Research Article
Austin J Urol. 2014;1(3): 5.
Can Herpes Simplex Virus Vector-Mediated Gene Transfer of Kynurenine Aminotransferase Reduce Urethral Resistance in Spinal Cord Injured Rats?
Limin Liao1,2*, Chunsong Jia1,2 and Naoki Yoshimura3
1Department of Urology, China Rehabilitation Research Center, China
2Department of Urology, Capital Medical University, China
3Department of Urology, University of Pittsburgh, USA
*Corresponding author: Limin Liao, Department of Urology, China Rehabilitation Research Center, No 10 Jiaomen Beilu, Beijing 100068, China
Received: August 07, 2014; Accepted: September 15, 2014; Published: September 20, 2014
Abstract
Aim: To explore if replication-defective herpes simplex virus (HSVrd) vector-mediated gene transfer of kynurenine aminotransferase (KAT) can reduce urethral resistance in spinal cord injured rats.
Methods: The HSVrd-KAT II vector was constructed, Sprague-Dawley rats was spinalized with complete transection of the T10 spinal cord. Viral suspension of HSVrd or HSVrd-KAT II (1×107 plaque-forming units) was injected into 4 sites around the bladder base, cystometry was recorded. The KAT II positive neurons, ratio of KAT II/GADPH proteins and KAT II/β-actin mRNA in L6-S1 dorsal root ganglia (DRG) were examined.
Results: The number and amplitude of non-voiding bladder contraction (NVC), Detrusor Leak Point Pressur (DLPP) and Maximum Cystometric Capacity (MCC) were decreased significantly by 59.6-61.1%, 21.6-24.2%, 30.3- 34.4% and 44.1-46.5% (P<0.01), and the Bladder Emptying Efficiency (BEE) and the time to first NVC were increased significantly by 40.7-47.7% and 30.1- 49.0% (P<0.01), respectively, in the HSVrd-KAT II group compared to sham or HSVrd group. The vectors are transported to L6-S1 dorsal root ganglia and up-regulate the expression of KAT II.
Conclusion: The HSVrd-KAT II vectors injected into the bladder wall can reduce the urethral resistance, improved DSD and BEE in SCI rats, possibly due to suppressing C-fiber bladder afferents and N-Methyl-D-Aspartate Receptors (NMDAR) blockade in the DRG and spinal cord.
Keywords: Herpes simplex virus; Gene therapy; Kynurenine aminotransferase; Urethral resistance; Spinal cord injury; Rat
Introduction
Detrusor-Sphincter Dyssynergia (DSD) caused by spinal cord injury (SCI) usually lead to an increased urethral resistance, low compliant, elevated pressures in the bladder, hydronephrosis and kidney dysfunction [1,2]. The goal of management for neurogenic bladder is to protect renal function by reducing the bladder pressure during the storage and voiding phase through inhibiting Detrusor Over activity (DO) and reducing urethral resistance.
The glutamatergic system via N-methyl-D-aspartate receptors (NMDARs) activation plays an important role in the control of micturition. MK-801, a potent non-competitive NMDAR antagonist, reportedly increases bladder capacity, reduces bladder contractions [3], inhibits external urethral sphincter contraction and increases the maximum flow rate [4,5] in SCI rats. These results suggest that blocking NMDARs might improve SCI-induced DO and DSD.
Kynurenic acid (KYNA), the only known endogenous antagonist of glutamate receptors, is able to block NMDARs [6,7]. A study showed that KYNA intrathecal application to the sacral cord completely inhibits bladder contractions in cats [8]. In the rat and human brain, over 70% of KYNA is formed from L-kynurenine catalyzed by kynurenine aminotransferase (KAT) II [9,10]. Human KAT II cDNA is about 1.5 kb, encoding a mitochondrial protein of 425 amino residues (NP_057312). Thus, gene delivery of KAT II that increases KYNA, thereby suppressing NMDAR mediated action in micturition, could be a useful approach for the treatment of SCI-induced DO and DSD.
The prosperous researches using replication-defective herpes simplex virus vectors (HSVrd) to treat neuropathies and pain imply the bright future for them in the treatment of bladder dysfunction [11]. HSVrd vectors are non-toxic and neurotropic gene transfer tools so that they could express therapeutic genes after latent infections in dorsal root ganglia (DRG) [12]. These characteristics guarantee the vectors to specifically deliver the KAT II gene to targeted afferent pathways after bladder wall injection with limited side effects in the treatment of SCI-induced neurogenic bladder. Previous reports showed HSVrd-mediated gene transfer of glutamic acid decarboxylase, the enzyme synthesizing GABA, delivered by bladder wall inoculation can reduce DO and DSD in SCI rats [13,14]. In our previous publication [15], we confirmed that HSVrd-mediated gene transfer of KAT II could improve DO in SCI rats. Here we would report if the HSVrd vectors encoding KAT II (HSVrd-KAT II) injection into the bladder wall could reduce urethral resistance.
Materials and Methods
The HSVrd-KAT II vector was constructed by recombining the RG223277 plasmid (Origene Technologies, Beijing, China) human KAT II cDNA into the ICP4, ICP27 and ICP 34.5 deleted HSVrd vector (Sino Geno Max Co., Beijing, China) with 6 His tag, which derived from wild HSV-I virus. Both HSVrd and HSVrd-KAT II express the viral reporter protein RFP under the control of EF1a promoter. The vectors were propagated in the OG01 cell line (Sino Geno Max Co.), which derived from Vero cell (ATCC CCL-81) and supplies ICP4 and ICP27 in trans.
Forty-eight male Sprague-Dawley rats (Academy of military medical sciences, Beijing, China) weighing 240-280 g were spinalized with complete transection of the T 10 spinal cord under 2% Isofluorane anesthesia according to the experimental protocol approved by the ethic committee of China Rehabilitation Research Center. Postoperatively, the rats were treated with ampicillin (100 mg/ kg, i.m.) for three days. The bladder of spinalized rats was expressed manually three times daily until reflex voiding recovered (9-14 days), then once daily afterwards. One week post spinalization, rats were divided into three groups randomly and received bladder wall injection under 2.0% Isofluorane anesthesia: (1) with normal saline (sham; n=16); (2) with HSVrd control vector (HSVrd; n=16) and (3) with HSVrd-KAT II (HSVrd-KAT II; n=16). After exposure of the rat bladder via midline abdominal incision, a total of 40 μl normal saline, viral suspension of HSVrd or HSVrd-KAT II (1×107 plaque-forming units) was injected into 4 sites around the bladder base and 4 sites around the middle of the bladder wall using a 30-gauge syringe (10 μl, Hamilton). After the abdomen was closed, the rats received ampicillin (100 mg/kg, i.m.) treatment for three days.
Three weeks post bladder injection, the rat bladder dome was exposed via a midline abdominal incision and the ureters were ligated at the level of the aortic bifurcation under 2% isoflurane anesthesia. The bladder was emptied and a PE-50 catheter was inserted from the bladder dome to record intravesical pressure. The intravesical catheter was connected to an a PT 100 Pressure Transducer (Chengdu Technology & Market Co., Sichuan, China) for pressure monitoring and to a WZ-50C66T micro infusion pump (Smiths Medical Instrument Co., Zhejiang, China) for bladder infusion through a three-way stopcock. After the abdomen was closed, the rats were allowed to recover from anesthesia for 1-2 h, and then normal saline at room temperature was infused into the bladder at 0.08 ml/min. Once fluid release from urethra occurred, infusion was stopped and residual volume was measured. The data was recorded and analyzed by BL-420F software (Chengdu Technology & Market Co.). Cystometric parameters were recorded after at least two stable micturition cycles. The number and amplitude of all non-voiding bladder contraction NVC, detrusor leak point pressur [(DLPP), cm H2O] and maximum cystometric capacity [(MCC), ml], time to first NVC [(TF), min), leakage volume [(LV), ml] were measured. NVCs were defined as rhythmic intravesical pressure increases greater than 7 cm H2O from baseline pressure without release of fluid from the urethra [13]. MCC was defined as the cystometric volume at DLPP. The bladder emptying efficiency [(BEE), %] was defined as a percentage of leakage volume compared to the MCC.
After cystometry, rats were perfused by 4% paraformaldehyde and L6-S1 DRGs were removed. Cryostat sections (10 μm) of DRG were mounted onto poly-L-lysine-coated slides. Primary antibodies of rabbit anti-human KAT II (H-301) antibody, mouse anti-DsRed (N9) antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and secondary antibodies of goat anti-rabbit IgG conjugated TRITC, Alexa Fluor 488 conjugated rabbit anti- mouse IgG (1: 200; Invitrogen, Carlsbad, CA) were used for the detection of KAT II and RFP. A negative control was prepared without primary antibody. In randomly selected DRG sections (n=3 sections per animal), the intensity of RFP or KAT II immuno reactivity was rated on a four point scale (0-3) and the neurons that exhibited grade 2 or 3 were regarded as positively stained cells. The ratio of positively stained cells/total DRG cells per section was calculated.
L6-S1 DRG were homogenized with RIPA buffer (Sigma- Aldrich, Shanghai, China) containing protease inhibitor cocktail (Sigma-Aldrich) and centrifuged at 10 000 rpm for 10 min at 4°C, and 30 μg protein in the supernatant was separated in a 12% Tris- HCl PAGE-Gel (Bio-Rad laboratories Inc), transferred onto a nitrocellulose membrane (Bio-Rad laboratories Inc), incubated with rabbit anti-human KAT II (H-301) antibody and GAPDH (H- 12) monoclonal antibody (1: 500 and 1: 1 000, respectively) (Santa Cruz Biotechnology) for 2 h at room temperature, followed by HRP-conjugated goat anti-rabbit antibody and HRP-conjugated goat anti-mouse antibody (1:5 000; Santa Cruz Biotechnology) for 1 h at room temperature. The intensity of each band was determined by Versa Doc™ image acquisition and analysis software (Bio-Rad laboratories Inc). The ratio of KAT II/ GAPDH was calculated.
Total RNA was extracted from L6-S1 DRG using TRIzol reagent (Invitrogen) and reverse transcribed into cDNA using TIANScript RT Reagent kit (Tiangen Biotech, Beijing, China). KAT II levels were examined with quantitative real-time PCR with TIANScript RT Reagent kit (Tiangen Biotech) and SYBR Premix Ex Taq II (Invitrogen) using a TL988 II System (Tianlong, Xian, China). Primers for KAT II (forward primer: 5’-TAGTAACCAGAAGGATGCAA-3’; reverse primer: 5’-GCTGAAGAGAAGGATGCTC-3’) and β-actin (forward primer: 5’-ACACCCGCCACCAGTTC-3’; reverse primer: 5’-TGACCCATACCCACCATC-3’) were used. Amplification of cDNA was performed as following: 95°C 2 min, then 45 cycles of 94oC 30s, 54oC 30s, and 72oC 30s. The reaction system was as following: SYBR Premix Ex Taq II 1μl, 10×PCR Buffer 2.5μl, dUTP 0.5μl, each primer 0.5μl, cDNA 2μl and Taq DNA polymerase 0.5μl with RNase Free ddH2O added up to 25μl. Each sample was tested three times and melting curve analysis was performed to confirm primer specificity. Standard curves were constructed from serial dilution of cDNA in each tissue and quantification of the samples was achieved from the standard curve. The ratio of KAT II/β-actin mRNA was compared between the two groups.
The experiments were performed on female Sprague-Dawley rats (100-140g; Hilltop Animal Care, Pittsburgh, PA) in accordance with the requirements and recommendations in the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 1985) approved by the University of Pittsburgh (Pittsburgh, PA) Institutional Animal Care and Use Committee (IACUC). Under 2.0% isoflurane anesthesia, a low dorsal midline incision was performed to remove L6-S1 DRG quickly. DRG were minced, placed in medium consisting of 4.5 ml NEUROBASAL A Medium (Invitrogen, Carlsbad, CA), 5μl 5% B27 supplements (Invitrogen), 45μl 1% Penicillin- Streptomycin-Neomycin solution (Sigma-Aldrich), 0.45ml FBS (Invitrogen), 5mg trypsin (Worthingtown Biochemical; Lakewood, NJ ) and 10mg collagenase type 4 (worthing town Biochemical), and incubated at 37°C for 20–30 min. After trituration using a pipette, the cell suspension was centrifuged for 5 min at 416g. The cell pellet was then re suspended in the medium consisted as above without Trypsin and Collagenase type 4. Neurons were placed on poly-L-lysine-coated 35-mm culture dishes and cultured at 37°C in a 5% CO2 incubator. Vector-untreated neurons were studied after 24–72 h in culture. HSVrd or HSVrd-KAT II-treated neurons (multiplicity of infection: 1:10) were studied after 48-72 h transinfection.
Data are expressed as mean ± SE. One-way ANOVA, Two related samples Wilcoxon tests, Mann-Whitney U tests or Independent t-test was used with P < 0.05 considered statistically significant.
Results
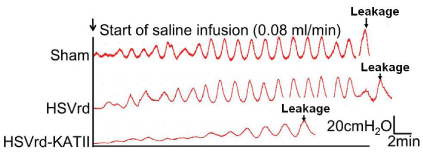
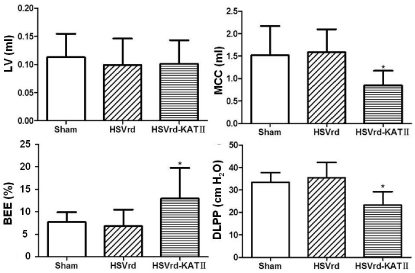
At one week after complete transection of the T 10 spinal cord in male rats, normal saline (sham group; n=16), HSVrd control vector (HSVrd group; n=16) or HSVrd-KAT II (HSVrd-KAT II group; n=16) was injected into the rat bladder wall. At three weeks after injection, the cystometry and the gene expression of KAT II in L6-S1 DRG were evaluated. Representative traces of cystometry in three groups are shown in Figure 1. In sham and HSVrd groups, all rats showed high amplitudes of NVCs before fluid leak from the urethra. There were no significant differences in cystometric parameters between sham and HSVrd groups (Table 1). However, the number and amplitude of NVCs, DLPP and MCC were decreased significantly by 59.6-61.1%, 21.6-24.2%, 30.3-34.4% and 44.1-46.5% (P<0.01), and the BEE and the TF were increased significantly by 40.7-47.7% and 30.1-49.0% (P<0.01), respectively, in the HSVrd-KAT II group compared to sham or HSVrd group (Table 1). The DLPP decreased significantly and the BEE increased significantly suggested that the urethral resistance or bladder outflow resistance reduced in the HSVrd-KAT II group (Figure 2).
Representative traces of cystometry in rats with spinal cord transection.
The traces show that the number and amplitude of non-voiding bladder contractions (NVCs), Detrusor Leak Point Pressur (DLPP) and Maximum Cystometric Capacity (MCC) were decreased in the HSVrd-KAT II group, whereas the time to the first NVC (TF) was significantly prolonged compared with the sham or control HSVrd group.Figure 1 :Representative traces of cystometry in rats with spinal cord transection.
The traces show that the number and amplitude of non-voiding bladder contractions (NVCs), Detrusor Leak Point Pressur (DLPP) and Maximum Cystometric Capacity (MCC) were decreased in the HSVrd-KAT II group, whereas the time to the first NVC (TF) was significantly prolonged compared with the sham or control HSVrd group.
Urethral resistance reduced in the HSVrd-KAT II group with spinal cord transection.
In the HSVrd-KAT II group, the detrusor leak point pressur (DLPP) decreased significantly (P<0.01) and the Bladder Emptying Efficiency (BEE) increased significantly (P<0.01) suggested that the urethral resistance or bladder outflow resistance reduced. LV: Leakage Volume; MCC: Maximum Cystometric Capacity; BEE=LV/MCC×100%.Figure 2 :Urethral resistance reduced in the HSVrd-KAT II group with spinal cord transection.
In the HSVrd-KAT II group, the detrusor leak point pressur (DLPP) decreased significantly (P<0.01) and the Bladder Emptying Efficiency (BEE) increased significantly (P<0.01) suggested that the urethral resistance or bladder outflow resistance reduced. LV: Leakage Volume; MCC: Maximum Cystometric Capacity; BEE=LV/MCC×100%.
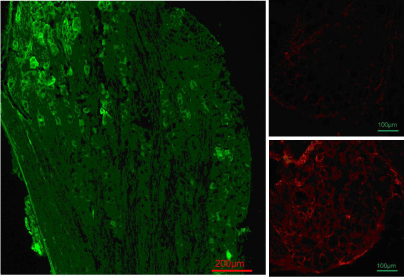
Viral positive neurons shown by red fluorescent protein (RFP) immuno reactivity were found in 37.2 ± 8.6% (HSVrd group) and 39.6 ± 5.7% (HSVrd-KAT II group) of neuronal profiles in DRG sections. The KAT II positive neurons in DRG were significantly increased from 8.1 ± 4.6% of DRG neuronal profiles in the HSVrd group to 32.4 ± 7.2% in the HSVrd-KAT II group (P<0.01) (Figure 3).
DRG immunofluorescent staining in HSVrd and HSVrd-KAT II groups.
Primary antibody of mouse anti-DsRed (N9) antibody and secondary antibody of Alexa Fluor 488-conjugated rabbit antimouse immunoglobulin G were used for the detection of RFP (green color). The primary antibody of rabbit anti-human KAT II (H-301) antibody and the secondary antibody of goat anti-rabbit immunoglobulin G-conjugated tetramethylrhodamine were used for the detection of KAT II (red color). (Left panel) Anti-vector reporter RFP immunofluorescent staining in an L6 dorsal root ganglia section of an HSVrd-KAT II-trans infected rat. The positive neurons with RFP were in 37.2 ± 8.6% of dorsal root ganglia cells in the HSVrd group and 39.6 ± 5.7% of dorsal root ganglia cells in the HSVrd-KAT II group. There were no significant difference between the two groups (P>0.05). (Right panels) KAT II immuno reactivity in an L6 dorsal root ganglia section of an HSVrd-inoculated rat (upper photomicrograph) or an HSVrd-KAT II-inoculated rat (lower photomicrograph). The number of KAT II-positive neurons in dorsal root ganglia sections was increased significantly from 8.1 ± 4.6% in the HSVrd vector group to 32.4 ± 7.2% in the HSVrd-KAT II group (P<0.01).Figure 3 :DRG immunofluorescent staining in HSVrd and HSVrd-KAT II groups.
Primary antibody of mouse anti-DsRed (N9) antibody and secondary antibody of Alexa Fluor 488-conjugated rabbit antimouse immunoglobulin G were used for the detection of RFP (green color). The primary antibody of rabbit anti-human KAT II (H-301) antibody and the secondary antibody of goat anti-rabbit immunoglobulin G-conjugated tetramethylrhodamine were used for the detection of KAT II (red color). (Left panel) Anti-vector reporter RFP immunofluorescent staining in an L6 dorsal root ganglia section of an HSVrd-KAT II-trans infected rat. The positive neurons with RFP were in 37.2 ± 8.6% of dorsal root ganglia cells in the HSVrd group and 39.6 ± 5.7% of dorsal root ganglia cells in the HSVrd-KAT II group. There were no significant difference between the two groups (P>0.05). (Right panels) KAT II immuno reactivity in an L6 dorsal root ganglia section of an HSVrd-inoculated rat (upper photomicrograph) or an HSVrd-KAT II-inoculated rat (lower photomicrograph). The number of KAT II-positive neurons in dorsal root ganglia sections was increased significantly from 8.1 ± 4.6% in the HSVrd vector group to 32.4 ± 7.2% in the HSVrd-KAT II group (P<0.01).
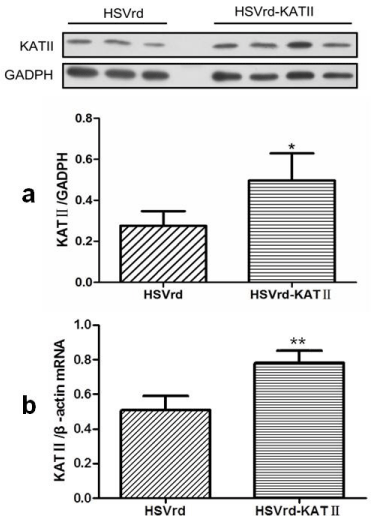
The ratio of KAT II/GADPH proteins, which was used to assess KAT II expression in L6-S1 DRG by Western Blot, was significantly increased by 78.6% in the HSVrd-KAT II group (0.50 ± 0.13) compared to the HSVrd group (0.28 ± 0.07) (P<0.05) (Figure 4A). The ration of KAT II/β-actin mRNA, which was used to assess KAT II mRNA expression in L6-S1 DRG by quantitative real-time PCR, was significantly increased by 52.9% in the HSVrd-KAT II group (0.78 ± 0.06) compared to the HSVrd group (0.51 ± 0.08) (P<0.01) (Figure 4B).
KAT II protein and mRNA expressions in HSVrd and HSVrd-KAT II groups.
L6-S1 dorsal root ganglia in rats of HSVrd and HSVrd-KAT II groups were removed, and KAT II protein (a) and mRNA (b) levels were tested. *P<0.05 and **P<0.01 compared with the HSVrd group, respectively.Figure 4 :KAT II protein and mRNA expressions in HSVrd and HSVrd-KAT II groups.
L6-S1 dorsal root ganglia in rats of HSVrd and HSVrd-KAT II groups were removed, and KAT II protein (a) and mRNA (b) levels were tested. *P<0.05 and **P<0.01 compared with the HSVrd group, respectively.
Discussion
This study indicated that bladder wall injection of HSVrd-KAT II reduced the urethral resistance in rats with T 10 SCT as the DLPP decreased and BEE increased significantly. The reductions in NVC number and amplitudes and prolongation of the time to first NVC also indicated the suppression of DO, and improvement of DSD. It is well known that two types of afferent fibers, namely Ad and C-fibers, carry sensory information from the bladder to the spinal cord [16,17]. In cats and rats, Ad-fiber bladder afferents trigger normal micturition via a long latency supraspinal pathway passing through the pons [16,18]. In SCI rats, C-fiber afferents appear to initiate DO, although Ad bladder afferents still trigger the voiding reflex [16,18], because desensitizing C-fiber afferents by systemic capsaicin administration suppressed NVCs in SCI rats [19]. This capsaicin effect is similar to that seen in the current experiments (i.e., reduced NVCs) following KAT II gene therapy in SCI rats, suggesting that HSVrd-KAT II therapy inhibits DO by suppressing C-fiber bladder afferents whose activity is enhanced in the SCI condition. Previous studies also indicated that hyperexcitability of C-fiber bladder afferents is involved in DSD after SCI because C-fiber desensitization by capsaicin pretreatment reduces urethral contraction pressure during bladder contraction in SCI rats [20]. In this study, although the urethral pressure change during bladder contractions was not directly evaluated, bladder emptying efficiency was increased along with decreased DLPP in HSVrd-KAT II- treated SCI rats, suggesting that HSVrd-mediated KAT II gene delivery reduces urethral resistance during voiding, resulting in improved voiding efficiency. In addition, the inhibition of external urethral sphincter contraction during bladder contractions may contribute to the significant decrease of volume thresholds inducing voiding after HSVrd-KAT II injection because low urethral resistance can induce earlier voiding at a lower bladder pressure. Overall, suppression of C-fiber bladder afferent activity after HSVrd-mediated KAT II gene delivery might also have an inhibitory effect on DSD in SCI rats although further studies are needed to confirm this point.
Glutamate released from primary afferents is a major excitatory neurotransmitter that conveys nociceptive information to the spinal cord dorsal horn neurons [21,22]. Previous studies showed that bladder wall injection of HSVrd vectors encoding glutamic acid decariboxylase, the synthetizing enzyme of GABA, suppressed DO and DSD in SCI rats via stimulation of inhibitory GABA receptors in the spinal cord [13,14]. Therefore, this study also suggests that HSVrd-KAT II injected into the bladder wall are transported through bladder afferent pathways not only to produce KYNA in the DRG neurons to suppress afferent activity, but also to release KYNA into the spinal cord, thereby suppressing NMDAR mediated excitation of the micturition reflex in the spinal cord [15]; the latter may also be another reason for the decreased urethral resistance.
In brief, this study found that HSVrd-KAT II vectors injected into the bladder wall are transported to L6-S1 DRG, which contains bladder afferent neurons, and reduced the urethral resistance, improved DSD and bladder emptying efficiency in SCI rats, possibly due to suppressing C-fiber bladder afferents and NMDAR blockade in the DRG and spinal cord.
Acknowledgment
This study was supported by National Natural Scientific Foundation of China (81270847), China National Technology R&G Program (2012BAI34B02) to Liao.
References
- De Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol Urodyn. 2010; 29: 63-76.
- Castro-Diaz D, Taracena Lafuente JM. Detrusor-sphincter dyssynergia. Int J Clin Pract Suppl. 2006; 17-21.
- Yoshiyama M, Nezu FM, Yokoyama O, Chancellor MB, de Groat WC. Influence of glutamate receptor antagonists on micturition in rats with spinal cord injury. Exp Neurol. 1999; 159: 250-257.
- Watanabe T, Constantinou CE. Analysis of pressure/flow characteristics in the female rat and their pharmacologic modulation. Neurourol Urodyn. 1996; 15: 513-527.
- Yoshiyama M, Roppolo JR, De Groat WC. Alteration by urethane of glutamatergic control of micturition. Eur J Pharmacol. 1994; 264: 417-425.
- Elmslie KS, Yoshikami D. Effects of kynurenate on root potentials evoked by synaptic activity and amino acids in the frog spinal cord. Brain Res. 1985; 330: 265-272.
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001; 21: 7463-7473.
- Iwabuchi N. Sacral glutamatergic transmission in the descending limb of the micturition reflex in the cat. Fukuoka Igaku Zasshi. 1997; 88: 30-38.
- Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J Neurosci Res. 1997; 50: 457-465.
- Kiss C, Ceresoli-Borroni G, Guidetti P, Zielke CL, Zielke HR, Schwarcz R. Kynurenate production by cultured human astrocytes. J Neural Transm. 2003; 110: 1-14.
- Yoshimura N, Miyazato M, Sasaki K, Yokoyama H, Oguchi T, Chancellor MB, et al. Gene therapy for lower urinary tract dysfunction. Int J Urol. 2013; 20: 56-63.
- Manservigi R, Argnani R, Marconi P. HSV Recombinant Vectors for Gene Therapy. Open Virol J. 2010; 4: 123-156.
- Miyazato M, Sugaya K, Goins WF, Wolfe D, Goss JR, Chancellor MB, et al. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther. 2009; 16: 660-668.
- Miyazato M, Sugaya K, Saito S, Chancellor MB, Goins WF, Goss JR, et al. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector mediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. J Urol. 2010; 184: 1204-1210.
- Jia C, Yoshimura N, Liao L. Herpes simplex virus vector-mediated gene transfer of kynurenine aminotransferase improves detrusor overactivity in spinal cord-injured rats. Gene Ther. 2014; 21: 484-489.
- Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992; 319: 615-623.
- Mallory B, Steers WD, De Groat WC. Electrophysiological study of micturition reflexes in rats. Am J Physiol. 1989; 257: R410-421.
- De Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006; 152: 59-84.
- Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995; 678: 40-48.
- Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, De Groat WC, et al. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004; 171: 478-482.
- Li JQ, Chen SR, Chen H, Cai YQ, Pan HL. Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem. 2010; 112: 162-172.
- Jin XG, Chen SR, Cao XH, Li L, Pan HL. Nitric oxide inhibits nociceptive transmission by differentially regulating glutamate and glycine release to spinal dorsal horn neurons. J Biol Chem. 2011; 286: 33190-33202.