
Case Report
Austin J Urol. 2024; 10(1): 1084.
Invasive Urothelial Carcinoma of the Bladder with Inverted Growth Pattern: A Case Report
Reda Tariqi*; Ilyas Soufiani; Hamza El Abidi; Imad Boualaoui; Ahmed Ibrahimi; Hachem El Sayegh; Yassine Nouini
Department of Urologic Surgery “A” Ibn Sina University Hospital, Mohammed V University, Rabat, Morocco
*Corresponding author: Reda Tariqi Department of Urologic Surgery “A” Ibn Sina University Hospital, Mohammed V University, Rabat, Morocco. Email: Dr.tariqireda@gmail.com
Received: April 23, 2024 Accepted: May 21, 2024 Published: May 28, 2024
Abstract
Most urothelial neoplasms of the bladder present in an exophytic papillary form, but some present in an inverted growth form, in which case the invasive feature can be difficult to identify. We present a case of invasive urothelial carcinoma of the bladder associated with an inverted growth pattern, in which the patient underwent total cystoprostatectomy with ilio-obturator lymph node dissection and a Briker-type urinary diversion.
Keywords: Urothelial carcinoma; Inverted growth pattern; Invasive carcinoma; Cystectomy
Introduction
Most urothelial neoplasms of the bladder exhibit an exophytic growth with delicate finger-like or complex and fused papillae, but some present an inverted or endophytic growth pattern. Distinguishing low-grade inverted papillary urothelial carcinoma from inverted papilloma or low-grade inverted papillary urothelial neoplasm with low malignant potential can be challenging [1,2]. This distinction is crucial as the therapeutic options are vastly different, but it is often impossible to differentiate these entities due to morphological overlap. Moreover, there has not been a classification system for inverted neoplasms, nor a well-established system until now.
Case Presentation
A 73-year-old man reports macroscopic haematuria and right-sided hydronephrosis.
Cystoscopy revealed a 5 cm polyp at the bladder floor showing a sessile and solid tumour with an infiltrative appearance. A complete resection of 30 g is performed (8 blocks).
Diagnosis: Invasive papillary urothelial carcinoma extending into the muscle (Stage T2).
Histological description: On some sections, there is a papillary urothelial carcinoma with features of low grade. On other sections, the tumor exhibits inverted, endophytic growth, raising the question of invasion. Multiple well-defined nodules or tumor clusters are observed, with relatively regular contours, in the stroma but also separating the bundles of well-represented bladder muscle seen in the resection material. These tumor aspects, in contact with smooth muscle fibers, are also visible on multiple sections, confirming the invasive nature of the tumor (Stage T2). Despite its invasive nature, the carcinoma remains low grade, with cells displaying mildly atypical nuclei. Focally, some high-grade features may, however, be discussed (Figure 1).
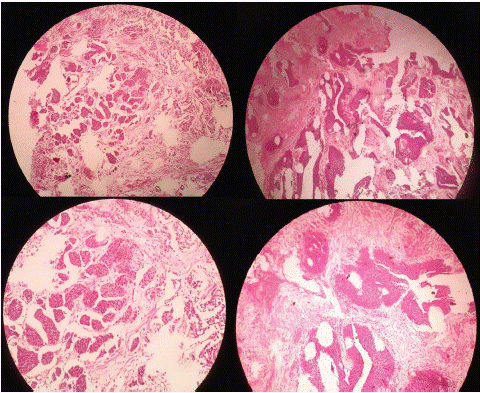
Figure 1: Bladder with invasive urothelial carcinoma arising in a background of urothelial carcinoma with an inverted growth
pattern. Note invasive nests with irregular borders.
Thoraco-abdomino-pelvic CT only reveals the lesion on the bladder floor without any associated lesions (Figure 2).
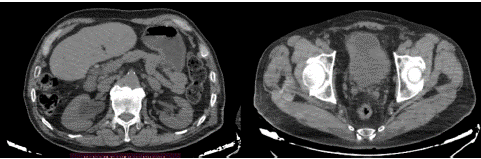
Figure 2: Thoraco-abdomino-pelvic CT reveals the lesion on the bladder floor with right-sided hydronephrosis.
Subsequently, the patient underwent a total cystoprostatectomy with ilio-obturator lymph node dissection and a Briker-type urinary diversion. The cyto-anatomopathological study of the surgical specimen confirmed muscle invasion with peri-vesical fat invasion, classified as pT3N0.
Discussion
Most urothelial neoplasms of the bladder exhibit an exophytic papillary form, but some display an inverted growth pattern. The World Health Organisation (WHO) issued a precise histological classification system for papillary urothelial neoplasms in 2004, but not for inverted type. [3] The recommendations from the International Consultation on Urologic Disease (ICUD) in 2012 apply to inverted/endophytic papillary lesions as follows: 1) inverted papilloma (IP), 2) inverted low-grade urothelial papillary neoplasm (IPUNLMP), 3) non-invasive inverted low-grade urothelial papillary carcinoma (IPUCLG-NI), 4) non-invasive inverted high-grade urothelial papillary carcinoma (IPUCHG-NI), 5) invasive inverted high-grade urothelial papillary carcinoma (IPUCHG-I). [4] However, only the atypical cellular morphology was considered for classification in the 2012 ICUD recommendations, and supporting data for this new classification system are lacking.
Urothelial carcinoma with an inverted growth pattern was initially described decades ago, notable for its morphological features overlapping with inverted urothelial papilloma and its potential for concealed invasion due to its deceptive growth pattern [5]. However, little has been added to the literature to help us understand this ambiguous mode of growth, and it has since been largely forgotten. Nevertheless, it is crucial to be familiar with these lesions as they can sometimes resemble each other, increasing the risk of diagnostic errors [6]. This is a common issue associated with inverted growth urothelial carcinoma and its benign imitator, inverted urothelial papilloma. To differentiate between the two, one must recognize subtle histological differences and the presence of invasion. Inverted urothelial papilloma, a benign tumor, typically presents with a smooth or dome-shaped surface with cords and trabeculae composed of ordered and monotonous cells with occasional peripheral palisading growing endophytically beneath the surface. In contrast, inverted growth urothelial carcinoma may present with an exophytic papillary lesion on the surface and, below, thickened trabeculae with endophytic growth consisting of cytologically atypical cells with increased mitotic figures [7,8]. Sometimes, distinguishing between the two can become extremely challenging, especially when these features are obscured, as in the case of limited biopsy samples with significant crushing or cauterization artifacts, or in the case of tangential sections.
Inverted growth patterns are a known architectural variant in the literature [1,9]. Attention has been drawn to their deceptive nature, especially when the invasive component consists of minimally atypical elements, organized in regular masses, and lacking the usual signs of invasion (irregular cells or small masses, paradoxical differentiation with well-visible cytoplasm, inflammatory stromal reaction). They are related to the so-called large nests recently described by Epstein, who proposed to distinguish them as a true entity [10]. Like the classic nest variant, the cells are minimally atypical and grouped in large nodules with often regular contours, invading the stroma and muscle.
A more recent study proposed grouping tumors described under these different terms - inverted, endophytic growth, and large nests - into a single category, emphasizing that the definition should be primarily architectural (large nodules or inverted papillae ± anastomosed, with regular or irregular contours); for this team, cytonuclear atypies may be observed [11]. The same team does not recommend using the term "large nests," as these tumors have little in common with the classic nest variant. In particular, it seems that inverted growth forms, often associated with nodular invasion, have a relatively more favorable prognosis than classic urothelial carcinomas [12], unlike classic nest carcinomas, which are known to be very aggressive.
Morphological criteria and immunohistochemistry can be used as ancillary methods to classify urothelial neoplasms with a prominent endophytic growth pattern. Amin et al analyzed 18 urothelial carcinomas with an endophytic growth pattern and established a set of morphological criteria to distinguish inverted papillomas from urothelial carcinomas with a prominent endophytic growth pattern [1]. Inverted papillary urothelial carcinoma tends to present with an exophytic papillary surface, irregularly wide cords or trabeculae, possible invasion, cytological atypies, and the absence of maturation, spindles, or palisades. Terada et al [13] reported that inverted variants of Urothelial Carcinoma (UC) exhibit cytological atypies and thickened trabeculae that are absent in inverted papilloma. Immunohistochemically, p53 expression and a high Ki-67 labelling index were observed in the inverted UC variant, whereas they were negative and very low, respectively, in the inverted papilloma. Sun and colleagues also studied immunohistochemistry of Ki-67, p53, CK20, cyclin D1, and UroVysion fluorescence in situ hybridization (FISH) for gains of chromosomes 3, 7, or 17 or homozygous loss of 9p21 to distinguish non-invasive low-grade urothelial carcinoma with an inverted growth pattern (ILGNUC) from inverted papilloma [8]. They demonstrated that Ki-67 and CK20 expression were higher in ILGNUC than in inverted papilloma. Moreover, 24 out of 38 (63.2%) ILGNUC cases were positive for UroVysion FISH, while all inverted papillomas were negative. Morphologically, ILGNUC presented with thick and irregular cords, large nests, and more mitotic figures in the upper layers, whereas inverted papilloma consisted of thin anastomosed cords and exhibited microcysts, peripheral palisading, streaming, and fewer mitotic figures.
The reported observation here perfectly illustrates the staging challenges posed by large rounded nodules or inverted papillae consisting of minimally atypical cells. The muscle invasion described here is entirely in line with the right hydronephrosis present in this patient, always suggestive to the clinician of advanced-stage disease. The cystectomy performed on the patient will confirm the massive invasion of the muscle with invasion of the peri-vesical fat (pT3 stage). The low-grade aspects with minimally atypical nuclei and tumor cells often grouped in stratified nodules complicate the staging evaluation, but the extent of the observed features in contact with muscle bundles and on multiple sections leads to the correct diagnosis, essential for appropriate patient management.
Conclusion
Invasive urothelial carcinomas with inverted/endophytic growth can be challenging to identify when the invasive component is composed of large, rounded, regular masses with few cytological atypies; Recognition of muscle invasion is crucial for appropriate management; This tumor group could represent a variant of urothelial carcinoma, also described under the term large nests.
References
- Amin MB, Gomez JA, Young RH. Urothelial transitional cell carcinoma with endophytic growth patterns: a discussion of patterns of invasion and problems associated with assessment of invasion in 18 cases. Am J Surg Pathol. 1997; 21: 1057–68.
- Sun JJ, Wu Y, Lu YM, Zhang HZ, Wang T, Yang XQ, et al. Immunohistochemistry and fluorescence in situ hybridization can inform the differential diagnosis of low-grade noninvasive urothelial carcinoma with an inverted growth pattern and inverted urothelial papilloma. PLoS One. 2015; 10: e0133530.
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. WHO classification of tumours of the urinary system and male genital organs. 3rd ed Lyon: IARC Press. 2004.
- Amin MB, Smith SC, Reuter VE, Epstein JI, Grignon D, Hansel DE, et al. Update for the practicing pathologist: The International Consultation on Urologic Disease-European association of urology consultation on bladder cancer. Mod Pathol. 2015; 28: 612–3.
- Heejin Banga, Heejung Parkb, Sanghui Parkc, Euno Choic, Min-Sun Choc, Sun Hee Sungc, et al. Clinicopathologic study of 60 cases of urothelial neoplasms with inverted growth patterns: Reclassification by international consultation on urologic disease (ICUD) recommendations. Annals of Diagnostic Pathology. 2020; 44: 151433.
- Christina M. Gutierrez, Mehrdad Alemozaffar, Adeboye O Osunkoya. Invasive high-grade urothelial carcinoma of the bladder, renal pelvis, ureter, and prostatic urethra arising in a background of urothelial carcinoma with an inverted growth pattern: a contemporary clinicopathological analysis of 91cases. Human Pathology. 2019; 92: 18–24.
- Cheng L, MacLennan GT, Bostwick DG. Urologic surgical pathology. 4th ed. Philadelphia, PA: Philadelphia, PA: Elsevier, Inc. 2020.
- Sun JJ, Wu Y, Lu YM, Zhang HZ, Wang T, Yang XQ, et al. Immunohistochemistry and fluorescence in situ hybridization can inform the differential diagnosis of low-grade noninvasive urothelial carcinoma with an inverted growth pattern and inverted urothelial papilloma. PLoS One. 2015; 10: e0133530.
- Jones TD, Zhang S, Lopez-Beltran A, Eble JN, Sung MT, MacLennan GT, et al. Urothelial carcinoma with an inverted growth pattern can be distinguished from inverted papilloma by fluorescence in situ hybridization, immunohistochemistry, and morphologic analysis. Am J Surg Pathol. 2007; 31: 1861-7.
- Cox R, Epstein JI. Large nested variant of urothelial carcinoma: 23 cases mimicking von Brunn nests and inverted growth pattern of noninvasive papillary urothelial carcinoma. Am J Surg Pathol. 2011; 35: 1337-42.
- Brimo F, Dauphin-Pierre S, Aprikian A, Kassouf W, Tanguay S, Ajise O, et al. Inverted urothelial carcinoma: a series of 12 cases with a wide morphologic spectrum overlapping with the large nested variant. Hum Pathol. 2015; 46: 1506-13.
- Jimenez RE, Gheiler E, Oskanian P, Tiguert R, Sakr W, Wood DP, et al. Grading the invasive component of urothelial carcinoma of the bladder and its relationship with progression-free survival. Am J Surg Pathol. 2000; 24: 980—7.
- Terada T. Inverted variant of urothelial carcinoma of the urinary bladder: a report of three cases and a proposal for a new clinicopathologic entity. Int J Clin Exp Pathol. 2013; 6: 766–70.