
Case Report
Austin J Trop Med & Hyg. 2015;1(1): 1003.
Fascioliasis and Hepatic Decompensation in a Pegylated Interferon Treated Chronic Hepatitis C Patient
Ashraf Metwaly1, Mohamed H Emara1* and Sameh Saber2
1Tropical Medicine Department, Zagazig University, Egypt
2Radiodiagnosis Department, Zagazig University, Egypt
*Corresponding author: Mohamed H Emara, Tropical Medicine Department, Faculty of Medicine, Zagazig University, Zagazig, PO box 44519, Egypt
Received: November 17, 2014; Accepted: December 19, 2014; Published: January 05, 2015
Abstract
Fascioliasis is a zoonotic parasitic disease that affects the liver and the biliary system and is endemic in many countries. We reported a 49-year-old chronic HCV patient who developed virological breakthrough and hepatic decompensation while under treatment with Pegylated interferon and ribavirin. The deterioration was possibly linked to superimposed Fasciola infection. Multiple courses of anti-Fascioliasis drugs achieved stabilization of the patient's condition while interferon therapy was permanently discontinued.
Keywords: Fascioliasis; Hepatic Decompensation; Chronic Hepatitis C; Pegylated Interferon
Introduction
Human Fascioliasis is a zoonotic disease caused by the trematodes of the genus Fasciola (Fasciola hepatica and Fasciola gigantica). Fascioliasis is distributed worldwide, with cases reported from more than 50 countries [1]. The liver fluke eggs are passed out in feces. Each egg hatches, releasing a larva called miracidium. The miracidia larvae invade aquatic snails (the genus Lymnaea). Inside the snails, they develop into cercariae. Cercariae exit the snails and adhere to aquatic plants where they form cysts called encysted metacarcariae. Animals or humans are infected through ingestion of water plants contaminated with encysted metacarcariae. The flukes penetrate through the intestinal walls, enter the abdominal cavity, and migrate to the host's liver and bile ducts, causing parenchymal injury with necrosis, biliary fibrosis, dilatation, or obstruction [2].
Chronic Hepatitis C (HCV) is a global health problem that affects more than 170 million people worldwide [3], particularly in Egypt, where high prevalence rates were reported reaching up to 20% [4]. Years ago a nationwide control program for HCV was conducted with more and more cases being treated by the standard of care Pegylated interferon and ribavirin combination therapy.
Case Presentation
A 49-year-old male patient of rural residence (Table 1) was treated for chronic HCV by Pegylated interferon alpha 2a 180 ug /s.c once weekly and ribavirin 1000 mg/day. The treatment was planned for 48 weeks (according to PCR assessments at weeks 12 and 24), without genotype testing. The patient achieved early virologic response (HCV RNA was undetectable) by week 12 of the combination therapy. By week 20 of therapy the patient experienced increasing fatigue and he began noticing minimal lower limb edema and jaundice. By this time the patient denied fever, vomiting, and bleeding tendency. On examination he was jaundiced, with minimal lower limb edema, and was a febrile. Investigations showed serum total bilirubin 7.4 mg/dL, direct bilirubin 5.6 mg/dL, ALT 124 IU/L, AST 158 IU/L, albumin 3.1 gm/dL, prothrombin concentration 52%, hemoglobin 9.3 gm/ dL, platelets 121 x 103 /mm, white blood cell 3.3x103/mm without eosinophillia, alpha fetoprotein 1.2 u/mL, Cancer Antigen (CA) 19-9 4.1 u/mL, Carcino-Embryonic Antigen (CEA) 30 u/mL. HBs Ag, HBc Ig M and HAV Ig M were negative. Abdominal ultrasound examination showed hepatic mass adjacent to the common bile duct with minimal intrahepatic biliary radicles dilatation and mild ascites. The mass was further evaluated by triphasic CT and it lacked the typical criteria of hepatocellular carcinoma (Figure 1). The mass was aspirated twice and histopathological examination was irrelevant. In the third trial, attempts for tru-cut biopsy showed part of a flat worm in the histopathological examination (Figure 2) and raised the suspicion of Fascioliasis. By this time serological examination for Fascioliasis using the Indirect Haemagglutination Assay (IHA) test was positive with a titre of 1/640. Qualitative HCV RNA was examined and was positive (virological breakthrough). The combination therapy was permanently discontinued due to hepatic decompensation by the week 21. Due to unavailability of triclabendazole in the Egyptian market, the patient was first treated by Myrrh (Mirazid, Pharco Pharmaceuticals, Egypt) which is an oleo-gum resin from the stem of Commiphora mol mol tree (Family Burseraceae). However, no improvement was observed in both Fasciola serology and the patient's general condition. Another treatment trial was made using Praziquantel (Biltricide, Alexandria Co. for Pharmaceuticals & Chemical industries, Egypt) which was given in a dose of 40 mg/kg body weight, yet no improvement was noticed. An alternative trial was made using a veterinary preparation (TRIMEC, Pharma Swede, Egypt) which is composed of ivermectin (1 mg) and triclabendazole (50 mg) per each mL. Two successive doses (10 mg triclabendazole / kg body weight) of TRIMEC were given to the patient at two weeks interval [5]. The patient achieved improvement of both the general condition (fatigue, jaundice, lower limb edema) and dramatic decrease in IHA titer (1/80). Follow up ultrasound and CT (6 months later) showed absence of the liver mass and only mildly dilated CBD was present with normalization of all liver biochemistry by the end of the six months. The patient was kept on the best supportive care and permanently stopped interferon based antiviral therapy and awaits the availability of oral HCV treatments.
Age (years)
49
Abdominal ultrasound
Mild hepatomegaly
Liver Biopsy
Fibrosis
Activity
Others
F2
A2
No granulomas, steatosis nor parasites
Basal HCV RNA level (IUx 105)
3.12
ALT (IU)/L
64
AST (IU) /L
72
Albumin (gm/dL)
3.9
Bilirubin (mg/dL)
1
Prothrombin concentration (%)
82
Table 1: The patient's base line characteristics.
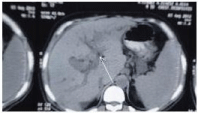
Figure 1: Triphasic computed tomography scan of the liver showing the mass related to the biliary system (arrow) with mild intrahepatic biliary radicles dilatation.
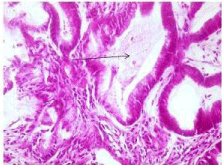
Figure 2: Histopathological examination of the liver showing part of a flat worm (arrow).
Discussion
The case presented here is important due to many reasons. Firstly, it documents the occurrence of this zoonotic infection in humans despite the great efforts to control parasitic infections which succeeded to decrease the burden of major parasitic diseases in our community, the best example is the reduction of schistosomiasis disease burden [6]. This may be explained by the fact that; the major Egyptian life setting is a rural community where the life cycle of Fasciola and other parasitic diseases can be completed easily. Fascioliasis as a zoonotic disease requires the control of the disease among domesticated farm animals which represent the main reservoir for human infection.
Secondly, it clarified the possible detrimental effect of Fascioliasis on the course of chronic HCV. Preliminary reports emphasized the non-detrimental impact of Fasciola infection in the course and management of patients infected with acute hepatitis C [7] and chronic hepatitis B virus [8], furthermore eradication of infection by interferon therapy was achieved in both cases [7,8]. Relying on the current case, the situation seems quite different; Fascioliasis superimposing chronic HCV under Pegylated interferon probably enhanced the chance of both virological breakthrough and hepatic decompensation. The explanation of this observation is probably related to the immunological responses.
Persistence of HCV infection is due to impaired T helper (Th) 1 immune response, while eradication of HCV infection requires a strong Th1 immune response. HCV and interferon operate through the Th1 immune response and enhancement of the innate immunity [9]. Th2 cytokines generally inhibit the development of Th1 activity [10]. Also, Th2 populations and cytokines were found to be higher in HCV interferon non responders at, during and after treatment when compared with Th1 that was higher in interferon responders [11]. IFN alpha modulates the balance of Th1/Th2 type cytokines favoring the induction and maintenance of Th1-like cells [12]. Whereas; Fasciola, like other parasites, induces a Th2 immune response [8]. Th2 cytokines are strongly induced in the chronic phase of Fascioliasis [13]. Moreover, it has been demonstrated that the secretion of Th1 cytokines, IFN-gamma and IL-2 is completely suppressed in the highdose Fasciola infection in mice [14]. Enhanced induction of Th2-type cytokines and down regulation of Th1 responses during infection with F. hepatica could be expected to interfere with chronic hepatitis treatment and favors persistence of infection [8].
Thirdly, the atypical presentation of the liver flukes in this case. Fasciola usually presents in two different phases. The acute (hepatic) phase, during which the disease is presented with fever, abdominal pain, tender hepatomegaly, malaise, urticaria and marked eosinophillia. The second phase, the chronic (biliary) phase, is manifested with dyspeptic symptoms and intermittent right upper quadrant pain with or without cholestasis. In this case the presentation by hepatic mass adjacent to the CBD that mimicked cholangicarcinoma and consequently hepatic decompensation is an infrequent presentation of this trematodes. Atypical presentations were reported by many other investigators [15-17], but the aggressive atypical presentation documented here is infrequent and may largely be related to the co-infection by HCV and the side effects related to the interferon therapy.
Fourthly, the diagnostic challenge. Diagnosis of Fascioliasis in the acute phase mainly relies on the clinical manifestations and the systemic eosinophilic response while the chronic phase depends on detection of the parasite eggs in stool that is of low sensitivity. In the current case the clinical suspicion was lacking due to many reasons including decreasing awareness for this infection, lack of fever and absence of eosinophillia in the blood smear. Histopathological diagnosis of Fasciola is an incidental event [18], while serological testing is valuable both in diagnosis and therapy monitoring [7,8]; in particular its value is enhanced due to low sensitivity of stool egg detection as shown in the current case.
Viral and host-related factors are the two main factors that influence the efficacy of HCV antiviral treatment. Consequently, the association between superimposed Fasciola infection and both virological breakthrough and hepatic decompensation in the present case is probable and not definite. This is particularly true because some important viral and host data are lacking especially the viral genotype and IL28B gene status.
In conclusion, Fasciola infection superimposing chronic HCV under combination therapy may increase the chance of both virological breakthrough and hepatic decompensation probably by an immunological mechanism.
Acknowledgement
To all our colleagues particularly Dr. Mohamed I Radwan and Dr. Hesham R. Abdel-Aziz.
References
- Esteban JG, Bargues MD, Mas-Coma S. Geographical distribution, diagnosis and treatment of human fascioliasis: a review. Res Rev Parasitol. 1998; 58: 13-42.
- Harinasuta T, Pungpak S, Keystone JS. Trematode infections. Opisthorchiasis, clonorchiasis, fascioliasis, and paragonimiasis. Infect Dis Clin North Am. 1993; 7: 699-716.
- Yuan HJ, Lee WM. Nonresponse to treatment for hepatitis C: current management strategies. Drugs. 2008; 68: 27-42.
- Mohamed M. Epidemiology of HCV in Egypt. The Afro-Arab Liver journal. 2004; 3: 41-52.
- Hakyemez IN, Aktas G, Savli H, Kucukbayrak A, Gurel S, Tas T. A Fascioliasis Case: a not Rare Cause of Hypereosinophilia in Developing Countries, Present in Developed too. Mediterr J Hematol Infect Dis. 2012; 4: 2012029.
- Salem S, Mitchell RE, El-Alim El-Dorey A, Smith JA, Barocas DA. Successful control of schistosomiasis and the changing epidemiology of bladder cancer in Egypt. BJU Int. 2011; 107: 206-211.
- Sahin M, Isler M, Senol A, Demirci M, Aydin ZD. Does Fasciola hepatica infection modify the response of acute hepatitis C virus infection to IFN-alpha treatment? World J Gastroenterol. 2005; 11: 7688-7689.
- Demirci M, Isler M, Cicioglu Aridogan B, Senol A, Korkmaz M. Coinfection of chronic hepatitis B and fasciolosis. Infection. 2004; 32: 54-56.
- Spaan M, Janssen HL, Boonstra A. Immunology of hepatitis C virus infections. Best Pract Res Clin Gastroenterol. 2012; 26: 391-400.
- Jacobson Brown PM, Neuman MG. Immunopathogenesis of hepatitis C viral infection: Th1/Th2 responses and the role of cytokines. Clin Biochem. 2001; 34: 167-171.
- Shinohara M, Ishii K, Takamura N. Long-term changes of peripheral blood CD4-positive T cell subsets (Th1, Th2) in chronic hepatitis C patients with a sustained response or no response to IFN. Hepatol Res. 2003; 27: 260-265.
- Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med. 1993; 178: 1655-1663.
- Clery DG, Mulcahy G. Lymphocyte and cytokine responses of young cattle during primary infection with Fasciola hepatica. Res Vet Sci. 1998; 65: 169-171.
- Brady MT, O'Neill SM, Dalton JP, Mills KH. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect Immun. 1999; 67: 5372-5378.
- Arslan F, Batirel A, Samasti M, Tabak F, Mert A, Ozer S. Fascioliasis: 3 cases with three different clinical presentations. Turk J Gastroenterol. 2012; 23: 267-271.
- Onder H, Ekici F, Adin E, Kuday S, Gumus H, Bilici A. An incidental case of biliary fascioliasis with subtle clinical findings: US and MRCP findings. Radiol Oncol. 2013; 47: 125-127.
- Emara MH, Radwan MI, Ibrahim IM. Video Case: Fascioliasis: Uncommon cause of Recurrent Biliary Colic. Afro-Egypt J Infect Endem Dis. 2013; 3: 113.
- Yen TJ, Hsiao CH, Hu RH, Liu KL, Chen CH. Education and imaging: hepatobiliary and pancreatic: chronic hepatic abscess associated with fascioliasis. J Gastroenterol Hepatol. 2011; 26: 611.