Review Article
Austin J Urol. 2014;1(1): 3.
The Role of Interleukin–10 in Suppression of Bacillus Calmette–Guérin Induced T Helper Type 1Immune Response and Anti-Bladder Cancer Immunity
Yi Luo*
Department of Urology, University of Iowa Carver College of Medicine, USA
*Corresponding author: Luo Y, Department of Urology, University of Iowa, 375 Newton Road, 3204 MERF, Iowa City, IA 52242, USA
Received: March 25, 2014; Accepted: April 16, 2014; Published: April 18, 2014
Abstract
Bladder cancer is a common urologic cancer and intravesical Mycobacterium bovis bacillus Calmette-Guérin (BCG) is the mainstay in the treatment of superficial bladder cancer. However, the current BCG therapy is not optimal with respect to its efficacy and side effects. Accumulating evidence suggests that proper induction of T helper type (Th) 1 immunity is required for effective immunotherapy of bladder cancer with BCG. Interleukin (IL)–10, a Th2 cytokine, down–regulates the Th1 immune response and is associated with BCG therapy failure. Therefore, blocking IL–10 activity could be beneficial for bladder cancer patients undergoing BCG therapy. We evaluated BCG in combination with IL– 10 neutralizing or receptor 1 (IL–10R1) blocking monoclonal antibodies (mAb) and found that the combination therapies induced enhanced Th1 immune responses and anti–bladder cancer immunity in preclinical animal models. The mechanistic studies revealed that BCG in combination with anti–IL–10R1 mAb induced specific antitumor immune responses. Our observations suggest that BCG immunotherapy for bladder cancer can be enhanced by addition of IL– 10 blocking agents. This paper reviews our recent progress in animal studies on bladder cancer treatment by BCG and IL–10 blocking agent combination therapies.
Keywords: Bladder cancer; BCG; IL–10; Immunotherapy; Th1
Abbreviations
BCG: Mycobacterium Bovis Bacillus Calmette–Guérin; CIS: Carcinoma In Situ; DTH: Delayed–Type Hypersensitivity; IFN: Interferon; IL: Interleukin; IL–10R, IL–10 Receptor; mAb: Monoclonal Antibody; NMIBC: Non Muscle Invasive Bladder Cancer; Th: T Helper type
Introduction
Bladder cancer is a common urologic malignant disease. At the time of diagnosis, 20–25% of cases are muscle invasive (stage T2 or higher) and are typically treated with surgical resection. The remainders are Non Muscle Invasive Bladder Cancer (NMIBC) including tumors confined to the epithelial mucosa (Ta), tumors invading the lamina propria (T1), and carcinoma in Situ (CIS). Transurethral resection of bladder tumor (TURBT) is the primary treatment for Ta and T1 lesions. Intravesical therapy is used as adjuvant treatment to prevent recurrence and progression of the disease after TURBT and is also the treatment of choice for CIS. Intravesical instillation of Mycobacterium bovis bacillus Calmette–Guérin (BCG) is widely used as the standard therapy for NMIBC. Bladder cancer is dominated by a T helper type (Th) 2 polarized immune pathologic response. BCG therapy can shift the Th2 environment toward a Th1 milieu, leading to effective anti–bladder cancer immunity in the majority of patients. BCG therapy typically results in 50–60% effectiveness against small residual tumors and a 70–75% complete response rate for CIS. However, BCG therapy is associated with 40–50% disease recurrence and a lack of therapeutic response in some patients. In addition, up to 90% of patients experience various side effects and occasionally even life–threatening complications such as sepsis. Therefore, the current BCG therapy is not optimal with respect to its efficacy and safety. To improve BCG therapy, efforts have been made to enhance the induction of Th1 immune responses, since this response is known to be essential in BCG–mediated bladder cancer destruction [1]. To date, two major strategies have been used; one to combine BCG with Th1– stimulating cytokines in treatment and the other to block the Th2 immune pathway during BCG treatment. We have demonstrated that both strategies are favorable for treating bladder cancer in humans and⁄or in animal models.
Supplement with Th1–stimulating cytokines enhances BCG treatment for bladder cancer
Given that the effect of BCG on treating bladder cancer is immune mediated, decades of research have focused on adjunctive immune therapies including cytokines with Th1–stimulating activities such as interferon (IFN)–α, interleukin (IL)–2 and IL–12. We have investigated BCG in combination with intravesical IFN–α or IL–12forbladder cancer treatment and found the combination therapies to be safe and effective [2–4]. Particularly, we have observed that BCG combined with intravesical IFN–α is favorable for patients who failed BCG mono therapy [4]. The mechanisms underlying the effect of combination therapies are multifaceted. In addition to the augmentation of Th1 immune responses, blocking BCG–induced Th2 cytokines (e.g. IL–10) is believed to play an important role in the effect of combination therapies [2,3].These BCG and cytokine combination therapies provide an opportunity for the use of lower and safer doses of BCG, while preserving or even enhancing BCG efficacy in the treatment of bladder cancer.
Blockage of IL–10 enhances BCG–induced Th1 immune response and anti–bladder cancer immunity
BCG treatment in IL–10−⁄− and IL–10 neutralized mice
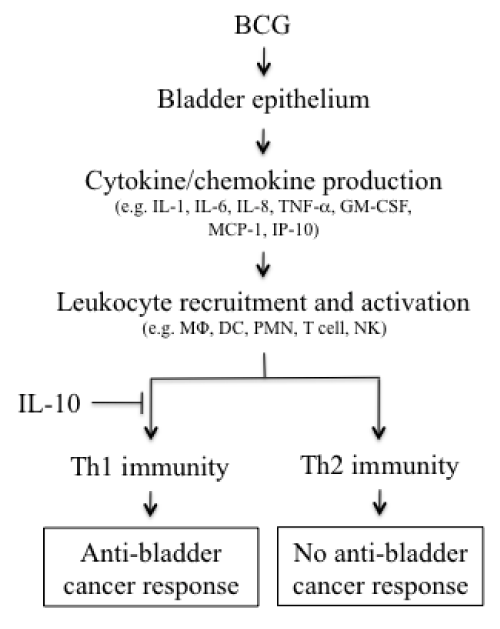
Much progress in BCG immunotherapy of bladder cancer has been made through preclinical studies using animal models of bladder cancer. In addition to BCG in combination with Th1– stimulating cytokines, we have investigated BCG in combination with IL–10 blocking agents for treating bladder cancer in animal models.IL–10 is classified as a Th2 cytokine and regulates growth and/or differentiation of various types of cells to control immune responses and tolerance in vivo [5]. It has been known that IL–10 plays an important inhibitory role in both bladder cancer immune surveillance and BCG therapeutic control of bladder cancer (Figure 1) [1,6], although it can promote antitumor responses in certain types of other cancers. The development of a dominant Th1 cytokine profile (e.g. IFN–γ, IL–2 and IL–12) has been observed to be associated with the therapeutic effect of BCG, whereas the presence of a high level of Th2 cytokines (e.g. IL–10) has been observed to be associated with BCG therapy failure [7]. A tendency toward higher ratios of IFN–− vs. IL–10 has also been observed for BCG responders [7,8]. To date, all animal studies have supported the Th2 cytokine dominance observed in human bladder cancer. Studies have shown that IFN–− and IL–12 but not IL–10 are required for local tumor surveillance in a mouse model of bladder cancer [9]. Studies have also shown the inhibitory role of IL–10 in BCG treatment of bladder cancer in animal models. Mice genetically deficient in IL–10 (IL–10−⁄−) developed a massive local immune response, coinciding with increased therapeutic efficacy, after intravesical BCG treatment (Table 1) [9]. In agreement with these observations, our early studies showed that absence of IL– 10 abrogated either by systemic administration of an anti–IL–10 neutralizing monoclonal antibody (mAb) or by the use of IL–10− ⁄− mice resulted in enhanced delayed–type hypersensitivity (DTH) responses that were associated with increased mononuclear cell infiltration and Th1 cytokine production (e.g. IFN–−) in the BCGtreated bladders (Table 1) [7]. Under the condition of aggravated DTH responses, a significant enhancement in BCG–induced antibladder cancer immunity was observed (Table 1) [7]. In addition to the in vivo studies, we have also observed the inhibitory effect of IL– 10 on BCG–induced macrophage cytotoxicity against bladder cancer cells in vitro through the use of anti–IL–10 neutralizing mAb and IL– 10−⁄− macrophages [10].
Figure 1 :Suggested cascade of immune responses in bladder mucosa induced by intravesical BCG instillation. Attachment of BCG to bladder epithelial cells including carcinoma cells triggers the release of cytokines and chemokines, resulting in the recruitment and activation of multiple leukocyte types in the bladder wall. The cytokine environment leads to differentiation of Th1 or Th2 immunity in the bladder wall. The therapeutic effect of BCG depends on the proper induction of Th1 immunity. IL-10, a Th2 cytokine produced by T cells, macrophages and epithelial cells, inhibits Th1 immunity. Therefore, blocking IL-10 could result in a Th1-dominated immunity that is essential for BCG-mediated bladder cancer destruction. MF: macrophage; DC: dendritic Cell; PMN: Poly Morphonuclear Neutrophil; NK: Natural Killer cell.
BCG treatment in IL–10 receptor blocked mice
We recently evaluated the effect of IL–10 blockage at the receptor level on BCG induction of Th1 immune responses and anti–bladder cancer immunity (Table 1) [11,12]. Mice treated with intravesical BCG plus systemic administration of an anti–IL–10 receptor 1 (R1) mAb showed significantly increased IFN–− mRNA and protein in the bladder and urine, respectively, in a dose–dependent manner [11]. Accordingly, mice implanted with bladder cancer cells and treated with BCG in combination with anti–IL–10R1 mAb showed substantially improved tumor–free (40% vs. 20% in BCG–treated mice and 0% in non–treated mice) and survival rates (100% vs. 80% in both non– and BCG–treated mice) in a 22–day experimental period [11]. Studies further revealed that the combination therapy with a reduced dose (1/3 full–dose) of BCG significantly prevented bladder cancer metastasis to the lung (0% vs. 36% in non–treated mice and 53% in BCG–treated mice, p = 0.02) during an extended 76–dayexperimental period [12]. This observation suggests that BCG could be used at a reduced dose when combined with an IL–10 blocking agent to minimize BCG side effects while preserving or even enhancing BCG efficacy. The observed effects of anti–IL–10R1 mAb are presumably due to its direct inhibition on IL–10 signaling, leading to a Th1 enriched microenvironment in the bladder, a condition essential for the therapeutic control of bladder cancer by BCG. The mechanistic studies further revealed that the combination therapy induced specific antitumor immunity. MB49 cells, a parental line of MB49–Luc cells used to establish the bladder cancer model in our studies, is known to contain a serine mutation at codon 12 of the K–ras oncogene [13]. This gene mutation results in the abundant expression of mutated p21 K–ras protein that is immunogenic and capable of inducing ras mutation–specific immune responses in mice when immunized properly [13]. Indeed, splenocytes from mice treated with BCG plus anti–IL–10R1 mAb showed increased IFN–− production and cytotoxic T lymphocyte (CTL) activity in response to the mutant but not wildtype rasstimulation in vitro [12]. The induction of specific antitumor immunity may explain the observed effect of the combination therapy on preventing bladder cancer metastasis to the lung in the animal model.
Table 1. Immune Induction by Intravesical BCG under IL-10 Blockage
Strain
IL-10 Activity
Immune Response
Reference
C57BL/6 IL-10-/-
Genetic defect
Cell infiltration,Th1 cyt, DTH, antitumor
7
C57BL/6 IL-10-/-
Genetic defect
Cell infiltration, antitumor
9
C57BL/6 IL-10+/+
IL-10 neutralization
Cell infiltration,Th1 cyt, DTH, antitumor
7
C57BL/6 IL-10+/+
IL-10R1 blocking
Th1 cyt, CTL, antitumor
11,12
Th1 cyt: Th1 Cytokine; DTH: Delayed-Type Hypersensitivity; CTL: Cytotoxic T Lymphocyte; IL-10R1: IL-10 receptor 1.
Table 1: Immune Induction by Intravesical BCG under IL-10 Blockage
Conclusion
Our studies have suggested that blocking IL–10 production and⁄or activity may provide therapeutic benefits for BCG–based immunotherapy of bladder cancer, particularly for high–risk patients with NMIBC, when combined with BCG. A humanized form of IL– 10 blocking antibody warrants future clinical evaluation of the safety and efficacy of BCG combination therapy. As research continues, we anticipate that a new BCG therapy with improved efficacy and limited side effects will be available for bladder cancer, one of the most frequently occurring and most expensive cancers to treat.
Acknowledgments
This work was supported by Pfizer.
References
- Brandau S, Suttmann H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother. 2007; 61: 299-305.
- Luo Y, Chen X, Downs TM, DeWolf WC, O'Donnell MA. IFN-alpha 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guérin immunotherapy.J Immunol. 1999; 162: 2399-2405.
- O'Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK. Role of IL-12 in the induction and potentiation of IFN-g in response to bacillus Calmette-Guérin. J Immunol. 1999; 163: 4246-4252.
- O'Donnell MA, Boehle A. Treatment options for BCG failures. World J Urol. 2006; 24: 481-487.
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001; 19: 683-765.
- Mocellin S, Wang E, Marincola FM. Cytokines and immune response in the tumor micro environment. J Immunother. 2001; 24: 392-407.
- Nadler R, Luo Y, Zhao W, Ritchey JK, Austin JC, Cohen MB, et al. Interleukin 10 induced augmentation of delayed-type hypersensitivity (DTH) enhances Mycobacterium bovis bacillus Calmette-Guérin (BCG) mediated antitumour activity. Clin Exp Immunol. 2003; 131: 206-216.
- Saint F, Patard JJ, Maille P, Soyeux P, Hoznek A, Salomon L, et al. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette-Guerin treatment for superficial bladder cancer. J Urol. 2002; 167: 364-367.
- Riemensberger J, Böhle A, Brandau S. IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol. 2002; 127: 20-26.
- Luo Y, Han R, Evanoff DP, Chen X. Interleukin-10 inhibits Mycobacterium bovis bacillus Calmette-Guerin (BCG)-induced macrophage cytotoxicity against bladder cancer cells. Clin Exp Immunol. 2010; 160: 359-368.
- Bockholt NA, Knudson MJ, Henning JR, Maymi JL, Weady P, Smith GJ III, et al. Anti-IL-10R1 monoclonal antibody enhances BCG-induced TH1 immune responses and antitumor immunity in a mouse orthotopic model of bladder cancer. J Urol. 2012; 2228-2235.
- Newton MR, Askeland EJ, Andersen ED, Chehval VA, Wang X, Askeland R, et al. Anti-interleukin-10R1 monoclonal antibody in combination with BCG is protective against bladder cancer metastasis in a murine orthotopic tumor model and demonstrates systemic antitumor immunity. Clin Exp Immunol. 2014 [In press].
- Luo Y, Chen X, Han R, Chorev M, DeWolf WC, O’Donnell MA. Mutated ras p21 as a target for cancer therapy in mouse transitional cell carcinoma. J Urol. 1999; 162: 1519-1526.