Research Article
Austin J Trauma Treat. 2014;1(1): 5.
Impact of Brain and Skull Injuries on Physiology, Infectious Complications and Outcomes in Patients with Polytrauma
Ladislav Mica*, Hanspeter Simmen, Clément Werner, Michael Plecko and Kai Sprengel
Division of Trauma Surgery, University Hospital of Zürich, Switzerland
*Corresponding author: Ladislav Mica, Division of Trauma Surgery, University Hospital of Zürich, Rämistrasse 100, 8091 Zürich, Switzerland
Received: October 06, 2014; Accepted: November 01, 2014; Published: November 03, 2014
Abstract
Brain and skull injuries in patients with polytrauma lead mostly to adverse outcomes. We investigated how such injuries influenced the physiology, infectious complications and outcomes. A total of 1465 patients with polytrauma were included in this retrospective cohort study with an Injury Severity Score (ISS) ≥ 16 and an age ≥ 16 years. The patients were subdivided into six groups according to the Abbreviated Injury Score (AIS) of the head. Marshall, Goris, Sequential Organ Failure Assessment (SOFA), Murray and Systemic Inflammatory Response Syndrome (SIRS) scores were calculated retrospectively. Infections were determined according to clinical signs and bacteremia. Data were analyzed using SPSS® 22.0; analysis of variance was used for continuous normally distributed data, the Kruskal–Wallis test was used for categorical data, and P < 0.05 was considered significant. The Marshall score increased along with the head AIS (P < 0.01). The Goris (P < 0.01) and SOFA (P < 0.01) score also increased significantly with increased head AIS. In the severe AIS groups the incidence of pneumonia was high (60%; P = 0.003) without correlation with the AIS of the thorax. Ventilator-assisted days increased significantly (P < 0.01) as well as the death rate (P < 0.01) along with the head AIS severity. The mortality reached 80% in the group with the maximum head AIS. These injuries have an adverse impact on physiology and outcome in polytrauma patients without being associated with the overall injury pattern. However, there appeared to be side effects of intensive-care-unit therapy on the patients’ physiology.
Keywords: Brain scull injury; Polytrauma; Marshall score; Goris score; SOFA score; SIRS score; Infection
Abbreviations
AIS: Abbreviated Injury Scale; ANOVA: Analysis of Variance; APACHE: Acute Physiology and Chronic Health Evaluation; ATLS: Advanced Trauma Life Support; AUC: Area Under the Curve; CI: Confidence Interval; ICU: Intensive Care Unit; IRB: Institutional Review Board; ISS: Injury Severity Score; NISS: New Injury Severity Scale; ROC: Receiver Operator Characteristic; SD: Standard Deviation; SIRS: Systemic Inflammatory Response Syndrome; SOFA: Sequential Organ Failure Assessment
Introduction
The proper management of patients with polytrauma is challenging and often involves an individual plan and time of treatment. Brain and skull injuries are very often a part of the injury pattern in such patients and contribute significantly to adverse outcomes. The proper management of acute isolated brain and skull injuries involves decompression and stopping the hemorrhage [1]. The patient’s possible recovery depends on the amount of destroyed brain parenchyma and the degree of posttraumatic swelling with compression of the pons and medulla oblongata. As a monotraumatic injury, the management might be straightforward; however, the impact of a brain and skull injury on the patient’s physiology under polytraumatic conditions remains unclear. Patients with polytrauma are at high risk of suffering bleeding complications based on the coagulopathy of trauma-induced shock [2,3]. Sustained bleeding reduces the patient’s temperature and oxygen transport capacity, and promotes anaerobic glycolysis leading to a decreased tissue pH. Sustained bleeding endangers perfusion and oxygenation of the brain tissue. Multilocular bleeding gives the surgeon multiple problems in the trauma bay. Even in the most experienced hands, brain and skull injuries remain difficult to treat, and the outcome is often uncertain [4]. Initially well-recovering patients may develop secondary damage such as bleeding or necrosis, and this can lead to further swelling of the brain and even to death [4]. Therefore, the management of brain and skull injuries takes precedence over other traumatic injuries. These might remain untreated, undergo a damage control procedure [5] or receive delayed definitive surgery. Taken together, the trauma and the treatment of brain and skull injury in a patient with polytrauma could have an impact on their physiology and susceptibility to infections because of delayed definitive care. The data on this topic are very scarce; however, knowing the nature of the most common complications could lead to improved treatment protocols in Intensive Care Units (ICUs). The main goal in this retrospective cohort study was to investigate the nature of the impact of brain and skull injury on the physiology and infectious complications in patients with polytrauma.
Materials and Methods
Patient sample
One thousand four hundred and five patients with polytrauma admitted consecutively to the emergency room of the University Hospital of Zürich (Switzerland) in the period 1996–2011 were included in this retrospective cohort study. The inclusion criteria were an Injury Severity Score (ISS) ≥ 16 points, age ≥ 16 years, and admission within at least 24 h of incurring the polytrauma. The cohort was subdivided into six groups (Table 1) according to the Abbreviated Injury Scale (AIS) of the head. All patient data were collected retrospectively. All data were retrieved from patient records with the approval of the local institutional review board according to the University of Zürich guidelines as well as the World Medical Association Declaration of Helsinki. The study was conducted according to our guidelines for good clinical practice (Permission: “RetrospektiveAnalysen in der ChirurgischenIntensivmedizin” Nr. St. V. 01-2008).
AIS head score group
0
1
2
3
4
5
6
P-value
Total
n
380
77
114
204
285
395
10
0.072†
1465
Age (y)
42.6 ± 17.4
40.8 ± 17.6
41.7 ± 19.1
41.3 ± 18.3
45.2 ± 19.8
42.7 ± 20.5
49.4 ± 25.0
0.224*
42.8 ± 19.1
Sex (male/female)
285/95
55/22
81/33
143/61
194/91
300/95
10/0
0.001†
1068/397
AIS face
0.3 ± 0.8
0.6 ± 1.0
0.6 ± 1.2
0.9 ± 1.1
0.9 ± 1.2
0.7 ± 1.2
0.9 ± 1.5
< 0.001*
0.6 ± 1.1
AIS thorax
2.4 ± 1.7
2.9 ± 1.3
2.3 ± 1.6
2.4 ± 1.5
2.1 ± 1.6
1.7 ± 1.7
0.9 ± 1.4
< 0.001*
2.2 ± 1.7
AIS abdomen
2.3 ± 2.2
2.1 ± 1.8
1.8 ± 1.9
1.2 ± 1.8
0.9 ± 1.6
0.9 ± 1.6
0.0 ± 0.0
< 0.001*
1.4 ± 1.9
AIS extremities
2.0 ± 1.6
2.1 ± 1.4
2.2 ± 1.4
2.0 ± 1.4
1.5 ± 1.4
1.2 ± 1.3
0.7 ± 1.2
< 0.001*
1.7 ± 1.4
AIS pelvis
0.9 ± 1.4
0.9 ± 1.4
1.0 ± 1.4
0.9 ± 1.4
0.5 ± 1.0
0.5 ± 1.1
0.0 ± 0.0
< 0.001*
0.7 ± 1.3
AIS skin
0.5 ± 0.9
0.6 ± 0.7
0.9 ± 0.9
0.7 ± 0.9
0.5 ± 0.7
0.4 ± 0.8
0.4 ± 0.5
< 0.001*
0.5 ± 0.8
ISS
29.4 ± 10.3
28.9 ± 8.6
27.9 ± 10.2
29.0 ± 10.5
32.4 ± 10.2
38.9 ± 11.8
69.0 ± 13.3
< 0.001*
32.6 ± 11.8
NISS
38.2 ± 14.2
33.6 ± 10.4
31.8 ± 11.8
32.3 ± 12.2
39.2 ± 9.5
53.2 ± 12.0
72.4 ± 6.7
< 0.001*
41.1 ± 14.7
APACHE
13.8 ± 10.0
11.1 ± 8.2
10.2 ± 6.7
14.9 ± 9.6
14.9 ± 8.6
20.3 ± 8.0
20.1 ± 10.3
< 0.001*
15.5 ± 9.4
Blood pH
7.27 ± 0.17
7.31 ± 0.12
7.33 ± 0.08
7.28 ± 0.16
7.31 ± 0.12
7.29 ± 0.13
7.31 ± 0.12
.004*
7.29 ± 0.14
Blood lactate (mmol/L)
3.7 ± 3.2
2.7 ± 2.1
2.8 ± 2.0
3.3 ± 3.0
3.1 ± 2.6
3.5 ± 2.9
3.5 ± 2.5
.025*
3.4 ± 2.9
Base excess (mmol/L)
–5.67 ± 7.05
–3.48 ± 4.12
–3.23 ± 4.02
–5.13 ± 6.48
–3.44 ± 5.41
–4.83 ± 5.33
–5.53 ± 4.86
< 0.001*
–4.64 ± 5.94
Hematocrit (%)
30.6 ± 9.4
32.5 ± 8.0
33.6 ± 8.4
31.9 ± 9.3
33.8 ± 8.4
32.6 ± 9.0
30.7 ± 10.6
< 0.001*
32.2 ± 9.0
Table 1: Patient sample characteristics at admission.
Diagnostic protocols
Unstable patients underwent resuscitative procedures according to the Advanced Trauma Life Support (ATLS) standards of the American College of Surgeons. Hemodynamically stable patients received diagnostics according to the clinical findings or a whole-body Computed Tomography (CT) scan in uncertain situations. Hemodynamically unstable patients received focally oriented diagnoses with immediate problem solving according to the ATLS protocols.
Primary care
The treatment of all patients admitted was done according to the ATLS guidelines and previously assessed trauma management protocols after appropriate indications had been identified [6–8].
Scoring systems
The maximal values during hospitalization of the Murray, Goris, Marshall, and Sequential Organ Failure Assessment (SOFA) scores were used to evaluate the physiological impairment of the patient [9– 12]. The acute physiology and chronic health evaluation (APACHE II) score was used to evaluate the overall physiological impairment of the patient at admission [13]. The ISS and the new injury severity scale (NISS) were used to define the severity of any trauma [14,15]. The 2005 version of the AIS was used to describe injuries in specific anatomical regions.
Laboratory parameters
Blood lactate, pH, hematocrit and base excess were measured at regular intervals using a blood-gas analyzer (ABL 800 Flex; Radiometer GmbH, Thalwil, Switzerland).
Statistical analysis
Data are presented as the mean ± Standard Deviation (SD) for continuous variables and as percentages for categorical variables. Cases with an incomplete data set were discarded from this study. A two-tailed Kolmogorov–Smirnov test was used for normality testing; if P < 0.05, the data were considered as normally distributed. The data for the AIS groups of the brain and skull injuries were compared using the Kruskal–Wallis test for categorical data and with one-way Analysis of Variance (ANOVA) for continuous data. The data were considered significant when P < 0.05. Pearson’s correlation was calculated and was given as Pearson r with the corresponding p-value (2-tailed) The predictive quality of brain and skull injuries for any increase in the Goris, SOFA, Murray and Marshall scores was subjected to Receiver Operator Characteristic (ROC) analysis, and results are reported as the area under the curve (AUC) ± standard error with a corresponding Confidence Interval (CI) of 95%. The data were analyzed using SPSS for IBM statistical software (version 22.0; IBM Corp., Armonk, NY, USA).
Results and Discussion
Patient sample
All patients admitted to the trauma bay who met the inclusion criteria were included in this study. The admission data were subjected to the Kolmogorov–Smirnov test, and all data tested positively for normality (P < 0.05, for all noncategorical data; Table 1). There were significantly more men than women (P < 0.001). There was no significant difference in age between the groups. The mean age was 42.8 ± 19.1 years at admission (range between study groups, 40.8– 49.4). According to the AIS of the head the ISS values (range between study groups, 27.9 ± 10.2 – 69.0 ± 13.3; P < 0.001) and NISS grades (range between study groups, 31.8 ± 11.8 – 72.4 ± 6.7, P < 0.001) increased significantly (Table 1). Interestingly, the AIS values from the different anatomical regions, especially the thorax (range between study groups, 0.9 ± 1.4 – 2.9 ± 1.3, P < 0.001) and abdomen (range between study groups, 0.0 ± 0.0 – 2.3 ± 2.2, P < 0.001) decreased significantly according to the increasing AIS head score and reached their minima in group 6 at 0.9 ± 1.4 and 0.0 ± 0.0, respectively (Table 1). The APACHE II score increased along with the AIS head score (range between study groups, 10.2 ± 6.7 – 20.3 ± 8.0), reaching a maximum in AIS group 5 at 20.3 ± 8.0 (P < 0.001; Table 1). The pH, lactate, base excess and hematocrit values were significantly different between the six study groups but without any particular association with the AIS scores (Table 1).
Scoring each patient’s health
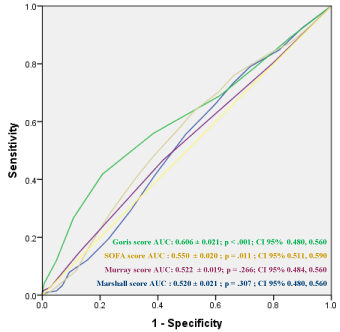
The Marshall score increased along with the AIS head scores, as could be expected, reaching a maximum in group 6 at 9.6 ± 3.4 (P < 0.001; Table 2). The Goris score (range between study groups, 3.6 ± 2.1 – 7.2 ± 1.5; P < 0.001) and SOFA score (range between study groups, 5.5 ± 4.4 – 11.8 ± 3.2; P < 0.001) also increased along with the AIS head scores but did not correspond to the injury pattern shown in Table 1 (Table 2). Pulmonary function estimated by the Murray score revealed no significant differences between the head score groups (P = 0.263). The SIRS score was similar over all head score groups (range, 2.1 ± 1.1 – 2.2 ± 1.2, P = 0.715). The maxima of the Goris, SOFA, and Murray scores revealed no clear tendencies. The predictive quality of brain scull injuries for the rise of the investigated scores was only for theGoris score significant (AUC: 0.606 ± 0.021; P < 0.001) (Figure 1).
Figure 1: The predictive power of brain and skull injuries for the changes in Goris, SOFA, Murray and Marshall Scores based on the area under the curve (AUC) of receiver operator characteristic (ROC) curves.
AIS head
0
1
2
3
4
5
6
P-value
Marshall score
4.9 ± 3.9
4.3 ± 3.3
3.9 ± 3.8
5.6 ± 3.4
5.1 ± 3.4
6.4 ± 2.7
9.6 ± 3.4
< 0.001*
Day of Marshall score maximum
1.4 ± 2.2
1.5 ± 1.7
1.8 ± 2.1
2.0 ± 2.5
2.3 ± 3.4
1.9 ± 3.0
3.2 ± 3.1
0.005*
Goris score
3.6 ± 2.1
3.6 ± 2.3
3.4 ± 2.3
4.3 ± 2.3
4.5 ± 2.5
5.3 ± 2.3
7.2 ± 1.5
< 0.001*
Day of Goris maximum
1.2 ± 1.9
1.3 ± 1.7
1.7 ± 2.4
1.6 ± 2.3
1.9 ± 3.2
1.5 ± 2.4
1.8 ± 1.9
0.042*
SOFA score
5.5 ± 4.4
5.5 ± 3.8
4.9 ± 4.5
7.0 ± 4.2
7.1 ± 4.3
8.6 ± 3.4
11.8 ± 3.2
< 0.001*
Day of SOFA score maximum
1.3 ± 2.1
1.4 ± 1.5
1.7 ± 2.2
1.9 ± 2.4
1.9 ± 2.7
1.5 ± 2.1
3.0 ± 1.9
< 0.001*
Murray score
1.4 ± 1.0
1.1 ± 0.9
1.5 ± 1.5
1.4 ± 1.0
1.4 ± 1.1
1.5 ± 1.4
1.9 ± 1.2
0.263*
Day of Murray score maximum
1.8 ± 2.5
2.0 ± 2.5
2.8 ± 3.5
2.7 ± 4.3
2.8 ± 4.0
2.0 ± 2.8
3.3 ± 3.1
0.007*
SIRS score
2.1 ± 1.2
2.3 ± 1.1
2.1 ± 1.1
2.2 ± 1.2
2.1 ± 1.3
2.1 ± 1.4
1.7 ± 1.9
0.715*
Day of SIRS score maximum
2.1 ± 2.9
2.9 ± 5.0
2.2 ± 2.6
2.8 ± 3.6
3.2 ± 4.6
2.4 ± 3.8
2.3 ± 2.7
0.023*
Table 2: The analysis of physiological scoring systems and the days when they peaked.
Infectious complications
Analysis of infections revealed only a significant increase in pneumonia along with the AIS head scores, with a maximum incidence of 60% (range, 13–60%; P = 0.003; Pearson’s r = .049; Table 3). Bacteremia showed a maximum incidence of 15% in group 5 (overall range, 0–12%; P = 0.016; Pearson’s r = 0.077) with significant correlation to the AIS head scores (P = 0.005; Table 3). The other infectious foci were randomly distributed between the six AIS head score groups (Table 3).
AIS head
0
1
2
3
4
5
6
P-value
Pearson’s r/P
Pneumonia
17%
13%
19%
16%
15%
23%
60%
0.003†
0.049/0.072
Abdominal infection
2%
0%
3%
1%
2%
1%
0%
0.586†
–0.002/0.933
Wound infection
6%
1%
4%
6%
6%
7%
20%
0.294†
0.020/0.474
Bone infection
1%
0%
0%
1%
1%
1%
0%
0.881†
0.004/0.891
Urinary tract infection
7%
4%
5%
4%
5%
8%
10%
0.705†
0.005/0.848
Catheter infection
4%
3%
5%
4%
6%
8%
0%
0.143†
0.065/0.020
Central nervous system infection
2%
4%
4%
3%
2%
3%
0%
0.834†
0.006/0.815
Bacteremia
6%
7%
7%
4%
8%
12%
0%
0.016†
0.077/0.005
Table 3: Analysis of the infection rate in the patient sample.
Outcomes
The analysis of time spent in the ICU revealed significant differences (range between study groups, 5.8 ± 5.6 – 7.8 ± 9.0 days; P = 0.004; Pearson’s r = 0.016; Table 4). Analysis of the ventilator-associated days showed a clearer picture with a significant increase according to the AIS head score (range between study groups, 2.4 ± 3.4 – 4.7 ± 4.9 days; P < 0.001; Pearson’s r = 0.146; Table 4). The numbers of hospitalization days were inversely related to the AIS head scores, with the lowest AIS head score group having the longest hospitalization (range between study groups, 20.4 ± 19.0 – 7.6 ± 10.5 days; P < 0.001; Pearson’s r = –0.273; Table 4). The mortality rate also showed an increase to 80% in group 6 (overall range, 5–80%, P < 0.001; Pearson’s r = 0.380; Table 4).
AIS head score
0
1
2
3
4
5
6
P-value
Total
Pearson’s r/P
ICU [days]
5.8 ± 5.6
7.9 ± 5.6
5.9 ± 6.2
7.8 ± 9.0
7.3 ± 8.3
5.9 ± 7.8
5.9 ± 7.3
0.004*
6.5 ± 7.4
0.016/0.534
Ventilator [days]
2.4 ± 3.4
3.6 ± 4.8
2.3 ± 3.4
4.4 ± 6.6
4.4 ± 6.9
4.3 ± 5.9
4.7 ± 4.9
< 0.001*
3.7 ± 5.7
0.146/0.001
Hospitalization [days]
20.4 ± 19.0
20.3 ± 13.3
18.3 ± 13.7
17.9 ± 15.2
14.7 ± 13.3
9.0 ± 11.4
7.6 ± 10.5
< 0.001*
15.6 ± 15.5
–0.273/0.001
Death [% of each group]
17
5
9
18
32
65
80
< 0.001†
32
0.380/0.001
Table 4: Analysis of outcomes.
Discussion
Brain and skull injuries are very often associated with accompanying injuries and are very common in patients with polytrauma [16]. Higher AIS scores of the head at admission were associated with increased AIS values from other anatomical regions, suggesting a higher whole body impact upon trauma. However, these higher head AIS values were associated with lower AIS values from other anatomical regions, suggesting a direct isolated impact on the head. Unfortunately, the head tends to be involved in any kind of trauma in humans because of its mass and the weak neck muscles. The analysis of the Murray score showed no significant differences between the six study groups. An increase in this score could be expected according to the AIS head score because of ICU-associated brain therapy with hyperventilation and changing blood gases. Furthermore, significant differences were only found in the Goris and SOFA scores, which increased according to the severity of brain and skull injury. This association with the injury pattern might only partly explain the continuous rise of the Goris and SOFA scores, especially because in the higher AIS head score groups, there were low AIS scores for the abdomen and thorax. This suggests that there were physiological changes associated with brain and skull injury therapy such as hyperventilation with hyperoxygenation and the extensive use of norepinephrine to assure sufficient cerebral tissue oxygenation and perfusion pressure. The Glasgow Coma Scale [17] is usually artificially lowered to 3 by sedation during brain and skull therapy to stabilize neuronal networks and to reduce their electric activity. Rheological properties of the blood might be improved with a reduced hematocrit and a lower international normalized ratio for blood clotting; however, this reduced hematocrit usually results from the anemia associated with critical illness [18]. These alterations in blood properties might be accepted clinically to a certain extent; however, they play a role in deciding the Goris and SOFA scores. Interestingly, the injured brain is an organ with high levels of cytokine production by astrocytes and microglia, but this does not influence the SIRS score [19], which might be masked by ICU treatment of the brain and skull injuries. The heart rate, hyperventilation and temperature (usually hypothermia) are changed during brain and skull therapy. The Goris, SOFA and SIRS scores reached their maxima at about the second day of treatment in the ICU, suggesting side effects of the therapies used. The same was shown by the ROC analysis, with a low ability to predict the severity of the brain and skull injuries on physiological scores. The analysis of infectious complications as frequent problems during the management of patients with polytrauma showed a good correlation only for urinary tract infections and bacteremia. However, this distribution seemed to be random, without any particular association with the severity of brain and skull injuries.
Outcomes of therapy for brain and skull injuries
Controlled ventilation was required for the patients with more severe brain and skull injuries, which was mirrored by the increasing numbers of days on ventilation according to the higher AIS head scores. Interestingly, the duration of hospitalization decreased with the severity of brain and skull injuries. This might be reflected by the mortality rate, as brain and skull injuries contribute to the mortality of patients with polytrauma when a certain severity is reached. We can speculate about the extent to which the physiological changes are caused by the brain and skull injury or are side effects of ICU therapy. These data indicate that the higher the AIS head score, the more such therapy-related factors contributed to the scoring systems used in this study. Reflections made on improvement of the therapy of traumatic brain injuries might not show the golden path but a harsh way between Scylla and Charybdis.
Conclusive Clinical Recommendations
The Physiological changes and infectious complications in this study might be rated as paratherapeutic and are only evitable by proper nursing and hygiene of the ICU patient. Rigorous hygiene and supportive systemic therapy could reduce the adverse effects of a brain scull injury in polytrauma patients.
Hypothetical Sources of Bias
All patients were selected retrospectively. The documentation of all parameters followed Good Clinical Practice guidelines. The collection of data was performed by many different persons under the guidance of the personnel selecting the patients for treatment. The time period of 15 years could have led to a bias in the treatment of brain and skull injuries, and several parameters have changed during this time. The scores and values were calculated from a single Excel® spreadsheet (Microsoft®, Office® 2010, Redmond, WA, USA).
References
- Brain Trauma Foundation, American Association of Neurologic Surgeons, Congress of Neurological Surgeons. AANS/CNS joint section on neurotrauma and critical care. J Neurotrauma. 2007; 24: S1-106.
- Maegele M, Schöchl H, Cohen MJ. An update on the coagulopathy of trauma. Shock. 2014; 41: 21-25.
- Frith D, Brohi K. The acute coagulopathy of trauma shock: clinical relevance. Surgeon. 2010; 8: 159-163.
- Raj R, Siironen J, Kivisaari R, Hernesniemi J, Skrifvars MB. Predicting Outcome after Traumatic Brain Injury: Development of Prognostic Scores Based on the IMPACT and the APACHE II. J Neurotrauma. 2014; 31: 1721-1732.
- Jaunoo SS, Harji DP. Damage control surgery. Int J Surg. 2009; 7: 110-113.
- Wang K, Liu B, Ma J. Research progress in traumatic brain penumbra. Chin Med J (Engl). 2014; 127: 1964-1968.
- Ertel W, Trentz O. Causes of shock in the severely traumatized patient: emergency treatment. Goris RJA, Trentz O, editors. In: The Integrated Approach to Trauma Care: The First 24 Hours. Berlin, Springer. 1995; 78-87.
- Trentz O, Friedl HP. Therapeutic sequences in the acute period in unstable patients. Goris RJA, Trentz O, editors. In: The Integrated Approach to Trauma Care: The First 24 Hours. Berlin, Springer. 1995; 172-178.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988; 138: 720-723.
- Goris RJ. The injury severity score. World J Surg. 1983; 7: 12-18.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995; 23: 1638-1652.
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996; 22: 707-710.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985; 13: 818-829.
- Baker SP, O'Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974; 14: 187-196.
- Champion HR, Copes WS, Sacco WJ, Lawnick MM, Bain LW, Gann DS, et al. A new characterization of injury severity. J Trauma. 1990; 30: 539-545.
- Zeckey C, Hildebrand F, Pape HC, Mommsen P, Panzica M, Zelle BA, et al. Head injury in polytrauma-Is there an effect on outcome more than 10 years after the injury? Brain Inj. 2011; 25: 551-559.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974; 2: 81-84.
- Shander A. Anemia in the critically ill. Crit Care Clin. 2004; 20: 159-178.
- Ferreira LC, Regner A, Miotto KD, Moura Sd, Ikuta N, Vargas AE, et al. Increased levels of interleukin-6, -8 and -10 are associated with fatal outcome following severe traumatic brain injury. Brain Inj. 2014; 28: 1311-1316.