
Review Article
Austin J Trop Med & Hyg. 2015;1(2): 1009.
Vector-Borne Diseases in Argentina: Current Situation and Prospects
Zaidenberg MO*
National Coordination for Vector Control, Argentina
*Corresponding author: Zaidenberg MO, National Coordination for Vector Control, Jurisdiction Salta, General Guemes 125, PB 4400, Salta, Argentina
Received: January 04, 2015; Accepted: March 16, 2015; Published: March 20, 2015
Abstract
Vector-borne diseases in Argentina are a major public health problem because they affect a significant portion of the population generally subjected to socio-economic deficiencies, as well as environmental, sanitation and health infrastructure etc. Three diseases with a different history and evolution, each at a particular stage of control, were selected for characterization: Dengue, Malaria and Chagas. Since its reemergence, Dengue annually poses a new threat to thousands living in urban centers with different factors involved, such as the type of serotype, the same sequence, the magnitude of the affected population and their experience immune, the capacity ot Sequence, the magnitude of the affected population and their experience immune, the capacity of the system to monitor emerging cases as well as their management and vector control. Chagas disease has other features that are closely linked to the rural, poor housing conditions and is widely distributed in most Argentine provinces. Malaria also has a heterogeneous scenario based on varying degrees of control advance. Malaria has a long history of involvement of large population groups in the country, particularly in the Northern provinces. The approach of pathology realized significant milestones that marked successive evolutionary stages that will converge simultaneously to the current path of elimination of indigenous transmission. Each challenge is pending the final approach to stop being the problem today.
Keywords: Control; Vector borne disease; Endemic
Introduction
Vector-Borne Diseases (VBD) are a group of diseases that bring a heavy burden of disease worldwide and particularly to areas belonging to developing countries, affecting populations generally characterized by precarious socio-economic condition, basic infrastructure problems, unplanned urbanization, environmental problems, insufficient or irregular water supply sanitation; all these elements seriously condition the social and health balance of these populations.
In this context, Argentina presents a heterogeneous scenario for this variety of diseases in its present epidemiological status, as well as in the evolution and future. In this work, we consider some of these diseases that have a particular epidemiological profile in the country: Dengue, Malaria and Chagas. Each of these conditions has a particular epidemiological evolution with a complex etiology frame, along with basic conditioning factors. They are characterized by affecting or having affected the health of thousands of people and are now in a different evolutionary process from the point of view of control.1. Dengue is in a potential threat status, with an uncertain epidemiological panorama due to a number of present conditions that are recurrent in each year nationally and internationally; 2. Chagas is an endemic disease whose epidemiological status has improved and its control has advanced; however, the advances are hindered by its great magnitude and expansion; 3. Malaria, another endemic disease with a long history in the country; a significant degree of progress has been made in its control and is currently in the process of certification of elimination of indigenous transmission.
Dengue
Dengue is an arbovirus infection caused by a virus that has variants, serotypes 1,2,3 and 4, and is transmitted by the mosquito Aedes aegypti in the region. The dengue reemerged in Argentina in 1998 in northern localities [1]. Since then, outbreaks and / or epidemics have occurred in urban areas of most Northern provinces of the country almost every year, except in 2001 and 2005. Four serotypes were diagnosed since 1998, which gradually appeared individually in each epidemic and sometimes with co-circulation of two or three viruses, 2 and 3 (2003,2007) and 1.2 4 (2010, 2013). The magnitude of epidemics varied, reaching the highest number of affected people in 2009 when more than 26,000 cases were diagnosed in 14 affected provinces (serotype 1) [2]. In general, the provinces that were initially affected were those that bordered with neighboring countries. Aedes aegypti is the vector involved in the transmission of dengue virus and about 38 million people (2010 Census, INDEC), live in areas at risk of transmission and more than 2500 towns and / or cities are distributed in areas considered of varying risk. Between the epidemiological weeks 1-50, 2014, 3193 suspected cases of dengue were reported nationwide, of which 490 were confirmed, 463 were indigenous and 27 imported; these numbers were below the reported and confirmed cases in the same period the previous year. The provinces with the highest number of reported cases were Salta (44%) and Misiones (11%), holding an adequate opportunity of confirmed notification) and travel history. Argentina, year 2014 (ibid). The provinces with the highest number of notifications were Salta (44%) and Misiones (11%) holding an adequate opportunity of confirmed notification) and travel history. Argentina, Year 2014 (ibid) (Table 1).
Years
1998
1999
2000
2001
2002
2003
2004
2005
2006
2007
2008
2009
2010
2011
2012
2013
2014
Cases
330
445
445
0
204
91
1491
0
287
208
40
26923
1338
285
275
2921
3178
Serotypes
2
2
1
1
1,2,3
3
2,3
2,3
1
1
1,2,4
1,2
2,3
1,2,4
1,4
Source: SNVS C2 and SIVILA
Table 1: Number of dengue cases reported in Argentina, years 1998 - 2014 (EW 45).
The role of migration and international travel in the spread of pathogens is crucial in generating epidemiological situations of viral transmission, which are expressed in outbreaks and / or epidemics of varying magnitude. Accordingly, since 1998, all the outbreaks and / or epidemics in the country have been associated with epidemics or migration of people from neighboring countries, where there were important epidemics. At the regional level, the Pan American Health Organization / World Health Organization issued a new epidemiological alert in 2014 of chikungunya and dengue fever due to the increased circulation of dengue in Central America and the Caribbean, which is already evident in El Salvador, Guatemala, Honduras and Dominican Republic [3] (Table 2).
(SD/D)
Week
Suspected
Incidence rate
Confirmed
Incidence rate
Serotype
Severe dengue
Dengue deaths
Pop. (thousands)
SD/D x 100
Argentina
48
3127
7,48
489
1,17
1,4
0
0
41803
0
Bolivia
42
2263
208,61
1158
10,67
4
354
0
10848
1,56
Brazil
41
574612
284.41
8423
4.17
1,2,3,4
644
377
202034
0,11
Paraguay
48
15986
231.08
2468
35.68
1,2,4
5
5
6918
0,03
Chile
42
25
0.55
0
0
0
0
4537
0
Uruguay
48
0
0
0
0
0
0
3419
0
Table 2: Number of Reported cases of Dengue and Severe dengue (SD) in the Americas, by country: EW 48 (updated Dec 19, 2014). Source Dengue cases, Americas. 2014, PAHO/WHO.
The epidemiological history of the country in relation to dengue shows a particular vulnerability of the northern provinces of Argentina, including Salta and Jujuy, bordering Bolivia, and Formosa and Misiones, bordering Paraguay and Brazil. All urban centers in the region are colonized by Aedes aegypti and exhibit entomological risk indices. Despite a low degree of certainty for assessing a critical threshold of transmission [4] these indices (House, Breteau and Containers) are widely used tools in control programs in Latin America and reveal values that raise great concern. Therefore, there is a need of a proactive interaction among stakeholders on both sides of the border to optimize the level of program actions to prevent and control. Argentina has signed through the National Plan for Dengue [5,6] a formalization for addressing the problem through the EGI-Dengue strategy, which basically involves regulatory guidelines in the following areas: Diagnosis, Environment, Patient care, Epidemiological surveillance, Entomological control, and Social communication. These elements, with the exception of Environment and Entomological surveillance, develop in an acceptable manner, both in the extent and coverage of diagnostic services, an epidemiological surveillance system that monitors systematically the onset of fever cases or suspects across the national network. This is a care network, which provides training in the area of patient care and establishes regulations for referral and counter-referral in the management of patients. The systematic implementation of this task is hindered by the high turnover of caregivers. This unstable situation makes it necessary to maintain a permanent policy of updating and training in the assistance of patients with acute febrile syndrome. Besides non-significant changes in the functioning of the laboratory network, this network is able to monitor the occurrence of acute febrile cases that are compatible with vector-borne diseases in the country.
A critical problem determining the recurrent risk for dengue is related to environmental concerns and efforts for entomological control. Among the relevant factors, Argentina presents the environmental and social determinants indicated by PAHO, 1994 [7], although unevenly. For years guidelines have been promoted that tend to the integrated environment management by municipalities, including: Focal Control [5,8]. Systematic activities in the home environment which aims to reduce or avoid the presence containers that contain or may contain water. This includes inspection of dwellings in search of containers and decision-making to prevent accumulation of water, including removing containers, turning them upside down, drilling them, placing them indoors, filling them with sand; frequently changing water, and cleaning and ordering the household environment. Chemical and biological larvicides are also used in the community setting. All these activities are accompanied by the transfer of information to solve the problem of the presence of containers in the house.
Elimination of pots, "descacharrado": activity generally developed by municipalities with heavy vehicles that travel across the town from across the town neighborhoods taking all kinds of larger vessels that can serve as water containers at home, (sanitary ware, toys, refrigerators, stoves, tires, etc.). In addition, municipalities deal with water sources and treatment of accumulated water in public places. These activities are partially accomplished with a wide range in the degree of compliance with these guidelines, ranging from municipalities that perform few environmental activities to those implementing community programs and plans that assist the community in specific aspects of the health system. Although preventive activities are undertaken, they are never sufficient and do not reach the necessary coverage to have a positive impact on reducing sources of water with or without Aedes aegypti breeding. Emergencies and outbreaks and / or epidemics are generally handled by the staff of the National Coordination for Vector Control of the Ministry of Health of the Nation, with the necessary equipment for applying insecticides using portable and heavy machinery ULV in the affected urban environments.
With regard to social communication, in recent years with seasonal variations, a strong emphasis on prevention dissemination in the media is made. However, like other experiences in Latin America, although the information disseminated is abundant at the local level, the effect has been shown to be scarce and ephemeral, which does not usually translate into effective and persistent behavior change in the community [9].
The panorama of dengue in Argentina is complicated considering the multiple factors involved and the need to be consistent in the actions at the local level. Increasing coverage and intensifying actions and the necessary interaction among all sectors of society are basic challenges to improve integrated environmental conditions of the communities involved.
Chagas
Chagas disease is a widespread zoonosis in America. Members of the epidemiological chain of the disease include the etiologic agent, a protozoan, Trypanosoma cruzi; a prevalent vector in Argentina is the Triatoma infestans, poor housing and the human host. It has a wide distribution in Argentina, affecting 19 of the 24 provinces. The latest estimates of cases, PAHO, 2006 [10] indicate that in Argentina there would be 7,300,000 cases of Chagas infection and more than 300,000 people with some degree of chagasic cardiomyopathy. Chagas seroprevalence in pregnant women and blood banks is about 3%. There are people with Chagas nationwide, considering that in addition to vector transmission, the existence of other routes of transmission and migration contribute to a wide distribution of infected people [11].
The struggle against Chagas disease has two strategic areas: vectorial and non-vectorial routes; the former comprises the actions whose target is the vector agent, whereas the latter includes mainly transfusion and congenital routes. Vector control of this endemic disease is mainly based on the use of residual insecticides, which have been applied in the endemic area irregularly, and inconsistently. However, with over thirty years of community applications of residual insecticides, with sustained work in some endemic jurisdictions committed to control activity, the impact of actions has been observed in different age groups, such as donors, soldiers, pregnant women and children under 15 years (Figure 1).
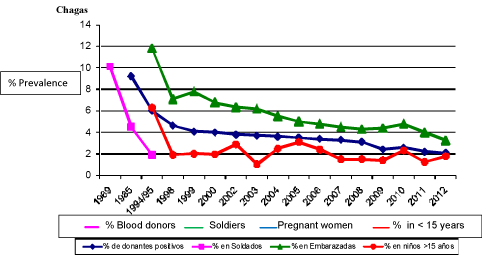
Figure 1: Serological indicators of the impact of the National Program actions, 1969 - 2012.
The Chagas National Program, ChNP, has formulated lines of action with general objectives of interrupting the transmission of Trypanosoma cruzi and reducing morbidity and mortality, epidemiological stratification of the endemic disease in every province, designing a strategy adjusted to every epidemiological scenario and defining the expected results. It should be noted that the certification of elimination of transmission is a process of objective assessment of epidemiological indicators, such as the situation of infestation in homes in an area and serological indicators of very low prevalence among young people. According to the degree of progress of control over the vector, the ChNP has produced a situation map of the provinces that has allowed stratification of the risk of transmission for each province (Figure 2).
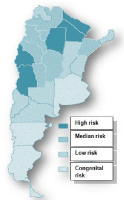
Figure 2: Risk stratification for Chagas by provinces in Argentina. National Program for Chagas, 2013.
High risk of vector-borne transmission
Chaco, Formosa, Catamarca, Santiago del Estero, San Juan and Mendoza provinces have a re-emergence of vector transmission due to an increase in house infestation and a high seroprevalence of vulnerable groups.
Moderate risk status
Cordoba, Corrientes, La Rioja, Salta and Tucumán show a situation intermediate risk, with a rate of reinfestation above 5% in some departments, and insufficient surveillance coverage in some cases.
Low risk status
In 2012, Misiones, and Santa fe and six departments of Santiago del Estero, certified the elimination of vectorial transmission of T. cruzi. Entre Rios, Jujuy, La Pampa, Neuquén and Rio Negro succeeding in recertifying the interruption of vectorial transmission.
Status of universal risk
The 24 jurisdictions of Argentina have risk of congenital transmission derived from internal and international migration from neighboring countries with high endemicity. Currently the provinces of Catamarca, Córdoba, Corrientes, Tucumán and Salta are in a process of consolidation of achievements in spraying of houses. An intermediate goal for 2016 includes achieving certification in Corrientes, Catamarca, Tucumán, Córdoba, La Rioja and Salta; achieving consolidation in San Juan and Mendoza; and partially achieving consolidation in Chaco, Santiago del Estero and Formosa (ChNP).
The most relevant weaknesses of and / or threats to the Program regarding Chagas vector route include the possibility of discontinuity of activities against the vector, low coverage, inadequate local supervision, insufficient human resources, complexity of peridomestic structures, and emergence of resistance areas.
On the other hand, the strengths / opportunities include: establishing the need to strengthen the policy priority of Chagas, a plan for monitoring insecticide resistance, the creation of the National Geographic Information System; human resource training activities related to Chagas and improvement of access to specific treatment, (ChNP). Epidemiological surveillance with community participation is an essential element in maintaining the addressed areas, so that recommendations emphasize the need to meet the operational needs of the worked areas. Approximately 600,000 households make up the total housing of the endemic area, many of which are poor houses (locally known as "ranchos", which precarious and vulnerable to colonization by the vector, [12].
Besides spraying or residual insecticides, endemic disease control also consists of improving housing and emphasizing on changes in community attitudes regarding the presence of the vector.
Non-vectorial transmission includes transfusion route for organ transplants and congenital route. The first pathway involves the area of transmission by blood transfusion. As mentioned above, the estimated prevalence is slightly above 2% (ChNP, 2013) in the general population and systematic monitoring of blood banks is performed and has legislation that determines the conditions for adequate control, (Report technical ChNP, 2012 [13]). Regarding congenital transmission all provincial jurisdictions have the tools for the serological diagnosis of pregnant women and monitoring of newborns. However, there is still an evident underdiagnosis. An estimate of ChNP indicates that annually about 1300 infants are born with congenital Chagas infection. In 2013, 89 infected infants were diagnosed, less than the 7% estimated for 1,300 infants in the year.
Another important area of intervention includes schoolchildren whose prevalence is about 2% in the endemic area, where there are more than 1,000,000 children under 15 years old. All this population should be diagnosed and treated if the reactivity of the diagnostic serology is demonstrated. This activity is a real challenge for the health authorities in the country, because there is a specific medication, Benznidazol,® which is produced in the country and so far the impact of the activity has been truly very low.
Argentina is a federal country, with each province being autonomous; accordingly each one implements a control policy based on priority needs as deemed by local authorities. According the level of decisions, regarding vector health situations can be found in provinces where there is a clear political commitment to the control strategy generated by the National Chagas Program and the to advance the level of control and maintenance of actions on the ground can be observed. There are other situations in which work progress are slower, which is evident in the continuing infestation levels and the significant prevalence of Chagas disease in younger age groups. These contrasting situations explain the heterogeneity of progress in the control of this endemic disease.
Malaria
The history of malaria in Argentina begins in the late 19 th century and extends to the present. Basically, it has been divided into four heterogeneous stages, each with characteristic identifying elements or facts [14,15]. The first stage, 1891-1939, comprises the beginnings of prevention and control. The second stage, 1940-1949, is marked by the systematic application of DDT in the houses of the endemic area. The third stage, 1950-2008, includes the development of sustainable control of the endemic disease and the fourth stage, 2009-2014, is the transition to the elimination of indigenous transmission of malaria.
Evolution
First stage, (1891 - 1939)
Since early 1900, primarily activities to combat malaria in Argentina were focused on two aspects: environment and care; the former included advising companies whose projects are prone to favor companies whose projects are prone to favor conditions for vector breeding, such as railways and route construction, reservoirs and works on rivers of water, whereas the latter involved the distribution and supply of quinine to accessible populations in urban and suburban areas. Between 1904 and 1911, several research articles were published on malaria etiology, transmission, environment, clinical manifestations, and treatment contributing to the characterization of malaria in the country. In 1911, the Act No 5195 of defense against malaria was passed and this was passed and this was a model law for the control of the endemic disease. In the same year, William Patterson, from the province of Jujuy, stated that Anopheles pseudopunctipennis was the only vector of malaria of epidemiological importance in northwestern Argentina. Available indicators evidenced the high malaria morbidity that affected the most vulnerable populations of central and particularly northern Argentina , including the provinces of Salta, Tucumán, La Rioja, and Catamarca [16] (Table 3).
YEARS
TUCUMÁN
SALTA
JUJUY
CATAMARCA
LA RIOJA
ALL
1912
19909
19790
8833
5420
1372
55324
1913
62145
39410
13505
10475
10475
128587
1914
96170
44977
14914
14598
3966
174625
1915
84209
43119
18615
13154
3728
162825
ALL 12-15
262433
147296
55867
43647
12118
521361
ANNUAL AVERAGE
65608
36824
13967
10912
3030
130340
Source : Jose Penna and Antonio Barbieri, El Paludismo y su profilaxis en la Argentina (Buenos Aires: DNH, 1916), 68-69.
Table 3: Malaria morbidity in endemic provinces of Argentina, 1912 - 1915.
YEARS
2000
2001
2002
2003
2004
2005
2006
2007
2008
2009
2010
2011
2012
2013
2014
Number of Cases per year
440
215
125
124
115
252
212
355
106
64
72
18
4
5
2
API
0,16
0,07
0,04
0,04
0,04
0,087
0,07
0,12
0,1
0,086
0,023
0,005
0,001
0,001
0.001
API: Anual Parasitolgy Index
Report: Programa Nacional de Paludismo, Argentina, Salta 2014.
Table 4: Annual malaria cases and Parasitology Index in Argentina, years 2000 - 2014 (EW 50).
From 1916 to 1935, malaria control received a major boost from European malarial schools, particularly Italian ones, based primarily on environmental sanitation projects. Major engineering works against malaria were also undertaken, such as waged piping, drains and landfills, and distribution of quinine in rural areas was increased [17]. A stage of qualitative growth led by Dr. Carlos Alvarado started in 1937, with the identification of the malaria endemic area and the affected population in northwestern Argentina. He understood that control measures transferred from Europe without proper adaptation to the prevalent local vector bionomics probably explained the failure of health efforts. Through a local experience, Dr. Alvarado achieved a significant reduction in breeding of Anopheles pseudopunctipennis and malaria morbidity in the area. However, the proposed changes in operational methodology did not alter the program decisions, which continued under the influence of the Italian school, (ibidem) (Figure 3).
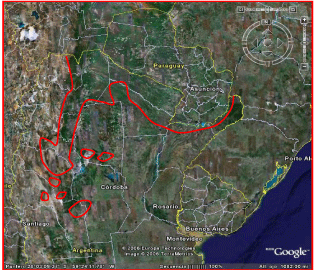
Figure 3: Distribution of Malaria endemic area in Argentina, 40s.
Report: Programa Nacional de Paludismo, Salta.
Second stage, 1939 - 1949
In the 30s, Alvarado worked in Argentina, addressing different pathologies, and especially Malaria. He is appointed head of the General Directorate of Malaria and reorganizes the strategic lines of control program. In the mid- 40s, a five-year Plan is created consisting of a traditional approach to the problem of malaria in the country. However, in 1947 the Division of Malaria changed the plan for another one based on the extensive use of DDT, (Dichloro Diphenyl Trichloroethane), for two years. This experience was borrowed from similar approaches in Central America, and consisted in the design of a new strategy incorporating substantial changes, especially reducing the time needed to comply with the original program. The sanitary engineering works are removed and a preventive plan is designed which included organizing DDT application service aimed at spraying with DDT houses in endemic areas. In just two years (1947-1949), in just two years (1947-1949, 4/5 of the endemic area was covered and more than 100,000 homes of the endemic area were sprayed, expanding to 80% of the original malaria area. The occurrence of about 5000 cases per year was reduced to 800 cases throughout the endemic area [18]. Thus, Argentina became the first country in Latin to use the DDT as a routine procedure. The appearance of DDT and other residual insecticides, introduced new elements into the strategic conception of malaria control and modified existing techniques known up to then in its fundamental aspects as this scenario, strategic objective, economic basis and cost estimations [19]. As was a originally planned, the care plan became a surveillance service.
Third stage: (1950 - 2008)
The basic components of the strategy proposed [18], since then were the establishment of a program to conduct epidemiological surveillance, with three elements:
- Research: passive and active detection, epidemiological investigation after confirmation of the case, classification of cases and tracking of positive cases.
- Healing: presumptive, radical and collective treatment.
- Environmental monitoring: house spraying.
This approach strategy still remains in full force at present with some non-significant operational variations. A gradual and sustained decline in the provinces considered endemic to the end of the 60s occurred with 212/285 average annual cases in 60s and 70s, until 1969, when most of the provinces ceased to present new cases, with the exception of Salta and Jujuy, which, continued to exhibit a persistent endemic level, with the predominance of the former over the years, and sporadic presentation of epidemics in the province of Misiones. At this stage, the evolution was heterogeneous and can be defined technically as "sustainable control", which basically consists of having the resources allocated in the most appropriate manner so as to maintain the endemic area with low transmission.
Resources for a residual disease in a country with multiple health priorities were assigned in diverse modes, sometimes insufficiently, whereas the budget of resources for programs was shared with other vector-borne diseases: dengue and Chagas.
The persistent endemic area was smaller than the original one, with particular expression in Salta, Jujuy, and Tucumán in the northwest of the country, and in Corrientes and Misiones in the northeast, where it manifested endemically.
The epidemiology of malaria was [14]:
- Low endemicity.
- The annual parasite index for more than two decades was less than 1%o in the last five years, it has remained below, 0.05%.
- The occurrence of cases is closely linked to migration flows in the area of the Bolivian-Argentine border.
- It exhibits seasonal variations.
- Its presentations is unstable.
- It affects all ages.
- The prevalent vector is Anopheles pseudopunctipennis.
- All prevalent cases in the endemic area are caused by Plasmodium vivax.
- The affected population is rural.
- The clinical expression is usually mild to moderate and subject to outpatient treatment.
The endemicity at this stage can be explained by the influence of migration flows in northwestern Argentina in the Argentine- Bolivian border, where movements ocurred in both directions, but particularly north-south, from populations that migrated to work in agricultural activities such as the sugar harvest, fruit and vegetable crops, cotton, beans, etc. These peoples often entered the country illegally, so follow-up was difficult. Moreover, in these conditions, they frequently resorted to self-medication or consultation with healers or pharmacies to try to remedy their symptoms; and only if symptoms persisted did they attend the formal health system, health centers or hospitals (ibidem). This situation hindered early detection and appropriate treatment. In the early 2000s, the occurrence of cases remained above 100 cases a year, with sporadic increases, as in 2007 in the town of Puerto Iguazú, when the country suffered the last malaria epidemic.
Fourth stage (2009 - 2014)
An important epidemiological consideration for this stage is to define the status of "elimination of indigenous transmission" in a country or region, which is basically achieve and sustain "zero impact" of new autochthonous cases. In the mid-90s, when a major epidemics occurred in the northern provinces of Salta and Jujuy, with over 2000 cases, a proactive strategy was implemented; it consisted of the development of operational prevention and control in more than 100 rural localities in the southern Tarija department, conducted by Argentine technical personnel, under the Argentine-Bolivian ARBOL II agreement, through technical operational intervention by intensified surveillance activities over a period of 40 days, and application with residual insecticides in the homes. This strategy achieved a significant reduction in the number of new cases in the region of San Martín and Orán departments, Salta; these operations were repeated every two or three years using a similar methodology until 2011. Since then have been between 200 and 300 cases per year, with annual variations; approximately 50% to 70% of the cases were imported from abroad, with a progressive decreasing trend, reaching 18 cases imported from abroad without indigenous cases in 2011 (Figure 3).
In 2008, the malaria endemic area in the country was updated, which at the time was focused on Oran and San Martin departments, in the north of Salta, with sporadic cases in the area that was categorized as stratum 1 [15], and a wider area where indigenous cases occurred up to three years ago, comprising the area of Jujuy branch, including the departments of Palpalá, El Carmen, San Pedro, General Belgrano; Anta department in Salta province, and the municipality of Puerto Iguazú, province of Misiones, in northeastern Argentina, stratum 2, of exclusive surveillance. Since 2011, the National Malaria Program developed intensified control activities with the aim of achieving the elimination of autochthonous malaria transmission in 2012. They activities include visits of houses of the risk area, with emphasis in the area of Argentine-Bolivian border, comprising about 4,000 households; searching for febrile patients, making hematologic samples, microscopic diagnosis and supervised treatment to patients with confirmed diagnosis. These activities are held monthly under normal conditions, and can be altered in the summer motivated when rains often make it impossible to travel on roads. In the same area, since 2012 hematological samples are taken from asymptomatic person by capillary puncture to perform PCR with thick drop. Sampling aims at detecting malaria in asymptomatic patients because in similar epidemiological situations [20] the disease has been shown to be present in submicroscopic populations usually not diagnosed by light microscopy. Other activities related to the process of elimination of transmission are entomological studies aimed to characterize various aspects of the distribution, seasonal fluctuations, bionomics, local anopheline habits [21-25], which it was completed through entomological samples along several years at appropriate sites, applications of human bait; even sensitivity studies of anopheline population of residents in the area of risk are still necessary.
As shown in Table 2, eighteen, four, five and two cases have been reported in 2011, 2012, 2013 and 2014, all imported from abroad and detected in the vicinity of the Argentine-Bolivian border. They were P. vivax cases diagnosed in peripheral operational bases and then confirmed by the reference laboratory; they were treated to following standards with conventional medication and monitored throughout treatment [26].
The next step, according to the guidelines of the World Health Organization, is the prevention of reintroduction of cases, which is, basically the early detection of cases carrying the parasite that enter the country. For this, it is critical to implement a proactive surveillance system suitable for the activity. For this purpose, it was determined that the technical staff should perform this activity continuously and recurrently. Another important activity is updating and / or training the health team in the differential diagnosis of acute fever in bordering areas, in order to include malaria among the possible diagnoses; finally, is necessary to interact appropriately with neighboring countries that hold a current case occurrence so as to be aware of a possible transmission situation in the border area information.
Conclusion
The three diseases analyzed might well make up socioenvironmental complex regarding this type of pathology. The different realities and advances made in each control, also reflected in the difficulties encountered and sometimes opposed to the progress of programs. As these diseases affect the health and quality of life of thousands of citizens, both from urban and rural areas, most of them without appreciable social visibility, only a strong political decision to hold the strategies defined for each area may favor the fulfillment of the objectives defined each disease.
References
- Zaidenberg M. Emergency dengue in Argentina. Dengue Epidemic in Salta. Journal of Epidemiology and Immunization. 2000; 3; 1-4.
- Integrated Surveillance Bulletin. Ministry of Health. Presidency of the Nation. 2014; 101: 11-12.
- Epidemiological Alert chikungunya and dengue fever in the Americas. August 29, 2014. Pan American Health Organization.
- Chadee DD, Doon R, Severson DW. Surveillance of dengue fever cases using a novel Aedes aegypti population sampling method in Trinidad, West Indies: the cardinal points approach. Acta Trop. 2007; 104: 1-7.
- National Plan for the Prevention and Control of Dengue and Yellow Fever. Ministry of Health. Presidency of the Nation. 2009.
- Strategy for integrated prevention and control of dengue in the region of the Americas. GT dengue. Second version. Pan American Health Organization and World Health Organization. 2003.
- Pan American Health Organization. Dengue and dengue hemorrhagic fever in the Americas: Guidelines for prevention and control. Washington DC: PAHO. Scientific Publication No. 548. 1994.
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New edition, Tropical Diseases Research, World Health Organization. 2009.
- Solis Ramirez MI, Quesada Quesada M, Gutiérrez Hernández I. Knowledge, attitudes and practices Against the dengue fever promoted by Costa Rican media. Costarr Rev Public Health. 2010; 19: 88-92.
- Quantitative estimation of Chagas disease in Americas.OPS / HDM / CD / 425-06. Department of Control of Neglected Tropical Diseases (NTD). Regional Office of Innovative and Intensified Disease Management (10M) World Health Organization WHO, NTD, IDM.
- Spillmann C, Burrone S, Coto H. Analysis of the epidemiological situation of Chagas disease in Argentina: Advances in control, 2012. Rev Argent of Public Health. 2013; 4.
- Zaidenberg M, Spillmann C, Carrizo Paez R. Control of Chagas in Argentina. Its evolution. Rev Argent Cardiol. 2004; 72: 375-38.
- Spillmann C. Report. Chagas National Programme Argentina, Cordoba. 2011.
- Zaidenberg M. Control of Malaria in Argentina Part I. RAZ and EIE. 2013; 8.
- Zaidenberg M. Control of Malaria in Argentina Part II. RAZ and EIE. 2014; 9.
- Penna J, Barbieri A. Malaria and its prophylaxis in Argentina. Buenos Aires: DNH. 1916; 68-69.
- Loncarica Kohn A, Agüero A, Sanchez NI. Nationalism and Internationalism in health science: the case of malaria control in Argentina, Asclepius. 1997; 49.
- Sierra Iglesias JP. Carlos Alberto Alvarado. Life and Work. Bicameral Commission examiner Works salteños authors. Salta. 1993.
- Alvarado CA. Control of Mosquito-Borne Diseases. Monthly of Pan American Sanitary Bureau (Washington). 1948: 1105: 182.
- Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol. 2014; 30: 183-190.
- Dantur MJ, Zaidenberg M, Almirón W. Seasonal fluctuation an pseudopunctipennis (Diptera: Culicidae) in a malarious area of Salta, Argentina. Entomol. Vect. 10 (4): 457- 468.2003. ISSN 0328-0381. Universidade Gama Filho.
- Juri MJ, Zaidenberg M, Almirón W. Spatial distribution of Anopheles pseudopunctipennis in the Yungas de Salta rainforest, Argentina. Rev Saude Publica. 2005; 39: 565-570.
- Dantur Juri MJ, Claps GL, Santana M, Zaidenberg M, Almirón WR. Abundance patterns of Anopheles pseudopunctipennis and Anopheles argyritarsis in northwestern Argentina. Acta Trop. 2010; 115: 234-241.
- Dantur Juri MJ, Zaidenberg M, Claps GL, Santana M, Almiron WR. Malaria transmission in two localities in north-western Argentina. Malar J. 2009; 8: 18.
- Dantur MJ, Galante G, Zaidenberg M, Almiron W, Claps G, Santana M. Longitudinal study of the species composition and spatio-temporal abundance of Anopheles larvae in a malaria risk area in Argentina. Memorias do Instituto Oswaldo Cruz. 2014; 1167-1181.
- Dantur JMJ, Zaidenberg M, Abril M, et al. Malaria epidemiology in northwestern Argentina: Dynamics of the transmission. Roemmers Annals Foundation. 2010-2012; 145 -157.