
Research Article
Austin J Trop Med & Hyg. 2015;1(1): 1002.
In vitro Assessment of Two commercial Honey Samples for Antibacterial and Antioxidant Activities
Amit Saha and Shyamapada Mandal*
Department of Zoology, University of Gour Banga, India
*Corresponding author: Shyamapada Mandal, Laboratory of Microbiology and Experimental Medicine, Department of Zoology, University of Gour Banga, Malda 732103, India
Received: November 17, 2014; Accepted: December 15, 2014; Published: January 05, 2015
Abstract
The current communication represents the antibacterial and antioxidant activities of two commercial honeys: Dabur Honey (DH) and Patanjali Honey (PH) against clinical isolates of Proteus vulgaris, Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. The antibacterial activities of PH and DH (both autoclaved and non-autoclaved), were determined alone and in combination with antibiotics, Gentamicin (GM) and Kanamycin (KM) against the test isolates. The autoclaved PH and DH (at concentrations 10x103 and 15x103μg/disc) had Zone of Inhibition Diameter (ZID) 9 - 17 mm and 9 - 13 mm, respectively, against the gram-negative bacteria (P. vulgaris, E. coli, Ps. aeruginosa); S. aureus was resistant to almost all concentrations of the honeys. The non-autoclaved honeys (at concentrations 10x103 and 15x103μg/disc) showed excellent activity against both gram-positive (S. aureus) and gramnegative bacteria tested (PH honey had ZID 10-27 mm, and DH honey had ZID10-30 mm). The IC50 values of PH and DH, in 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) system, were 132.24x103 μg/ml and 66.73x103μg/ml, respectively; both the honeys contained steroids, quinones and terpenoides. In combination with KM and GM the autoclaved honey samples (PH and DH) had synergistic activity against S. aureus and E. coli ATCC 25922 standard strain, while GM-honey and KM-honey combinations had synergistic interaction against Ps. aeruginosa and P. vulgaris, respectively. Thus, PH and DH alone and in combination with GM/ KM can be used against different bacterial strains causing infection to humans.
Introduction
Honey has been in use for its healing, nutritional and therapeutic properties since ancient times, and currently it has been proved experimentally that honey possesses anti-bacterial, anti-inflammatory and anti-oxidant properties, which may be beneficial in combating multi-drug resistant bacteria as well as in preventing many chronic inflammatory processes [1]. Honey, which is a healthy food stuff and nutrition, serves as a good source of natural antioxidant, and thus it is free radical scavenger reducing the formation of free radicals, or neutralizing them that produce beneficial effects in human health [2]. The improved status of serum total anti-oxidation among young females with regular use of honey revealed it is one of the most acceptable form of food to keep balance between antioxidants and prooxidants minimizing the onset of many diseases [3]. Various studies explained the mechanism of action of different honeys against antibiotic resistant bacteria in vitro and their antibio film activity, and an important clinical advantage is that resistance to honey has not yet been detected in microorganisms causing human infection [4,5].
Mandal et al. [6] determined the antibacterial activity of honey against clinical isolates of Escherichia coli, Pseudomonas aeruginosa and Salmonella enterica serovar Typhi. Das et al. [2,7] investigated the antioxidant properties of various unifloral honeys procured from West Bengal, India. Allen et al. [3,8] have revealed that the honey is effective against Methicillin-Resistant S. Aureus (MRSA), β-haemolytic streptococci and Vancomycin Resistant Enterococci (VRE). Cooper et al. [4,9] discovered the antibacterial activity of honey against strains of S. aureus from infected wounds. Visavadia et al. [5,10] conducted research on manuka (L. scoparium) honey, and showed its activity against several human pathogens, including E. coli, Enterobacter aero genes, Salmonella typhimurium, S. aureus. Hussein et al. [6,11] reported, Gelam honey has anti-oxidative and radical scavenging activities, which are mainly attributed to its phenolic content. Khalil et al. [7,12] discovered antioxidant property of Algerian honey, as indicated by their high phenolic, flavonoid, ascorbic acid and proline contents.
The importance of honey in medical science has already been described by Mandal et al [13]. Thus, honey, both natural and commercial, has been used traditionally over the years by the people in India as food, and as traditional medicine in the treatment of various health disorders, but only a few data based on the scientific studies are available to support the medicinal claims of commonly consumed honey in our part of the globe. Therefore, the current study has been undertaken to investigate the antibacterial and Antioxidative activities of two types of commonly consumed commercial honey samples: Dabur Honey (DH) and Patanjali Honey (PH) purchased from local market (Malda, India); to the best of our knowledge, this is the first study of its kind from our part of the globe.
Materials and Methods
Bacterial strains
The bacterial strains used in the study included E. coli, Ps. aeruginosa, P. vulgaris, and S. aureus; the E. coli ATCC 25922 strain was used as control. The identified bacterial isolates were kindly provided by Dr. N. K. Pal, Professor and Head, Department of Microbiology, Malda Medical College, Malda (India).
Honey samples and disc preparation
Two commercial honeys: Dabur Honey (DH) and Patanjali Honey (PH) were purchased from market (Malda, India), and were utilized in the study. One gram of honey diluted in 5ml of double distilled water (200μg/μl) was autoclaved at 121°C for 15 min. Similarly, non-autoclaved aqueous honey sample (200μg/μl) was also prepared for the study. The PH and DH were subjected to screening tests for bioactive compounds following standard protocol.
Both autoclaved and non-autoclaved honey samples (PH and DH) were utilized in disc preparation. The autoclaved blank paper discs (6 mm diameter; punched from what man No. 1 filter paper) were soaked either with diluted PH or DH, to prepare honey discs of different concentrations: 2.5x103, 5x103, 10x103 and 15x103μg/disc.
Antibacterial activity
The antibacterial activity of PH and DH were determined for the E. coli ATCC 25922, E. coli, Ps. aeruginosa, P. vulgaris, and S. aureus; by disc diffusion method using sterile Nutrient Agar (NA) plates, each of which were inoculated with 108 CFU from young broth culture of the test bacteria. The discs containing different concentrations of honey, as mentioned above, were placed aseptically on the inoculated NA plates, and incubated at 350C for 16-18 h. The sensitivity of the test bacterial isolates to PH and DH (autoclaved and non-autoclaved) were considered with Zone of Inhibition Diameter (ZID) =7 mm.
Antibiotic susceptibility testing
Antibiotic sensitivity testing was performed by disc diffusion method following the NCCLS (National Committee for Clinical Laboratory Standards) guidelines for the bacterial isolates the E. coli ATCC 25922, E. coli, Ps. aeruginosa, P. vulgaris, and S. aureus:, tested against Gentamicin (GM) and Kanamycin (KM). The antibiotic discs, GM (10 μg/disc) and KM (10 μg/disc), were purchased from Hi-Media, India.
In order to determine the combined effect of honey and antibiotic (GM/KM) against the test isolates, PH and DH (both non-autoclaved), two different amount of each of the honey samples:1x103 μg (5 μl) and 10x103μg (50 μl), were dropped on GM and KM discs on the NA plates inoculated with 108 CFU. The ZIDs were recorded after 16- 18 h incubation at 35°C. The combined antibiotic (GM/KM)-honey (PH/DH) activity was considered synergistic when the ZID from the combined action for a given bacterial strain was increased compared to the ZIDs obtained from the single action of both antibiotics and honey samples.
DPPH-free radical-scavenging assay for antioxidant activity
The free radical-scavenging activity for DH and PH was studied following the protocol of Habib et al. [14], through the evaluation of free radical-scavenging effect on 2, 2-Dipheny-1-Picrylhydrazyl (DPPH) radical. To 3.8 ml of methanolic DPPH solution (0.25 mM) aliquots (200 μl) of PH and DH (aqueous solution) at different concentration (25 x 103, 50 x 103, 75 x 103, 100 x 103, 125 x 103 and 150 x 103μg/ml) were mixed, and incubated in the dark for 30 min, following which the absorbance was measured colorimetric ally at 520 nm against methanol without DPPH as blank. The results were expressed as % inhibition of DPPH radical, which was calculated according to the equation: % inhibition of DPPH = Absorption (control) - (Absorption honey)/Absorption (control) x 100; where Absorption (control) is the absorbance of DPPH solution without the test sample (PH and DH). The IC50 values of PH and DH (honey concentration, mg/ml, which scavenges the DPPH radicals by 50 %) were calculated using linear regression of plots where the x-axisrepresented the concentration of honey and the y-axis represented the % inhibition (antioxidant activity).
Results
The ZIDs obtained due to the action of DH (both non-autoclaved and autoclaved), at different concentrations, against clinical isolates of E. coli, P.vulgaris, Ps. aeruginosa and S. aureus are presented in Table 1. The bacterial isolates were resistant to the autoclaved honey at concentrations 2.5x103 -5x103 μg/disc, while the ZIDs ranged 9-16 mm for clinical isolates of E. coli, P. vulgaris, Ps. aeruginosa and S. aureusat concentrations 10x103 -15x103 μg/disc; the S. aureus was resistant to the honey at concentration 15x103 μg/disc. The bacterial isolates of P. vulgaris and S. aureus were resistant to the non-autoclaved honey at concentrations 2.5x103-5x103 μg/disc; Ps. aeruginosa was resistant to the honey (2.5x103 μg/disc) and E. coli was resistant to the honey at concentration 5x103 μg/disc; the ZIDs ranged 10-24 mm for the clinical isolates of E. coli, P. vulgaris, Ps. aeruginosa and S. aureus at concentrations 10x103-15x103μg/disc.
Bacterial strain
ZID (mm) at different concentrations of honey (×103µg/disc)
Autoclaved
Non-autoclaved
2.5
5
10
15
2.5
5
10
15
E. coli ATCC 25922
6
6
13
17
13
18
24
27
E. coli (clinical)
6
6
14
16
8
6
11
11
P. vulgaris
8
6
9
10
6
6
10
12
Ps. aeruginosa
6
6
10
12
6
12
12
13
S. aureus
6
6
9
6
6
6
21
24
DH: Dabur Honey; the values 2.5, 5, 10 and 15 indicate the concentrations of honey (×103 µg/disc), and the values represented below each of the concentrations and against the bacterial isolates indicate the Zone of Inhibition Diameter (ZID; mm).
Table 1: Anti bacterial activity of different concentrations of DH (autoclaved and non-autoclaved) against clinical isolates of bacteria and the standard strain.
The ZIDs obtained due to the action of PH (both non-autoclaved and autoclaved), at different concentrations, against the test isolates are presented in Table 2. The bacterial isolate of S. aureus was resistant to the autoclaved honey at concentrations 2.5x103-5x103μg/ disc; in case of Ps. aeruginosa, E. coli and P. vulgaris ZID ranged 7-10 mm at same concentrations, while the ZIDs ranged 9-13 mm for all the clinical isolates of E. coli, P. vulgaris, Ps. aeruginosa and S. aureus at concentrations 10x103-15x103 μg/disc. The bacterial isolates of P. vulgaris and S.aureus were resistant to the non-autoclaved honey at concentration 2.5x103 μg/disc, while in case of Ps. aeruginosa and E. coli, ZIDs ranged 8-9 mm at the same concentrations. The ZIDs ranged 8-30 mm for clinical isolates of E. coli, P.vulgaris, Ps.aeruginosa and S. aureus in presence of 2.5x103-15x103μg/disc of non-auto claved honey. The susceptibility patterns of E. coli (ATCC 25922 strain), which was used as control, against all honey types are represented in Table 1 and Table 2.
Bacterial strain
ZID (mm) at different concentrations of honey (×103µg/disc)
Autoclaved
Non-autoclaved
2.5
5
10
15
2.5
5
10
15
E. coli ATCC 25922
8
7
9
10
9
11
14
20
E. coli (clinical)
8
10
11
13
9
17
22
21
P. vulgaris
9
10
13
12
6
10
10
20
Ps. aeruginosa
7
8
10
11
8
8
10
15
S. aureus
6
6
10
11
6
23
27
30
PH: Patanjali Honey; the indicative values for Zone of Inhibition Diameter (ZID; mm) and the honey concentrations are as mentioned in the Table 1.
Table 2: Anti bacterial activity of different concentrations of PH (autoclaved and non-autoclaved) against clinical isolates of bacteria and the standard strain.
The ZIDs obtained in antibiotic action alone and in combination with two honey samples against E. coli, P. vulgaris, Ps.aeruginosa and S.aureus isolates are presented in Figure 1. The isolates of E.coli and Ps.aeruginosa showed resistance and P. vulgaris showed intermediately susceptibility to KM; S.aureus was sensitive to KM. The ZIDs obtained due to the action of GM alone and in combination with two honey samples against the test isolates are presented in Figure 2.
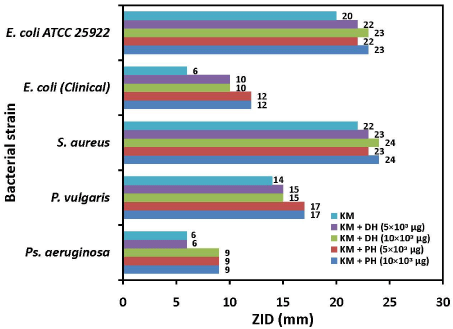
Figure 1: Combined antibacterial activity between KM (kanamicin) and honey samples against clinical bacterial isolates and the standard strain. PH: Patanjali Honey; and DH: Dabur Honey; ZID: Zone of Inhibition Diameter.
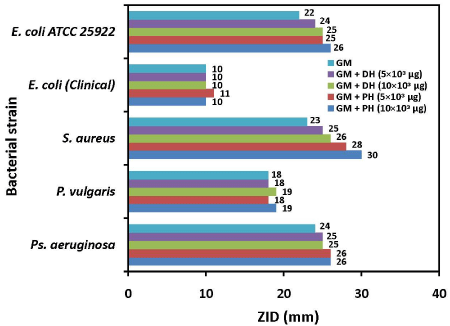
Figure 2: Combined antibacterial activity between GM (gentamicin) and honey samples against clinical bacterial isolates and the standard strain. PH: Patanjali Honey; and DH: Dabur Honey; ZID: Zone of Inhibition Diameter.
The scavenging activity (% inhibition) of the test honey samples (DH and PH) in DPPH system is represented in Figure 3. The lowest inhibition exerted by DH and PH honey samples were 17.24 % and 28 %, respectively, at concentration 25x103 μg/ml, while the highest scavenging activities (55.5 % and 71.78 %, respectively) were due to 125x103μg/ml of honey (DH, PH). The highest concentration of the honey samples used was 150x103μg/ml, at which the scavenging activities were decreased to 42.47 % and 68.43 %, respectively. The IC50 values calculated were 66.73x103μg/ml and 132.24x103μg/ml for DH and PH honeys, respectively.
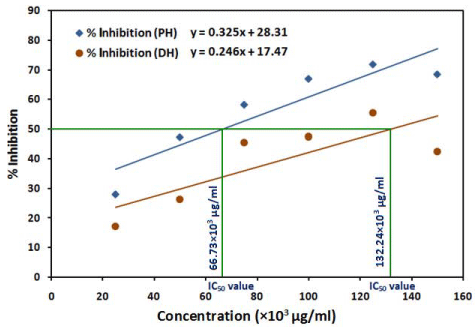
Figure 3: Antioxidant activity of PH: Patanjali Honey and DH: Dabur Honey in DPPH system.
Discussion
In the current study, both the PH and DH (autoclaved and non-autoclaved) had excellent antibacterial activities particularly at concentrations 10x103 and 15x103 μg/disc (Table 1 and Table 2). Rakhi et al. [15], studied the antibacterial activity of five types of natural honeys (Baidhyanath honey; BH, Uttarakhand honey, DH, Wings honey and Alwar) at different concentrations (20 - 100 %; v/v) against E. coli and S. aureus, and found that BH was more effective among all the others, and ZID ranged 14-28 mm for E. coli and 20-36 mm for S. aureus. The honey samples such as DH, crude honey-1 and crude honey-2 exhibited inhibitory effect against E. coli, Salmonella typhi, Ps.aeruginosa and P. vulgaris; the highest antimicrobial activity was found by crude honey-2 (100 %) against E. coli and S. typhi (ZID; 50 mm each) followed by Dabur honey against E. coli (ZID; 48mm) and S. typhi (ZID; 46mm) [16]. The Sesame Honey (SH) and Eucalyptus Honey (EH) exhibited excellent activities against Clostridium acetobutylicum DSM1731 with ZID 18 mm and 25 mm, respectively, while SH was effective against C. perfringens KF383123 strain showing ZID 29 mm [17]. The Karnataka raw honey had ZID 15-17 mm, while the Kerala raw honey had ZID 14-15 mm against the test bacteria; the DH showed least sensitivity (ZID; 8-13 mm) to a number of clinical bacteria [18]. As has been reported by Sharma et al. [19], the ZID due to the action of Himachal Pradesh raw honey was 15-17 mm, while the Rajasthan raw honey showed antibacterial activity with ZID 14-16 mm; ZID was 8-14 mm for DH, against Staphylococcus, Pseudomonas, Bacillus, Streptococcus, P. vulgaris and E.coli. High sugar concentration (approximately 80 % w/v), low pH (3.2-4.5 for undiluted honey) and production of hydrogen peroxide, which upon dilution of honey is produced by glucose oxidase originating from the bees, are probably responsible for the antibacterial activity [19]. The earlier studies indicated too that the honey contains enzymes such as glucose oxidase, diastase, invertase, catalase and peroxidase [20] and these enzymes may play role in the antibacterial activity of honey. Al-Waili [21] reported that the bacterial conjunctivitis caused by a variety of human bacterial pathogens, such as Proteus spp., S. aureus, E. coli, Ps. aeruginosa and Klebsiellaspp., was treated successfully with the topical application of honey. Ilechie et al. [22] documented the potency of Stingless Bee Honey (SBH), which was comparable with that of GM, and suggested that SBH might be a rational agent for the treatment of infective conjunctivitis, since the agent is less expensive and commonly available to the rural population.
The SH showed synergistic effect when combined with cefotaxime (CTX; 30 μg/disc) showing an increase in ZID against C. perfringens KF383123, from 29 mm for SH alone and 8 mm for CTX alone to 40 mm of the combined action; the SH had synergistic effect against C. acetobutylicumin combination with CTX, ciprofloxacin (CP; 5 μg/disc) and tobramycin (TOB ; 30 μg/disc), while the EH showed synergistic activity with CTX, CP, cephalexin (CN; 10 μg/disc), with TOB and Sulphamethoxazole (SMZ; 100 μg/disc) against C. perfringens [17]. MJawad [23] showed increase in the ZIDs of honey, compared to that of antibiotics ceftriaxone, CP and vancomycin, when used in combination with the antibiotics showing synergistic effect on methicillin resistant Staphylococci. Honey samples (5x103 and 10x103μg/ml) used in combination with KM, in the present study, had increased ZIDs compared to the single action of KM for almost all the isolates, but decreased ZID was seen compared to the single action of the honey samples for Ps. Aeruginosa and E. coli clinical isolates; in case of S.aureus and P. vulgaris along with the E. coli ATCC 25922 standard strain increased ZIDs were found in combined action compared to the single actions of both antibiotic and honey indicating synergism between honey and KM. The GM in combination with both the honey samples (PH and DH), in the current study, had greater activity (in terms of ZIDs) for Ps. aeruginosa, S. aureus and E. coli (ATCC 25922), but the ZIDs were similar or increased very slightly (not greater than 1 mm) for P. vulgaris and E. coli clinical isolates. The PH and DH had increased ZIDs for P. vulgaris, Ps. aeruginosa, S. aureus and E. coli (ATCC 25922) in combination with GM, showing synergistic activity against the isolates in combination with GM.
The radical scavenging activity of honey varied from23.81% to 100% in the DPPH reaction, as per the report of Wilczynska [24], who recorded that the dark honeys were highly active in DPPH system. Moniruzzaman et al. [25] showed strong correlation between the color intensities of the honey samples and their antioxidant parameters: phenolic acids, flavonoids, and reported the mean DPPH radical-scavenging activity of the Bangladeshi honey samples as 36.95 %; the highest DPPH radical-scavenging activity being 76.68 %.In the present study, in DPPH system, the honey samples had concentration dependant (25x103 - 125x103μg/ml) antioxidant activities that ranged 17.24 - 55.5 % for DH, and 28 - 71.78 % for PH; at the highest concentration the capacities were reduced to 42.47 % and 68.43 %, respectively (Figure 3). Chinwe et al. [26] reported that the IC50 value of the honey sample tested against DPPH was 12.74x103 μg/ml. The scavenging ability of multifloral honey samples expressed as IC50, with respect to the DPPH, which ranged 3.17x103-8.79x103μg/ml. Pontishoney from the northeast of Brazil had IC50 values 4.2x103- 106.72x103μg/ml, and most of the values were above 20x103μg/ml [27]. In a study conducted by Ferreira et al. [28], the antioxidant values ranged 106.67x103-168.94x103μg/ml, and according to the report of Beretta et al. [29], the values ranged 1.63x103-47.62x103μg/ml. Das et al. [7] determined the antioxidant activities of different unifloral honeys, and reported the dark brown Hizal honey as the most potent DPPH radical scavenger (IC50 = 23.92x103μg/ml). The honey samples used in the current study had IC50 66.73x103μg/ml and 132.24x103μg/ ml, respectively for DH and PH honeys. The antioxidant properties of honey are due to its both enzymatic (catalase, glucose oxidase and peroxidase) as well as non-enzymatic substances (ascorbic acid, a- tocopherol, carotenoids, amino acids, proteins, flavonoids and phenolic acids) [30-33]. In the present study, the presence of steroids, terpinoids and quinones were determined qualitatively.
Conclusion
Based upon the excellent antibacterial activity of the honey samples used, alone or in combination with the test antibiotics, in the current study, and the low IC50 values (66.73x103-132.24 x103 μg/ ml) it can be concluded that the honey, both PH and DH, can be used alone or in combination with KM and GM in combating bacterial antibiotic resistances, as well as a good source of antioxidants; the possible benefits in clinical implications of bacterial infections, however, warrant further investigation [34].
References
- Vallianou NG, Gounari P, Skourtis A, Panagos J, Kazazis C. Honey and its Anti-Inflammatory, Anti-Bacterial and Anti-Oxidant Properties. General Med. 2014; 2: 132.
- Maurya S, Kushwaha AK, Singh S, Singh G. An overview on antioxidative potential of honey from different flora and geographical origins. Indian J Nat Products Resources. 2014; 5: 9-19.
- Dave N, PritikaSanadhya, Patel VH. Effect of Feeding Honey on the Serum Antioxidant Status of Young Females. ResJ Pharma Biol ChemSc. 2014; 5: 645-637.
- Cooper R. Honey as an effective antimicrobial treatment for chronic wounds: is there a place for it in modern medicine? Chronic Wound Care Management Res. 2014; 1: 15-22.
- Ng WJ, Lim KY, Chong JY, Low KL. In vitro Screening of Honey against Enterococcus spp. Biofilm. J Med Bioeng. 2014; 3: 23-28.
- Mandal S, Mandal MD, Pal NK, Saha K. Antibacterial activity of honey against clinical isolates of Escherichia coli, Pseudomonas aeruginosa and Salmonella enteric serovar Typhi. Asian Pac J Trop Med. 2010; 3: 961-964.
- Das A, Mukherjee A, Dhar P. Characterization of antioxidants and antioxidative properties of various unifloral honeys procured from West Bengal, India. IOSR Journal Of Environmental Science, Toxicol Food Technol. 2013; 7: 56-63.
- Allen KL, Hutchinson G, Molan PC. The potential for using honey to treat wounds infected with MRSA and VRE. First World Healing Congress, Melbourne, Australia. 2000; 10-13.
- Cooper RA, Molan PC, Harding KG. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J R Soc Med. 1999; 92: 283-285.
- Kanatas A, Harris A. Re: Visavadia BG, Honeysett J, Danford MH. Manuka honey dressing: an effective treatment for chronic wound infections. Br J Oral Maxillofac Surg. 2006; [Epub ahead of print]. Br J Oral Maxillofac Surg. 2008; 46: 258.
- Hussein SZ, Mohd Yusoff K, Makpol S, Mohd Yusof YA. Gelam honey attenuates carrageenan-induced rat paw inflammation via NF- ?B pathway. PLoS One. 2013; 8: 72365.
- Khalil I, Moniruzzaman M, Boukra L, Benhanifia M, Islam A, Islam N, et al. Physicochemical and antioxidant properties of Algerian honey. Molecules. 2012; 17: 11199-11215.
- Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011; 1: 154-160.
- Habib HM, Ibrahim WH, Schneider-Stock R, Hassan HM. Camel milk lactoferrin reduces the proliferation of colorectal cancer cells and exerts antioxidant and DNA damage inhibitory activities. Food Chem. 2013; 141: 148-152.
- Rakhi K, Chute NG, Deogade MK. Antimicrobial activity of Indian honey against clinical isolates. Asiatic J Biotech Res. 2010; 1: 35-38.
- Gomashe AV, Narad MV, Gulhane PA. In Vitro assessment of the antimicrobial potential of honey against enteric pathogens. Int Res J SciEngg. 2014; 2: 153-157.
- Hegazi A, Sherein I, El-Moez A, Abdou AM, Allah AF. Synergistic Antibacterial Activity of Egyptian Honey and Common Antibiotics against Clostridium Reference Strains. Int.J.Curr.Microbiol.App.Sci. 2014; 3: 312-325.
- Raju EVN, Goli D. In-vitro comparative antimicrobial activity of commercial and raw honey against various bacteria isolated from ear discharge. SchAcad J Pharm. 2013; 2: 5-11.
- Sharma N, Negi S, Kumar A, Patil S, Kumar A. Comparative antimicrobial potential of raw & commercial honey against various bacteria isolated from wound & throat samples. Asian JBiochemPharma Res. 2012; 2: 31-39.
- Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: a review. J Am Coll Nutr. 2008; 27: 677-689.
- Al-Waili NS. Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. J Med Food. 2004; 7: 210-222.
- Ilechie AZ, Kwapong PK, Mate-Kole E, Kyei S, Darko-Takyi C. The efficacy of stingless bee honey for the treatment of bacteria-induced conjunctivitis in guinea pigs. J Experiment Pharmacol. 2012; 4: 63-68.
- MJawad RAH. Antimicrobial effect of bee honey on some pathogenic bacteria isolated from infected wounds in comparison to commonly used antibiotics. JBasrah Res(Sc). 2011; 37: 78-83.
- Wilczynska A. Phenolic content and antioxidant activity of different types of polish honey - a short report. Pol J Food NutrSci. 2010; 60: 309-313.
- Moniruzzaman M, Chua Yung A, Rao PV, Hawlader MNI, Azlan SAB, Sulaiman SA, et al. Identification of phenolic acids and flavonoids in monofloral honey from bangladesh by high performance liquid chromatography: determination of antioxidant capacity. BioMed Res Internat. 2014.
- Chinwe S, Alisi OA, Ojiako CU, Igwe CO, Ujowundu, Anugweje K. Antioxidant Content and Free Radical Scavenging Activity of Honeys of Apismelliferaof Obudu Cattle Ranch.Int J Biochem Res Rev. 2012; 2: 164-175.
- Liberato Mda C, de Morais SM, Siqueira SM, de Menezes JE, Ramos DN, Machado LK, et al. Phenolic content and antioxidant and antiacetylcholinesterase properties of honeys from different floral origins. J Med Food. 2011; 14: 658-663.
- Ferreira ICFR, Aires E, Barreira JCM, Estevinho LM. Antioxidant activity of portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem. 2009; 114: 1438-1443.
- Beretta G, Granata P, Ferrero M, Orioli M, Facino RM. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. AnalyticaChimicaActa. 2005; 533: 185-191.
- Al ML, Daniel D, Moise A, Bobis O, Laslo L, Bogdanov S. Physicochemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009; 112: 863-867.
- Bertoncelj J, Doberšek U, Jamnik M, Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007; 105: 822-828.
- Al-JadiAM, Kamaruddin MY. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004; 85: 513-518.
- Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011; 1: 154-160.
- Sufya N, Matar N, Kaddura R, Zorgani A. Evaluation of bactericidal activity of Hannon honey on slowly growing bacteria in the chemostat. Drug Healthc Patient Saf. 2014; 6: 139-144.