Rapid Communication
Hancke D and Suárez OV. The Need for a Better Understanding of the Transmission of Cryptosporidium in Urban Areas. Austin Trop Med Care. 2019; 2(1): 1002.
The Need for a Better Understanding of the Transmission of Cryptosporidium in Urban Areas
Hancke D* and Suárez OV
Laboratorio de Ecología de Roedores Urbanos, Departamento de Ecología, Genética y Evolución, IEGEBA (CONICET-UBA), Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Intendente Güiraldes, Ciudad Universitaria, Buenos Aires, Argentina
*Corresponding author: Diego Hancke, Laboratorio de Ecología de Roedores, Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Avenida Intendente Cantilo s/n, Ciudad Universitaria, Buenos Aires, Argentina; Email: diegohancke@ege.fcen.uba.ar
Received: October 10, 2019; Accepted: November 28, 2019; Published: December 05, 2019
Rapid Communication
Cryptosporidium is an intracellular protozoan parasite that infects the small intestine causing damage to the intestinal epithelium and disrupts absorption and barrier function, leading to mild-to-severe diarrhea [1]. Cryptosporidium has a worldwide distribution infecting humans and animals but in low and middle income countries, cryptosporidiosis is much more prevalent [2]. However, the burden of this parasitosis is underrated. According to Khalil et al (2018), Cryptosporidium infection was the fifth leading cause of diarrheal mortality in children younger than 5 years in 2016 and the estimation of the burden of Cryptosporidium is 2.5 times higher than previously reported by accounting childhood health beyond acute illness by decreasing growth. In addition, the co-infection of Cryptosporidium with other intestinal pathogens and respiratory cryptosporidiosis are other neglected topics [3]. There is currently no vaccine available, and the only approved treatment, the drug nitazoxanide, is not highly effective in immunocompromised individuals [4].
Characteristics of the biology of Cryptosporidium such as lifecycle completion within an individual host, zoonotic transmission, prolonged survival in environments, high probability of infection at low doses coupled with high excretion rate, substantial transmission through water and resistance to traditional water treatment technique are determinant to understand how environmental and social factors interact favoring the transmission of this parasite [5]. Among the factors that affect the incidence of Cryptosporidium, low socioeconomic status, crowded living conditions, presence of animals (pigs, cats, dogs, rodents, etc.), food storage, non-potable water consumption and rainy season are mentioned [2,6]. A model-based inventory of Cryptosporodium emissions to surface water developed by Hofstra et al [7] proposed large cities in China, India and Latin America, principally, as hot-spot areas. The authors suggested a more in-depth research attention regarding oocyst inactivation, specification for different genotypes, human and livestock emissions, the role of wildlife, wastewater treatment efficiency and runoff fraction for a better understanding of the causes of Cryptosporidium- related health problems.
Cities represent artificial ecosystems that are in constant transformation. According to reports from United Nations (UN), more than half of the world’s population lives in urban areas and the higher urbanization rates take place in less developed regions [8]. Uncontrolled urban growth result in large health inequities and several rural pathogens have adapted to urban environments [9]. The most problematic areas with regards to safe disposal of human faeces, and thus to Cryptosporidium emission to the environment, are likely to be urban slums [10]. Studies conducted in urban slums worldwide have shown that the main responsible of cryptosporidiosis cases were C. hominis and the IIc subtype of C. parvum, revealing that anthroponotic transmission dominate in this type of informal settlements [11–14]. These results are in concordance with a recent review performed by King et al demonstrating that anthroponotic transmission of Cryptosporidium predominates primarily in lowerincome countries with poor sanitation, highlighting that the need of improving sanitation provision may be the most important intervention to reduce the burden of the disease [15]. However, zoonotic transmission should not be neglected, since zoonotic species and genotypes of Cryptosporidium (C. parvum (not IIc), C. felis, C. meleagridis, C. muris, C. canis) were detected in human stool samples [11,14,16] and urban raw water samples [17,18]. Additionally, different genotypes of Cryptosporidium were detected in rats, dogs and cats, suggesting that urban pest and pet animals could be reservoirs for human cryptosporidiosis and potential sources of urban water contamination [19–22]. But studies involving a holistic approach considering the environment, animal reservoirs and human being in the transmission of Cryptosporidium in urban ecosystems are still lacking (Figure 1).
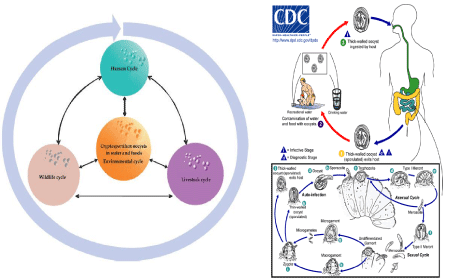
Figure 1: Life Cycle of Cryptosporidium: (a) Cryptosporidium spp. has
a worldwide distribution and the ability to infect a wide range of hosts,
including humans, and a broad variety of vertebrate. Humans can acquire
cryptosporidiosis through several transmission routes, such as direct
contact with infected persons or animals and consumption of contaminated
water (drinking or recreational) or food (extracted from [25]). (b) Sporulated
oocysts, containing 4 sporozoites, are excreted by the infected host through
feces and possibly other routes such as respiratory secretions. Zoonotic
and anthroponotic transmission occur through exposure to infected animals
or exposure to water contaminated by feces of infected animals. Following
ingestion (and possibly inhalation) by a suitable host, excystation (a) occurs
and sporozoites are released and parasitize epithelial cells (b, c) of the
gastrointestinal tract or other tissues such as the respiratory tract. In these
cells, the parasites undergo asexual multiplication (d, e, f) and then sexual
multiplication (g, h). Upon fertilization (i), oocysts (j, k) develop in the infected
host. Two different types of oocysts are produced, the thick-walled, which is
commonly excreted from the host (j), and the thin-walled oocyst (k), which
is primarily involved in autoinfection. Oocysts are infective upon excretion,
thus permitting direct and immediate fecal-oral transmission (extracted from
Center of Disease Control: https://www.cdc.gov/parasites/crypto/pathogen.
html)
To recommend strategies to predict, prevent, respond to, and mitigate infectious diseases, it is necessary to understand the link among human beings, animals and environment [23]. A One Health approach promises a better understanding of how to prevent and control emerging infectious diseases at the human-animal-ecosystem interface, requiring therefore a genuine cross-sectoral integration and sustained social and political willingness to achieve control [24]. Cryptosporidiosis is a globally neglected public health issue, particularly in large cities in the developing world. More information under an integrated vision about molecular characterization of the pathogen, zoonotic emissions and environmental drivers in urban areas are required to develop strategies to reduce the transmission of Cryptosporidium. In addition, consensus and updated methodologies that are compatible across countries are required to make the information more comparable and permit future collaborations.
References
- Khalil IA, Troeger C, Rao PC, Blacker BF, Brown A, Brewer TG, et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a metaanalyses study. Lancet Glob Heal. 2018; 6: e758–768.
- Bouzid M, Kintz E, Hunter PR. Risk factors for Cryptosporidium infection in low and middle income countries : A systematic review and meta-analysis. 2018; 4: 1–13.
- Abdoli A, Ghaffarifar F, Pirestani M. Neglected risk factors of childhood morbidity and mortality caused by Cryptosporidium infection. Lancet Glob Heal. 2018; 6: e1068.
- Vijayan K, Kaushansky A. Exciting contributions to the Cryptosporidium Renaissance. Cell Host Microbe. 2019; 26: 5–7.
- Lal A, Baker MG, Hales S, French NP. Potential effects of global environmental changes on cryptosporidiosis and giardiasis transmission. Trends Parasitol. 2013; 29: 83–90.
- Sarkar R, Kattula D, Francis MR, Ajjampur SSR, Prabakaran AD, Jayavelu N, et al. Risk factors for cryptosporidiosis among children in a Semi Urban slum in Southern India: A nested case-control study. Am J Trop Med Hyg. 2014; 91: 1128–1137.
- Hofstra N, Bouwman AF, Beusen AHW, Medema GJ. Exploring global Cryptosporidium emissions to surface water. Sci Total Environ. 2013; 442: 10-9.
- United Nations. The World’s Cities in 2016: Data Booklet.
- Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis. 2011; 11: 131–141.
- Vermeulen LC, De Kraker J, Hofstra N, Kroeze C, Medema G. Modelling the impact of sanitation, population growth and urbanization on human emissions of Cryptosporidium to surface waters - A case study for Bangladesh and India. Environ Res Lett. 2015; 10.
- Essid R, Menotti J, Hanen C, Aoun K, Bouratbine A, Parasitologie-mycologie L De, et al. I Genetic diversity of Cryptosporidium isolates from human populations in an urban area of Northern Tunisia. Infect Genet Evol. 2018; 58: 237–242.
- Ajjampur SSR, Sarkar R, Sankaran P, Kannan A, Menon VK, Muliyil J, et al. Symptomatic and Asymptomatic Cryptosporidium Infections in Children in a Semi-Urban Slum Community in Southern India. Am. J Trop Med Hyg. 2010; 83: 1110–1115.
- Bushen OY, Kohli A, Pinkerton RC, Dupnik K, Newman RD, Sears CL, et al. Heavy cryptosporidial infections in children in northeast Brazil : comparison of Cryptosporidium hominis and Cryptosporidium parvum. Trans R Soc Trop Med Hyg. 2007; 378–384.
- Mbae C, Mulinge E, Waruru A, Ngugi B, Wainaina J, Kariuki S. Genetic Diversity of Cryptosporidium in Children in an Urban Informal Settlement of Nairobi , Kenya. PlosOne 2015; 10: e0142055.
- King P, Tyler KM, Hunter PR. Anthroponotic transmission of Cryptosporidium parvum predominates in countries with poorer sanitation : a systematic review and meta-analysis. Parasites and Vectors. 2019; 12: 16.
- Gatei W, Wamae CN, Mbae C, Waruru A, Mulinge E, Waithera T, et al. Cryptosporidiosis: Prevalence, genotype analysis, and symptoms associated with infections in children in Kenya. Am J Trop Med Hyg. 2006; 75: 78–82.
- Feng Y, Li N, Duan L, Xiao L. Cryptosporidium Genotype and Subtype Distribution in Raw Wastewater in Shanghai, China : Evidence for Possible Unique Cryptosporidium hominis Transmission. J Clin Microbiol. 2009; 47: 153–157.
- Zhou L, Singh A, Jiang J, Xiao L. Molecular Surveillance of Cryptosporidium spp. in Raw Wastewater in Milwaukee: Implications for Understanding Outbreak Occurrence and Transmission Dynamics. J Clin Microbiol. 2003; 41: 5254–5257.
- Tan TK, Low VL, Ng WH, Ibrahim J, Wang D, Tan CH, et al. Occurrence of zoonotic Cryptosporidium and Giardia duodenalis species/genotypes in urban rodents. Parasitol Int. 2019; 69: 110–113.
- Zhao W, Wang J, Ren G, Yang Z, Yang F, Zhang W, et al. Molecular characterizations of Cryptosporidium spp. and Enterocytozoon bieneusi in brown rats (Rattus norvegicus) from Heilongjiang Province, China. Parasites and Vectors. 2018; 11: 1–7.
- Ayinmode AB, Obebe OO, Falohun OO. Molecular detection of Cryptosporidium species in street-sampled dog faeces in Ibadan , Nigeria. Vet Parasitol Reg Stud Rep. 2018; 14: 54–58.
- Li W, Li Y, Song M, Lu Y, Yang J, Tao W. Prevalence and genetic characteristics of Cryptosporidium , Enterocytozoon bieneusi and Giardia duodenalis in cats and dogs in Heilongjiang province , China. Vet Parasitol. 2015; 208: 125–134.
- Schneider MC, Machado G. Environmental and socioeconomic drivers in infectious disease. Lancet Planet Health. 2018; 2: e198–199.
- Degeling C, Johnson J, Kerridge I, Wilson A, Ward M, Stewart C, et al. Implementing a One Health approach to emerging infectious disease: Reflections on the socio-political, ethical and legal dimensions. BMC Public Health. 2015; 15: 1–11.
- Carneiro Santos HL, Mastropasqua Rebello K, Bergamo Bomfim TC. State of the Art and Future Directions of Cryptosporidium spp. IntechOpen. 2019.