mild and severe side effects.
This is probably because these immunizations were developed more swiftly than previously authorized vaccines, whose administrative approval frequently takes several years [12]. As a result, the objective of this study was to evaluate any adverse effects associated with the COVID-19 vaccines currently being given in Pakistan. We acquired data from individuals who received the Oxford-AstraZeneca or COVID-19 vaccines in Pakistan. Depending on the person's age, the kind of vaccine, and the dosages, each COVID-19 vaccine has been associated with between 60% and 90% of the documented adverse effects due to vaccination. In our study, 80% of the participants noted the same negative consequences [25].
This is likely because these vaccinations were created more quickly than previously authorized vaccines, often taking several years to receive administrative approval. Therefore, this study aimed to assess adverse effects related to the COVID-19 vaccinations presently being administered in Pakistan. We gathered information from people who got Oxford-AstraZeneca or assessed adverse COVID-19 vaccinations in Pakistan. Approximately 60% to 90% of the vaccination-related adverse reactions have been documented for each COVID-19 vaccine, including variations depending on the individual's age, kind of vaccine, and dosages. 80% of the subjects in our research mentioned identical adverse effects [25].
The most common adverse side effects included fatigue, pain following injections, fever, and headache [21,28]. 85% of those who received the immunizations reported having side effects that persisted for 12 to 48 hours [22,25]. Contrary to earlier studies, most of the individuals in our study reported fatigue and headaches, primarily because they were older than those in earlier studies. Elderly individuals had adverse effects more frequently than younger people, as has also been demonstrated in numerous studies [18,30]. Men and women both participated in this survey. The findings revealed that female candidates reported more symptoms after getting the COVID-19 vaccination than male applicants [10,26]. Additionally, those who have received different dosages of COVID-19 immunization have also experienced similar effects [7].
According to this study, the COVID-19 vaccination, used in Pakistan, has more serious adverse effects than the Pfizer vaccine. The Pfizer COVID-19 vaccine also produced a more severe reaction following a second dose [9]. Many people were affected by AstraZeneca's administration of the COVID-19 vaccination to Pakistan's citizens. We may require various vaccines that produce multiple types of immunity to properly build global protection [8]. Because they prevent disease at the population level, protecting those who have received the vaccination while also lowering transmission throughout the community, vaccines are crucial public health treatments. The COVID-19 vaccine can reduce your risk of getting the disease and spreading the virus that causes it. Additionally, vaccinations can protect individuals from dangerous infections. People with COVID-19 may extend it to family members who cannot be immunized and those who are more sensitive and may experience a severe COVID-19 disease. Consequently, illness prevention (defending immunity) is frequently the goal of vaccinations. Therefore, the main advantage of a proper vaccination is the avoidance of the illness caused by that condition [13].
Conclusion
The COVID-19 vaccines from Pfizer, AstraZeneca, Moderna, CanSino, Sinovac, and Sinopharm were evaluated for short-term side effects. The majority of individuals experienced tiredness and soreness at the injection site. Several states have started the vaccination process, giving them a significant advantage against COVID-19. In addition, only a few people required hospitalization or medical visits due to vaccination's adverse effects.
Author Statements
Conflict of Interest
There is no conflict of interest.
Funding Statement
There is no funding for this research.
References
- Abbas J, Wang D, Su Z, Ziapour A. The role of social media in the advent of COVID-19 pandemic: crisis management, mental health challenges, and implications. Risk Manag Healthc Policy. 2021; 14: 1917-32.
- Abbas S, Abbas B, Amir S, Wajahat M. Evaluation of adverse effects with COVID-19 vaccination in Pakistan. Pak J Med Sci. 2021; 37: 1959-64.
- Abdulla ZA, Al-Bashir SM, Al-Salih NS, Aldamen AA, Abdulazeez MZ. A summary of the SARS-CoV-2 vaccines and technologies available or under development. Pathogens. 2021; 10: 788.
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020; 26: 450-2.
- Andrzejczak-Grzadko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021; 25: 4418-21.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384: 403-16.
- Bernal JL, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-Astra Zeneca vaccines on Covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021; 373: m1088.
- Bernal JL, Andrews N, Gower C, Stowe J, Tessier E, Simmons R, et al. Effectiveness of BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on mortality following COVID-19. MedRxiv. 2021.
- Braun-Moscovici Y, Kaplan M, Braun M, Markovits D, Giryes S, Toledano K, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. 2021; 80: 1317-21.
- Feldman RA, Fuhr R, Smolenov I, Mick Ribeiro A, Panther L, Watson M, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019; 37: 3326-34.
- Ghram A, Moalla W, Lavie CJ. Vaccine and physical activity in the era of COVID-19 pandemic. Prog Cardiovasc Dis. 2021; 67: 33-4.
- Graham BS. Rapid COVID-19 vaccine development. Science. 2020; 368: 945-6.
- He Q, Mao Q, Zhang J, Bian L, Gao F, Wang J ,et al. COVID-19 vaccines: current understanding on immunogenicity, safety, and further considerations. Front Immunol. 2021; 12: 669339.
- Henry BM, Benoit SW, de Oliveira MHS, Hsieh WC, Benoit J, Ballout RA, et al. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): a pooled analysis and review. Clin Biochem. 2020; 81: 1-8.
- Jeon M, Kim J, Oh CE, Lee JY. Adverse events following immunization associated with coronavirus disease 2019 vaccination reported in the mobile vaccine adverse events reporting system. J Korean Med Sci. 2021; 36: e114.
- Li W, Yang Y, Liu ZH, Zhao YJ, Zhang Q, Zhang L, et al. Progression of mental health services during the COVID-19 outbreak in China. Int J Biol Sci. 2020; 16: 1732-8.
- Liu F, Liu H, Hou L, Li J, Zheng H, Chi R, et al. Clinico-radiological features and outcomes in pregnant women with COVID-19 pneumonia compared with age-matched non-pregnant women. Infect Drug Resist. 2020; 13: 2845-54.
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020; 191: 9-14.
- Lostroh P. Molecular and cellular biology of viruses. Garland Science. 2019.
- Mahase E. Covid-19: moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ. 2020; 371.
- Mellet J, Pepper MS. A COVID-19 Vaccine: big strides come with big challenges. Vaccines. 2021; 9: 39.
- Moll K, Lufkin B, Fingar KR, Ke Zhou C, Tworkoski E, Shi C, et al. Background rates of adverse events of special interest for COVID-19 vaccine safety monitoring in the United States, 2019-2020. Vaccine. 2023; 41: 333-53.
- Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis. 2021; 111: 219-26.
- Silberner J. US election: Biden announces Covid-19 task force, promising ”compassion, empathy, and concern”. Br Med J Publ Group. 2020; 371: m4327.
- Stanley KE, Thomas E, Leaver M, Wells D. Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 2020; 114: 33-43.
- Torreele E. The rush to create a Covid-19 vaccine may do more harm than good. BMJ. 2020; 370: m3209.
- Wadman M. Public needs to prep for vaccine side effects. American Association for the Advancement of Science. 2020; 370: 1022.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020; 323: 1061-9.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323: 1239-42.
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020; 17: 259-60.