Research Article
Austin Trop Med Care. 2020; 3(1): 1003.
Usefulness of IgM-ELISA Test for Screening of Leptospirosis in Cuba
Obregón AM*, Echevarría E, Lugo O and Soto Y
Department of Bacteriology and Micology, National Reference Laboratory on Leptospirosis and Brucellosis, Tropical Medicine Institute Pedro Kourí, Havana, Cuba
*Corresponding author: Obregón AM, Department of Bacteriology and Micology, Tropical Medicine Institute Pedro Kourí, Havana, Cuba
Received: November 28, 2019; Accepted: January 02, 2020; Published: Januray 09, 2020
Abstract
Introduction: Leptospirosis is a common cause of acute febrile illness in many tropical regions of the world. Early diagnosis is essential, since untreated cases can progress rapidly and mortality rates are high in severe cases. According to the observations of the Cuban National Reference Laboratory, non-reactive serologies are prevailing in most suspected cases of human leptospirosis.
Objective: To apply the IgM-ELISA test for screening of IgM antibodies using sera from patients with the acute phase of the illness.
Material and Methods: In the current study, 31 pairs of sera and 140 single sera from 337-suspected patients with leptospirosis were tested by two methods, a commercial IgM-ELISA test for Leptospira and Microagglutination Test (MAT).
Results: IgM-ELISA test results were concordant with MAT results in 90.0% (28/31) of paired sera and 88, 6% (124/140) of single sera. The following serogroups: Icterohaemorrhagiae 23,74% (18/76), Pomona 22.3% (17/76), Canicola 13.1% (10/76), and Ballum 5.2% (4/76) were the most frequently found in sera testing positive by IgM-ELISA. Positive IgM-ELISA sera were predominantly those taken from 5th to the 8th day of the acute phase of the illness. Some samples taken from day zero to the 28th day were also positive, suggesting a high sensitivity of this test.
Conclusion: IgM-ELISA test is useful for screening of human leptospirosis, particularly if using sera taken from days 5-8 of surveillance, which reduce the under reporting of leptospirosis cases in Cuba.
Keywords: Leptospirosis; Diagnosis; Igm-ELISA Test; Serology
Introduction
Leptospirosis is a common cause of acute febrile illness throughout the wet, tropical regions of the world. This disease is a spirochete zoonosis that causes a wide spectrum of clinical manifestations [1]. Early diagnosis is essential, since disease in untreated patients can progress rapidly, treatment is widely available and effective if started early, and mortality rates are high in severe leptospirosis. In addition, it is important to differentiate leptospirosis from other acute febrile illnesses [2].
Most cases of leptospirosis are diagnosed by serology because of limited capacity for culture and PCR in endemic areas of the world [3]. Serological methods can be divided into those which are genus-specific and those which are serogroup-specific. Conventional tests include the culturing of leptospires from clinical samples, the Microscopic Agglutination Test (MAT), which is the reference test and considered the global gold-standard, and the Enzyme-Linked Immunosorbent Assay (ELISA). Both serological tests, MAT and ELISA, detect antibodies against leptospires. However, both tests have several drawbacks, such as requiring technical expertise, and they may be laborious, unreliable, slow and/or expensive [4]. To address these issues, several rapid screening tests for detection of leptospiral antibodies during acute infection have been developed, although none are used in large-scale screening programs, and MAT and ELISA remain the preferred methods [5].
Detection of IgM antibodies by ELISA has been employed widely, most often using a conserved leptospiral antigen prepared from cultures of Leptospira biflexa, a species largely considered non-pathogenic to humans, although pathogenic species have also been used. Several IgM-ELISA preparations/kits are available commercially. Recombinant antigens have also been employed, but none has been evaluated for widespread screening programs [6]. The specificity of IgM-ELISA tests are variable, affected by the specific antigen used, the presence of antibodies resulting from previous exposure to Leptospira (in endemic regions), or by the presence of other diseases [7]. IgM antibodies become detectable during the first week of leptospirosis (5–7 days after the onset of symptoms), meaning that testing during that critical window allows the optimal laboratory confirmation of suspected leptospirosis diagnosis in order that treatment to be initiated at the most effective stage [5].
Around 1980, the first “in-house” IgM ELISA was reported for the diagnosis of human leptospirosis [8]. Later, another like it was produced for the detection of Leptospira-specific IgM antibodies, using a well grown culture of the Wijnberg strain (serovar Copenhageni, serogroup Icterohaemorrhagiae) [9]. Since then and to date, the use of IgM-ELISA test for diagnosis of human leptospirosis has gained wide acceptance and use in various settings [10-13].
In Cuba, where leptospirosis is important public health matter, the National Program for the Prevention and Control of Leptospirosis was created in 1981. Statistical analysis of surveillance data reveals an endemic-epidemic behavior of leptospirosis in Cuba, with a cyclical and seasonal nature. The climatic and geographic factors of the region influence this behavior and facilitate periodic outbreaks and epidemics [14].
The first “in-house” ELISA used in Cuba for human leptospirosis was developed in 1994 [15]. This antitotal-ELISA was designed for laboratory diagnosis in patients from established high-risk groups [15], as well as individuals vaccinated with Vaxspiral®, a new human vaccine against leptospirosis [16]. However, it was not until 2013 that a commercial SD-Leptospira IgM-ELISA test (BIO-LINE Standard Diagnostics, INC, Korea) was implemented for screening of leptospirosis [17].
Taking in account the laboratory’ results about human leptospirosis in Cuba, several non-reactive serologies by SD Leptospira IgM-IgG test (BioLINE Standard Diagnostics, INC, 2011 (http://www. standardia.com) or Hemaaglutination Test (HAT), are prevailing. These cases are not confirmed, not being notified to the National Control Program. The present paper discusses the results by the commercial SD-Leptospira IgM-ELISA, as rapid screening tests for leptospirosis. At the same time, this investigation tries to estimate the ideal time for taken a positive unique single serum, for this SDLeptospira IgM-ELISA test.
Material and Methods
Clinical material
For this laboratory study, a convenience sample from 337 patients suspected as having leptospirosis (31 paired sera and 140 single sera) was tested. These sera were submitted to the national laboratory for testing. The specimens came from blood samples that were taken from January 2016 to March 2017.
IgM-ELISA test
Sera were tested using a commercial SD-Leptospira IgM-ELISA test, from BIO-LINE Standard Diagnostics, INC, 2011 (http://www. standardia.com). Briefly, patient sera to be tested, negative control serum, and positive control serum were diluted 1:100 by adding 10 μL of serum to 990 μL of diluent solution. Negative control serum was run in triplicate (three wells of the plate) and positive control serum was run in duplicate (two wells). One hundred μL of the diluted sera and controls were added to wells, and the plate covered with adhesive paper. The reaction plate was incubated at 370C for 30 minutes. Subsequently, the plate was washed five times with 350 μL/well of wash buffer, allowing at least 10 seconds to pass between each wash. The final wash cycle was carefully aspirated. The conjugate antibody was diluted with the enzyme to 1:100 by adding 0.2 mL of conjugate to 20 mL of diluent; 100μl of the diluted conjugate was added to each well, and the plate was again covered with adhesive paper for a subsequent incubation at 370C for 30 minutes. Substrate A was mixed 1: 1 with substrate B, and 100 μL/well was added. The plate was incubated for 10 minutes at laboratory temperature (15- 250C). Finally, 100 μL/well of the reagent was added. The absorbance was read in a spectrophotometer at a wavelength of 450 nm, using a reference wavelength of 620 nm.
The Optical Densities (ODs) of the positive and negative control sera were calculated, and the average of each was taken. In the same way, the calculation of the averages ODs of the patient sera was performed. The cut-off value was determined by adding the constant of 0.7 to the value of OD of the negative control. The result was considered negative if the OD of the patient sample had a value lower than the cut-off. The result was considered positive if the OD had a value greater than the cut-off.
MAT
The microagglutination test was used as the reference test [5]. In MAT, patient sera are reacted with live antigen suspensions of multiple Leptospira serovars. After incubation at 28oC the serumantigen mixtures are examined microscopically for agglutination and the titers are determined. The specific antigens used in this analysis included serovars representative of all serogroups and locally common serovars (Table 1). The MAT endpoint titer was considered as the highest serum dilution where 50% of leptospires were agglutinated and 50% of leptospires appeared free, by comparison with the control suspension.
TABLECREATED
Table 1: List of serovars used as antigens by MAT.
All information used in this paper was obtained from the primary registry, and data management was conducted using Microsoft Excel package (MICROSFT OFFICE 7.0, Microsoft Corporation, and USA). Data analysis was performed in the Epidat 3.1 statistical package (Manufacturer and Headquarters). Concordance between results obtained from IgM-ELISA and MAT are estimated and presented as percentages, considered as a validity index, with 95% confidence intervals (CI) and considering a p-value ≤ 0.05 as statistically significant. All the procedures were developed as a Branch Project of the Ministry of Public Health, previously approved by the Specialized Scientific Commission of Microbiology and the Research Ethics Committee of the IPK. Good laboratory practices were strictly adhered to, including performing laboratory measures.
Results
Of the 31 patients tested using serum pairs, 22 (71.0%) were considered positive for leptospirosis by IgM-ELISA (Table 2).
TABLECREATED
Table 2: Concordance between testing results obtained through SD-Leptospira IgM-ELISA and MAT using sera from patients with suspected leptospirosis.
The median time between the paired sera collections was 3 days. For patients tested by single serum, 50.0% (70/140) were positive, for an overall prevalence anti-leptospiral IgM of 53.8% among patients tested during this study.
The concordance between the IgM-ELISA and MAT testing approaches was 90% (28/31) for paired sera and 89% (124/140) for single sera.
Specific subgroups of Leptospira implicated for infection in samples were: Icterohaemorrhagiae for 24% (18/76), Pomona for 22.3% (17/76), Canicola for 13.1% (10/76), and Ballum for 5.2% (4/76).
Figure 1 shows a temporal distribution of leptospirosis positivity obtained by testing single and paired sera using SD-Leptospira IgMELISA with reference to the number of days from symptom onset to sample extraction.
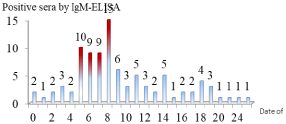
Figure 1: Distribution of positive sera by IgM-ELISA, according to the number
of days between the onset of clinical symptoms and the date of sample
collection.
SD-Leptospira IgM-ELISA was more sensitive (43/93) when detecting IgM antibodies on specimens collected between the 5th and 8th day after symptoms began. In a lesser extent, ELISA successfully detected sera from day zero to the 28th day. These results document the wide range of IgM antibody duration in serum during leptospirosis infection.
Discussion
Even though MAT is considered the international gold standard serology diagnostic for leptospirosis, it is inadequate for early disease detection. Importantly, it is insensitive when used to test early, acute-phase serum specimens, and confirmation of current acute infection requires testing of paired sera to document seroconversion. The detection and quantification of antibodies in serum, and thus the interpretation of MAT, depends very much on the presentation and timing of the specimens from the onset of clinical symptoms and relative to each other. These features may vary with individual prodrome or incubation period. If clinical symptoms are known to be already present, then an interval of 3–5 days between the paired sera may be adequate to detect rising antibody titers. However, if the date of symptom onset is not reported or not documented, an interval of between 10–14 days is recommended. Less often, seroconversion does not occur with such rapidity. For these reasons, the most appropriate application of MAT is in epidemiological sero-surveys [5]. Other testing algorithms, such as employing ELISA, are more useful in acute-phase diagnostics.
There is no international consensus established as to the ideal timeframe during the leptospirosis infection in which to collect serum samples for diagnostic serology. In particular, no sampling timeframe has been established for specimens destined for IgM antibody detection by ELISA. In prior studies, the highest concentration of IgM antibodies against leptospires have been observed during various windows, from between the 5th to the 8th day after the onset of clinical symptoms [18] to between the 10th to 25th day after onset [19]. Some objective and subjective factors may have influenced in the difference shows with others papers, some of them are the technical differences between the systems used in each study, the origin of the cases, the sample size, the type of antibodies detected by ELISA, the prevalence of the disease in each geographical region, the immune level of the people that acquire the natural infection, and the kinetics of antibodies in each individual. This new study in Cuba suggests that specimens collected during the earliest of these timeframes are most valuable for IgM detection by ELISA. The lack of consensus and variability in prior findings, along with the need to confirm the application of ELISA for acute-phase diagnosis of leptospirosis in place of MAT was the motivation for the current investigation.
In Cuba, there is already evidence of the utility of ELISA for detecting leptospirosis. One serology study tested 58 paired sera from patients with leptospirosis and compared them to sera from 200 supposedly healthy donors and 88 patients with nonleptospiral infections (14 patients with meningitis, 2 with bacterial acute respiratory infections, 34 with hepatitis B, 8 with serology positive to syphilis, 20 with toxoplasmosis, 5 with rubella, and 5 with measles). The sensitivity and specificity of the “in-house” antitotal- ELISA were 88.0% and of 90.5%, respectively, while the concordance between ELISA and MAT was 65% [15]. A second report, using the same “in house” ELISA tested paired sera from 100 confirmed leptospirosis patients, 200 supposedly healthy people, 25 suspected leptospirosis cases linked to an outbreak, 200 people considered highrisk (occupational exposure in rice fields), 40 healthy infants, and 35 children with pathologies related clinically to human leptospirosis. These antitotal-ELISA compared with the MAT demonstrates a range of positivity from 62 to 100% and the negativity from 50 to 90% [20]. In 2004, the same “in house” antitotal-ELISA was used on sera from 38 individuals who had received vaxSpiral® and 33 who received placebo vaccinations and found 32% of vaccinated (although this varied by vaccine dosage) and 9% of unvaccinated were seropositive. Finally in 2013, the first study to evaluate the SD-Leptospira IgMELISA in Cuba revealed a sensitivity of 95% and specificity of 41% [21], and in the second one, the 30% (11/33) of the sera were positive by SD-Leptospira IgM-ELISA, using suspect cases from different part of the country [17].
In any leptospirosis diagnostic test, it is important to understand the local epidemiology. Namely, locally circulating strains and serogroup types can drive the utility of specific tests. In Cuba, in sera tested positive by SD-Leptospira IgM-ELISA, the identified serogroups were predominantly Icterohaemorrhagiae (24%; n=18), followed by Pomona (22.3%; n=17), Canicola (13,1%; n=10), Cynopteri (8%; n=6) and Ballum (5.2%; n=4) serogroups. This demonstrates the broad reactivity of the SD-Leptospira IgM-ELISA to multiple pathogenic serogroups, an important advantage for the widespread and commercial implementation in the diagnosis of human leptospirosis [17] [22].
It is necessary to highlight that scientific works report multiple Leptospira serogroups circulating in Cuba during recent years. In 2002, Ballum, Pomona, Canicola, Pyrogenes, Autumnalis and Bataviae serogroups were identified (Rodríguez et al., 2002). In 2003, Ballum, Pomona, Canicola and Icterohamorrhagiae serogroups were associated with a leptospirosis epidemic in Santa Clara City [23]. Between 2006 and 2008, Pomona, Canicola, Icterohamorrhagiae, Ballum, and Hebdomadis/Lousiana were confirmed in Leptospira isolates [24], and isolates from 2007 were of Pomona (63%) and Canicola (31%) serogroups [25]. Another 2007 study found 293 patients associated with epidemic events had leptospirosis from Canicola, Ballum, Icterohamorrhagiae and Pomona serogroups [26]. Between 2007-2014, from 79 isolates (21 from Las Tunas province and 58 from Holguín province) consisted of Ballum Guangdong, Ballum Arborea, Ballum Ballum, Pomona Pomona, Pomona Mozdok, Pomona Proechimis, Canicola Canicola, Icterohamorrhagiae Copenhageni and Icterohamorrhagiae Icterohamorrhagiae [27]. The diversity of Leptospira on a local level is important to understand, with respect to disease detection; while MAT requires knowledge of and access to locally circulating types, ELISA may be more generally sensitive to detect all strains classified as pathogenic. In fact, the serogroups predominant in this study coincides with those reported by others in Cuba, validating the utility of ELISA for detecting patient’s positive for patients infected by locally relevant Leptsopsira subgroups and enable the implementation of measures for the prevention and control of the disease at the national level.
Conclusion
SD-Leptospira IgM-ELISA is a valuable and necessary tool for detection of Leptospira infection during the acute phase of disease. This method is classified as “fast” if compared with the culture as a gold technique, or with the MAT, as an international reference technique for the serodiagnosis of human leptospirosis. As demonstrated in this study, it is valid for antibody recognition over a wide range of sera specimens, with respect to the timing of sample collection, which in practice is highly variable. Specifically, IgM antibodies against leptospires were most effectively detected during the interval from the 5th to the 8th day after the onset of clinical symptoms. Thus, the IgM ELISA constitutes an efficient and scientifically sound diagnostic tool, having a high generic reactivity. This is consistent with prior reports justifying the use of IgM-ELISA systems for the serodiagnosis of human leptospirosis, particularly in Cuba, which show good sensitivity and specificity in cross-sectional and case-control studies.
We here in report that the Cuban scientific research community confirms the usefulness and validity of the IgM-ELISA system for the diagnosis of acute human leptospirosis, using both single serum or paired sera samples, when available. The implementation of ELISA for diagnosis, disease surveillance, and the evaluation of the reactogenicity and immunogenicity of the vaxSpiral® vaccine in Cuba has immense public health implications. National programs for detecting and confirming outbreaks and for the national serological disease surveillance will strengthen prevention and control of leptospirosis epidemics in Cuba. Similar programs could be developed for nearby and other similar environments to bolster public health efforts on a regional and global scale.
Acknowledgments
We gratefully acknowledge the assistance of all Cuban laboratory staff, for obtaining the samples from patients included in this study. Also, we would like to give, the thanks to Dr. Rebecca Fischer, for the review of this manuscript.
References
- Acha PN, Seyfres B. Zoonosis y enfermedades transmisibles comunes al hombre y a los animales. 3era ed. Washington, DC: OPS. 2001.
- Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and reemerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011; 17: 494-501.
- Koizumi N, Gamage CD, Muto M, Kularatne SA, Budagoda BD, Rajapakse RP, et al. Serological and genetic analysis of leptospirosis in patients with acute febrile illness in kandy, Sri lanka. Jpn J Infect Dis. 2009; 62: 474-475.
- Levett PN. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis. 2003; 36: 447- 452.
- Faine S, Adler B, Bolin C, Perolat, P. Guidelines for the control of leptospirosis. Geneva: World Health Organization. 2003; 1-150.
- Terpstra WJ, Ligthart GS, Schoone GJ. ELISA for the detection of specific IgM and IgG in human leptospirosis. J Gen Microbiol. 1985; 131: 377-385.
- Bercovich Z, et al. Evaluation of an ELISA for the diagnosis of experimentally induced and naturally occurring leptospira hardjo infections in cattle. Vet. Microbiol. 1990; 21: 255-262.
- Adler B, Murphy AM, Locarnini S.A, Faine S. Detection of Specific Anti- Leptospiral Immunoglobulins M and G in Human Serum By Solid Phase Enzyme-Linked Immunosorbent Assay. J. Clin. Microb. 1980; 11: 452-457.
- Céspedes M, Glenny M, Felices, V, Balda L, Suárez V. Prueba de ELISA indirecta para la detección de anticuerpos IgM para el diagnóstico de la leptospirosis humana. Rev Perú Exp Med Salud Pública. 2002; 19: 27-24.
- Céspedes M, Balda JL, Glenny M. Evaluación de dos ensayos de ELISA- IgM en la investigación de un brote de leptospirosis. Rev Perú Med. Exp. Salud Pública. 2008; 25: 333-335.
- Cumberland PC, Everard CO, Levett PN. Assessment of the efficacy of the IgM–ELISA and the MAT in the diagnosis of acute leptospirosis. Am J Trop Med Hyg. 1999; 61: 734-731.
- Da Silva MV, Camargo E.D. Teste immunoenzimatico (ELISA) para deteccao da anticorpos circulantes da clase IgA na leptospirose humana. Rev. Inst. Med. Trop. Sao Paulo. 1992; 34: 239-242.
- Da Silva MV, Camargo ED, Vaz AJ, Batista L. Immunodiagnosis of human leptospirosis using saliva. Trans. of the Royal Soc. of Trop. Med. and Hyg. 1992; 86: 560-561.
- Ministerio de Salud Pública. Programa Nacional de Prevención y Control de la Leptospirosis. La Habana: MINSAP. 1998; 3-5.
- Obregón AM, Otero A, Laferte J. ELISA for Detection of Human Leptospirosis. Mem Inst Oswaldo Cruz. 1994; 89: 365.
- Obregón AM, Martínez G, Martínez R, Llop A, Rodríguez I, Rodríguez J, et al. Respuesta serológica por ELISA y MAT en voluntarios cubanos vacunados con vaxSpiral®. Rev Cub Med Trop. 2004; 56: 148-151.
- Obregón AM, Fernández C, Rodríguez I, Rodríguez Y, Echeverría E, Rodríguez J, et al. Detección de anticuerpos IgM contra leptospiras por un sistema comercial ELISA-IgM. Rev Cubana de Med Tropical. 2013; 65: 202- 210.
- Adler B, Levett PN, Cameron CE, Picardeau M, Haake DA, Ellis WA. et al. Leptospira and leptospirosis. New York. 2015; 387: 1-289.
- Vanasco NB, Lottersberger J, Schmeling MF, Gardner IA, Tarabla HD. Diagnóstico de leptospirosis: evaluación de un enzimoinmunoensayo en fase sólida en diferentes etapas de la enfermedad. Rev Panam Salud Pública. 2007; 21: 388-395.
- Obregón AM, Calderón A, Espinosa I. Aplicación del sistema ELISA indirecto para la detección de anticuerpos totales a leptospira en diferentes grupos de estudio. Boletín Soc Venez de Microb. 1995; 15: 6.
- Rodríguez I, Martínez R, Zamora Y, Rodríguez JE , Fernández C, Obregón AM. Respuesta de anticuerpos IgG antileptospira en individuos inmunizados con vax-SPIRAL. Rev Cubana Med Trop. 2005; 57: 32-37.
- Tanganuchitcharnchai A, Smythe L, Dohnt M, Hartskeerl R, Vongsouvath M, Davong V. et al. Evaluation of the Standard Diagnostics Leptospira IgM ELISA for diagnosis of acute leptospirosis in Lao PDR. Trans R Soc Trop Med Hyg. 2012; 106: 563-566.
- Obregón AM, Fernández C, Rodríguez,I, Rodríguez J, Fernández N, Enrique G. Importancia de la confirmación microbiológica en un brote de leptospirosis humana en la ciudad de Villa Clara. Rev Cubana Med Trop. 2003; 55: 96-99.
- Zamora Y, Rodríguez Y, Obregón AM, Rodríguez I, Rodríguez J, Vasquez V, et al. Caracterización de cepas de leptospiras circulantes en Cuba en el periodo 2006-2008. International Course on Laboratory Methods for the diagnosis of Leptospirosis. La Habana. 2009.
- Obregón A.M, Fernández C, Rodríguez I, Rodríguez J, Zamora Y. Avances de laboratorio en el diagnóstico serológico y la investigación de la leptospirosis humana en Cuba. Rev Cubana Med Trop. 2007; 59: 63-67.
- Rodríguez I, Fernández C, Obregón A M, Zamora Y, Rodríguez J E, Rodríguez NM. et al. Confirmación microbiológica de 2 brotes emergentes de leptospirosis humana en Cuba. Rev Cubana Med Trop. 2007; 59: 19-23.
- Rodríguez Y, Echeverría E, Rodriguez J, Valdés Y, Rodriguez I, Obregón AM. Leptospira serovars circulating in eastern region of Cuba, from 2007 to 2012. J Vet Med Res. 2018; 5: 1143.