Abstract
Background: Thrombotic Microangiopathy (TMA) is a microvascular occlusive disorder caused by facilitation of endothelial damage and primary platelet aggregation. TMA is a fatal complication after Liver Transplantation (LT), which may be responsible for more than 90% mortality without treatment, which decrease to less than 10% with proper management in proper timing; it is important to diagnose and treat TMA following LT to reduce significant morbidity and mortality associated with this disease. The mainstay of treatment of TMA following LT is Plasma Exchange (PE).
Presentation of the case: Our case report of female patient 25 years – old developed Acute Liver Failure (ALF) caused by HEV infection, patient received Living Donor Liver Transplantation (LDLT), and developed TMA and received 3 sessions of plasma exchange, with significant improvement of all clinical and laboratory data. Conclusion: Early diagnosis of TMA and exclusion of rejection and other causes of thrombocytopenia following LT is the cornerstone of management. Schistocytes in blood smear and raised LDH are crucial criteria in diagnosis of TMA.
Keywords: Thrombotic microangiopathy; Liver transplantation; Hepatic failure; HEV infection; Plasma exchange
Abbreviations
TMA: Thrombotic Microangiopathy; TTP: Thrombotic Thrombocytopenic Purpura; HUS: Hemolytic Uremic Syndrome; ST- HUS: Hemolytic–Uremic Syndrome for Shiga Toxin; CNI: Calcineurin Inhibitors; LDH: Lactate Dehydrogenase; ULVWF: Ultra-Large Von-Willebrand Factor; CMV: Cytomegalovirus; PE: Plasma Exchange; FLF: Fulminant Liver Failure; POD: Post- Operative Day; AMR: Antibody Mediated Rejection; LDLT: Living Donor Liver Transplantation
Introduction
Thrombotic Microangiopathy (TMA) is a microvascular occlusive disorder caused by facilitation of endothelial damage and primary platelet aggregation [1]. Now, the concept of TMA includes two previous entities; thrombotic Thrombocytopenic Purpura (TTP) and Hemolytic Uremic Syndrome (HUS) [2,3]. Despite this diversity in names and entities, they have common clinical and pathological features, including micro-angiopathy hemolytic anemia, thrombocytopenia, and organ injury. The pathological feature is vascular damage [1]. It is known to be precipitated by bacterial and viral infections, autoimmune diseases, and is also associated with pregnancy, malignancy and certain medications such as Calcineurin Inhibitors (CNI), Gemcitabine and Quinine [2,3].
The disease is characterized by aggregation of platelets with micro thrombi in small vessels leading to obstruction of micro-circulation that result in mechanical destruction of red blood cells and ischemic injury to affected organs, most commonly the kidney and brain [1,4]. Fragmented erythrocytes (schistocytes) result from a turbulent flow in the microcirculation, which is partially occluded by platelet aggregation, and increased Lactate Dehydrogenase (LDH) level is more due to organ ischemia than from lysed red cells [1]. Endothelial injury appears to be important in initiating microthrombi formation, resulting in release of endothelial Ultra-Large Von Willebrand Factor (ULVWF), which increases platelet adhesiveness and causes thrombocytopenia [5]. In healthy individuals, a metalloproteinase, von Willebrand factor-cleaving metalloproteases splits ULVWF into smaller VWF fragments. Von Willebrand factor-cleaving metalloproteases (Known as ADAMTS-13) is synthesized mainly by hepatocytes and prevent entry of large multimers of VWF into the circulation. So, Any ADAMTS-13 deficiency or complement mutation or antibody inhibition or decreased activities of ADAMTS-13 by drugs or toxins can precipitate to TMA [4,5].
TMA is clinically defined as thrombocytopenia and hemolytic anemia after exclusion of other etiologies especially disseminated vascular coagulopathy [1]. Some authors proposed diagnostic criteria for TMA as follows: (1) Thrombocytopenia or rapid decline in platelet counts; (2) Hemolytic anemia; (3) Highly elevated levels of serum Lactate Dehydrogenase (LDH); (4) The presence of fractionated erythrocytes (schistocytes) in a blood smear; and (5) progressive renal failure in the form of raised creatinine; (6) Neurological deficits; and (7) Fever [1,4]. Although Presences of all these features are not required for diagnosis of TMA, However, Thrombocytopenia, Hemolytic anemia, Schistocyte in peripheral blood smear and raised LDH are considered diagnostic to TMA [4,6]. The occurrence and outcome of TMA in LT are not well described. It is important to diagnose and treat TMA following LT to reduce significant morbidity and mortality associated with this disease.TMA is a fatal complication after LT, which may be responsible for more than 90% mortality without treatment, which decrease to less than 10% with proper management in proper timing [4,7]. The first case was reported in 1984, and its frequencies are documented to be 3.8%-7.6% after LT [8]. Clinical features of TMA following LT don’t differ so much than the usual feature of TMA; thrombocytopenia, hemolytic anemia, schistocyte in peripheral blood smear, raised LDH, fever, renal impairment, occasionally neurological symptoms, this with organ specific symptom as raised liver enzymes and bilirubin [1,7,9].
Viral infection, including Hepatitis B (HBV), Hepatitis C (HCV), and Cytomegalovirus (CMV) and CNI use as immunosuppression following LT are considered to be precipitating factors for TMA development [7,10]. Nishi et al. mentioned 18 patients in his series who had TMA after LT, 10 of them due to viral infection (HBV and HCV associated cirrhosis), 9 out of 18 patients are transplanted due to HCV associated cirrhosis [11]. CMV infection following LT was reported as a possible cause of TMA. These viral diseases acting through inhibition of ADAMTS-13 [12]. There is a strong correlation between TMA post transplantation and Tacrolimus use as immunosuppression, although the exact mechanism is not yet known. Although in some series Tacrolimus level trough level is maintained within target level, and no apparent overdose was detected at the time of diagnosis of TMA, there is strong correlation between reduction or withdrawal of Tacrolimus and relive of TMA [2,13]. The mainstay of treatment of TMA following LT is Plasma Exchange (PE), which reduced mortality rates, from over 90% to10–20%. PE has the both effects ADAMTS13 replenishment and removal of inhibitory antibodies against ADAMTS13; therefore, it is considered as a standard management of TMA [14]. A small series of TMA after LT reported a favorable response with intravenous immunoglobulin infusion therapy [7]. In cases refractory to the standard treatments, replacement of CNI with other immunosuppressive agents, including mycofenolate mofetil, Pravastin, Limaprost Alfadex or anti-CD 25 antibodies [Rituximab], should be used as an alternative strategy [15]. Although re-transplantation may be a therapeutic option, this is strictly limited due to donor shortage and donor safety [7].
Case Report
We describe a case of a 25 years-old Indian female, presenting to the emergency department with the complaints of jaundice and abdominal discomfort of four- day duration and drowsiness since two days. No other relevant history was available. On examination, she had tachycardia with a pulse of 119 beat/min., febrile with temperature of 101*F and blood pressure of 110/80mmHg. Her Glasgow coma scale was E3 M5 V4. She was admitted to the intensive care unit, and a full set of laboratory investigations were sent. The significantly altered lab parameters showed a total serum bilirubin of 21.9 mg/dl, direct of 11.9 mg/dl, prothrombin time of >120 seconds, AST/ALT were 1082/404 U/L, platelets of 320.000/L with a normal serum creatinine. An ultrasound of the abdomen was done, which showed mild hepatomegaly and no features of cirrhosis of liver or portal hypertension. Based on these investigations and the clinical findings of grade II hepatic encephalopathy, she was diagnosed to be a case of Fulminant Liver Failure [FLF], fulfilling the Kings College Criteria [16]. A thorough etiological and septic workup was done and all anti encephalopathic measures were started. The cause of the FLF was Hepatitis E virus. It was concluded that the patient would require an urgent LT. She was evaluated by cardiology, pulmonary, gynecology and anesthesia doctors and was found fit to undergo LT. Before being shifted to the operative theater, a CT brain was done to confirm absence of radiological signs of coning of the brain. Her brother was the living liver donor, and his right liver lobe without the middle hepatic vein was utilized and implanted in the recipient. During the recipient surgery, 10 units of Packed RBC’s, two units of fresh frozen plasma and four units of platelets were transfused. The intraoperative findings were classic except portocaval shunt was done for two hours until the donor harvesting procedure finished. As the recipient was severely coagulopathic, it was decided to pack the abdomen with pressure sponges and re-explored again after 24 hours for removal of packs and achieved complete hemostasis. Post operatively, the patient was shifted to the liver intensive care unit and was vitally stable without any inotrope requirement. She was given Tacrolimus and methyl prednisolone imunossupressants. Her liver Doppler was normal post transplantation. On post-operative day POD6, she developed generalized tonic clonic seizures with an elevation in liver enzymes, Creatinine and serum bilirubin. MRI brain and EEG were found to be normal. Her elevation in liver enzymes was treated as an episode of rejection and pulsed steroids were started, and Tacrolimus was changed to Cyclosporin. Since the liver enzymes did not respond to the pulsed steroid dose, a liver biopsy was done showing no evidence of rejection, only congestion of liver with few areas of cholestasis. A peripheral blood smear and Lactate Dehydrogenase (LDH) levels were sent, and it showed the presence of schistocytes in blood smear and highly elevated LDH levels [2765 U/L]. This confirmed the diagnosis of TMA. It was decided to start her on plasma exchanges and monitor the response with serial LDH levels and peripheral blood smears. Cyclosporine was stopped and changed into Everolimus. The plasma exchanges were done with saline, albumin and fresh frozen plasma. After 3 cycles of plasma exchange in three consecutive days, the LDH levels reduced to upper limit of normal and the peripheral blood smears showed absence of schistocytes, a fall in liver enzymes and serum bilirubin levels (Figures 1 & 2). On following up her lab parameters, the liver function tests and bilirubin showed a falling trend and normalization by POD 20. The urine output improved and the creatinine decreased from a peak of 1.8 mg/dl to 0.7 mg/dl. She was discharged on POD 30.
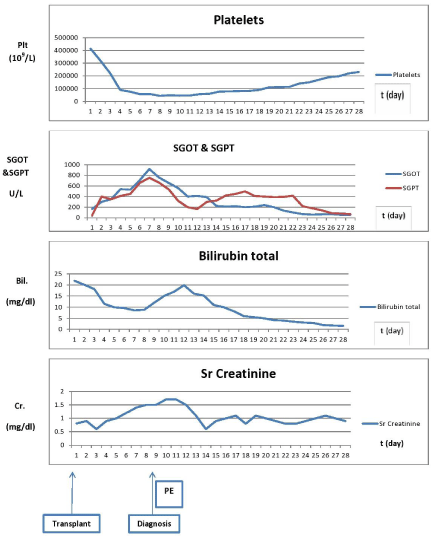
Figure 1: Trend of Platelet, SGOT, SGPT, Bilirubin, Creatinine before and after PE. Trends of Platelet, liver enzymes, bilirubin, and creatinine before and during the
attack and after PE treatment showing significant decline in platelet count and rise of liver enzymes, bilirubin and creatinine at the attack and improvement after PE.
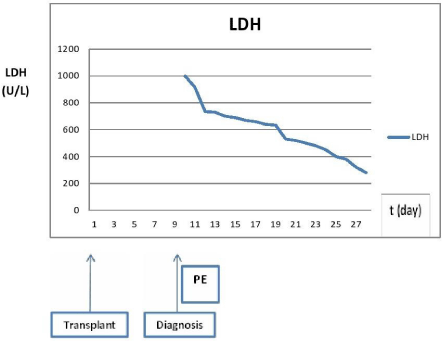
Figure 2: Trend of LDH before and after PE: Significant rise of LDH with the
attack of TMA with significant improvement with PE.
Discussion
TMA after liver transplantation is considered to be one of the serious complications, characterized by poor outcome and high mortality rate [17]. Endothelial injury, which occur, causes hemolytic anemia and platelet consumption, resulting in thrombosis in the microcirculation, especially in kidney and brain [17,18]. The pathophysiology is usually attributed to acquired or congenital decrease or deficiency of ADAMTS13, which may be drug induced, or induced by viral infection as HBV, HCV or CMV [9]. Immunosuppression as CNI may precipitate the initiation of the disease, despite CNI trough level correlates with neither TMA development nor progression. Differentiation between TMA and Antibody Mediated Rejection (AMR) at this point is very important as treatment differs accordingly [19]. Differentiation is based mainly on histopathological features of early graft biopsy and serological studies mainly schistocyte in the peripheral blood smear [4,7,20]. Viral infection either as a causative factor for LT or following it, has a role in TMA induction through either direct endothelial damage or ADAMTS13 inhibition, but the exact pathophysiology in viral associated TMA is still elucidated [12]. HCV is a small [50 nm in size], enveloped, single-stranded, positive sense RNA virus. It is the only known member of the family Flaviviridae. TMA in HCV positive graft recipient is attributed to inhibition of ADAMTS13 as a proposed mechanism of HCV-related TMA. HEV also is an RNA positive-sense single-stranded genome virus like HCV but related to family Hepeviridae so it is proposed to have the same mechanism and ability to induce TMA by ADAMTS13 inhibition [21]. Here in Our case, we reported TMA after LT due to HEV induced fulminant liver failure manifested by rising creatinine, neurological manifestations, raised liver enzymes with no histopathological evidence of rejection in liver biopsy raising the suspicion of TMA, which was confirmed by presence of schistocytes in peripheral blood smear and highly elevated LDH. Withdrawal of CNI and plasma exchange [three sessions in our case] led to rapid and significant improvement in platelet count, liver enzymes, and creatinine level, which confirms that PE is considered the first treatment option for TMA after LT. PE should be continued until disappearance of schistocytes in peripheral blood smear and normalization of LDH (Figures 2 & 3).
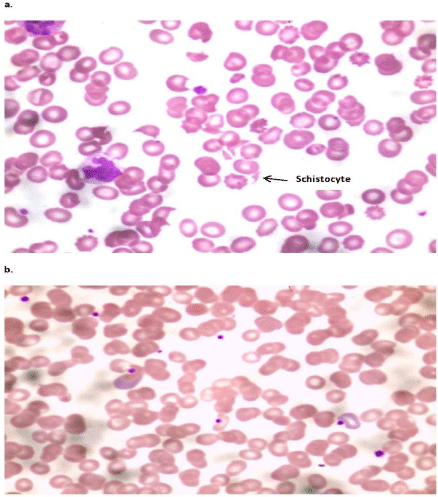
Figure 3: a: Blood film at time of diagnosis of TMA showing schistocyte with
low number of platelet; b: Blood film after three days PE showing absence of
schistocyte and relative increase in number of platelet.
Conclusion
HEV may be associated with TMA after LT, Schistocytes in blood smear and raised LDH are crucial criteria in diagnosis of TMA, however, early diagnosis of TMA and exclusion of rejection and other causes of thrombocytopenia following LT are the cornerstone of management, furthermore early conversion of CNI to another immunosuppression with PE may be associated with dramatic response and improvement of TMA.
Ethical Approval
This work was approved with the local ethical committee in Kokilaben Dhirubhai Ambani hospital. All the data or information which may point to the patient personality were masked or removed
Author’s Contributions
Ahmed Zidan, and Karan Julka, wrote the manuscript and treated the patient. Pankhi Dutta, and Nidhi Mehta, diagnosed the patient. Shailesh Sableand Kapildev Yadav searched for relivant literature. Barun Nath, Sorabh Kapoor and Vibha Varma treated the patient and revised the manuscript. Vinay Kumaran diagnosed the patient, revised and approved the manuscript for submission.
References
- Moake JL. Thrombotic microangiopathies. The New England Journal of Medicine. 2002; 347: 589-600.
- Nwaba A, MacQuillan G, Adams LA, Garas G, Delriviere L, Augustson B, et al. Tacrolimus-induced thrombotic microangiopathy in orthotopic liver transplant patients: case series of four patients. Internal Medicine Journal. 2013; 43: 328-333.
- Ramasubbu K, Mullick T, Koo A, Hussein M, Henderson JM, Mullen KD, et al. Thrombotic microangiopathy and cytomegalovirus in liver transplant recipients: a case-based review. Transplant infectious disease: An Official Journal of the Transplantation Society. 2003; 5: 98-103.
- Shindoh J, Sugawara Y, Akamatsu N, Kaneko J, Tamura S, Yamashiki N, et al. Thrombotic microangiopathy after living-donor liver transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012; 12: 728-736.
- Nakazawa Y, Hashikura Y, Urata K, Ikegami T, Terada M, Yagi H, et al. Von Willebrand factor--cleaving protease activity in thrombotic microangiopathy after living donor liver transplantation: a case report. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2003; 9: 1328-1333.
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. The New England Journal of Medicine. 2014; 371: 654-666.
- Hori T, Kaido T, Oike F, Ogura Y, Ogawa K, Yonekawa Y, et al. Thrombotic microangiopathy-like disorder after living-donor liver transplantation: a singlecenter experience in Japan. World Journal of Gastroenterology: WJG. 2011; 17: 1848-1857.
- Tamura S, Sugawara Y, Matsui Y, Kishi Y, Akamatsu N, Kaneko J, et al. Thrombotic microangiopathy in living-donor liver transplantation. Transplantation. 2005; 80: 169-175.
- Daly AS, Hasegawa WS, Lipton JH, Messner HA, Kiss TL. Transplantationassociated thrombotic microangiopathy is associated with transplantation from unrelated donors, acute graft-versus-host disease and venoocclusive disease of the liver. Transfusion and apheresis science: official journal of the World Apheresis Association: Official Journal of the European Society for Haemapheresis. 2002; 27: 3-12.
- Matsuda D, Toshima T, Ikegami T, Harimoto N, Yamashita Y, Yoshizumi T, et al. Thrombotic microangiopathy caused by severe graft dysfunction after living donor liver transplantation: report of a case. Clinical Journal of Gastroenterology. 2014; 7: 159-163.
- Nishi H, Hanafusa N, Kondo Y, Nangaku M, Sugawara Y, Makuuchi M, et al. Clinical outcome of thrombotic microangiopathy after living-donor liver transplantation treated with plasma exchange therapy. Clinical Journal of the American Society of Nephrology: CJASN. 2006; 1: 811-819.
- Lopes da Silva R. Viral-associated thrombotic microangiopathies. Hematology/oncology and Stem Cell Therapy. 2011; 4: 51-59.
- Rerolle JP, Akposso K, Lerolle N, Mougenot B, Ponnelle T, Rondeau E, et al. Tacrolimus-induced hemolytic uremic syndrome and end-stage renal failure after liver transplantation. Clinical Transplantation. 2000; 14: 262-265.
- Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004; 103: 4043-4049.
- Vasko R, Groenewold F, Korsten P, Muller GA, Koziolek M. Plasmapheresisrefractory thrombotic microangiopathy in a hematopoietic stem cell transplant recipient. Therapeutic Apheresis and Dialysis: Official Peer-Reviewed Journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2011; 15: 507-509.
- O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989; 97: 439-445.
- Paviglianiti A, Tomarchio V, Spurio S, Cerchiara E, Marchesi F, Tirindelli MC, et al. A case of transplantation-associated thrombotic microangiopathy with cardiac involvement successfully treated with plasma exchange. Indian Journal of Hematology & Blood Transfusion: An Official Journal of Indian Society of Hematology and Blood Transfusion. 2014; 30: 369-371.
- Tsai HM. Pathophysiology of thrombotic thrombocytopenic purpura. International Journal of Hematology. 2010; 91: 1-19.
- Noris M, Remuzzi G. Thrombotic microangiopathy after kidney transplantation. American Journal of Transplantation: Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010; 10: 1517-1523.
- Cortina G, Trojer R, Waldegger S, Schneeberger S, Gut N, Hofer J. De novo tacrolimus-induced thrombotic microangiopathy in the early stage after renal transplantation successfully treated with conversion to everolimus. Pediatric Nephrology. 2015; 30: 693-697.
- Yagita M, Uemura M, Nakamura T, Kunitomi A, Matsumoto M, Fujimura Y. Development of ADAMTS13 inhibitor in a patient with hepatitis C virusrelated liver cirrhosis causes thrombotic thrombocytopenic purpura. Journal of Hepatology. 2005; 42: 420-421.
