
Case Report
Austin J Urol. 2015;2(2): 1022.
Metastatic Urothelialcarcinoma to the Testis Presenting as Epididymo-Orchitis
Fuller TW, Ristau BT, Bandari and Benoit RM*
Department of Urology, University of Pittsburgh Medical Center, USA
*Corresponding author: Benoit RM, Department of Urology, University of Pittsburgh Medical Center, 1350 Locust Street, Pittsburgh, PA 15219
Received: February 06, 2015; Accepted: February 25, 2015; Published: February 26, 2015
Abstract
A 70 year-old male with a complicated past urologic history including right testicular abscess requiring orchiectomy, prostate cancer, non-muscle invasive Urothelial Carcinoma (UC) of the bladder, and distal ureteral UC presented with signs and symptoms consistent with epididymo-orchitis. It was refractory to multiple prolonged courses of parenteral antibiotics as well as surgical drainage of a paratesticular fluid collection. Ultimately, orchiectomy was performed and pathologic evaluation of the testis revealed metastatic urothelial carcinoma. The testes represent a very rare metastatic location for urothelial carcinoma when there is no other evidence of systemic disease.
Keywords: Urothelial carcinoma; Metastasis; Testis; Epididymo-orchitis; Orchiectomy
Abbreviations
UC: Urothelial Carcinoma; TUIP: Transurethral Incision of the Prostate; TURP: Transuretheral Resection of the Rostate
Introduction
We present a case of metastatic urothelial carcinoma to the testis in the absence of other identifiable metastatic disease. The most common sites of metastatic bladder UC in order of decreasing frequency are regional lymph nodes, bone, lung, liver, and peritoneum [1,2]. The testis represent an especially unusual site of metastasis due to the postulated limited metastatic access conferred by the bloodtestis barrier [3].
Case Report
A 70-year-old male with an extensive urologic history developed urinary retention several years after undergoing prostate brachytherapy. After medical therapy failed to resolve his retention, he elected to proceed with surgical intervention in hopes of obviating the need for intermittent catheterization. Given the significant risk of urinary incontinence resulting from surgical intervention to the prostate after pelvic radiotherapy, he initially underwent a Transurethral Incision of the Prostate (TUIP). This procedure failed to resolve his urinary retention and he was subsequently scheduled for Transurethral Resection of the Prostate (TURP). On preoperative examination the patient had a swollen, tender, erythematous, left hemiscrotum. He had a leukocytosis of 32,200. He was admitted for treatment of presumed epididymo-orchitis.
In addition to prostate cancer, he had a history of urothelial carcinoma involving both his upper urinary tract and bladder. He had undergone multiple trans-urethral resections of the bladder for superficial disease, including high grade disease invading the lamina propria (HGT1). Restaging transurethral resection of his bladder tumorat that time had confirmed the lack of muscle invasion. He had also undergone a distal ureterectomy for a 2.8 cm low grade tumor with focal invasion into muscle (T2N0MX). His distal ureterectomy occurred approximately 18 months prior to his episode of presumed epididymo-orchitis, while his last bladder tumor was resected approximately 12 months prior to this presentation.
On admission the patient was started on intravenous antibiotics and a urine culture was sent. His exam failed to improve, and his WBC did not decrease despite antimicrobial therapy and negative urine cultures. A scrotal ultrasound demonstrated a complex extratesticular fluid collection and a reactive hydrocele (Figure 1). Surgical intervention was considered as he failed to improve with more conservative therapy, but since the patient was hemodynamically stable, afebrile, and nontoxic in appearance; the patient elected to continue IV antibiotics rather than proceed with surgery. He was also reluctant to accept the risk of a left orchiectomy as he had a prior right orchiectomy for testicular abscess many years earlier.
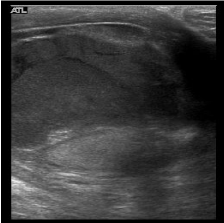
Figure 1: A complex layering septated fluid collection external to the left
testes. The testis is labeled in the dependent scrotum.
His leukocytosis increased to 40,000 as an outpatient despite IV antibiotics and his exam minimally worsened so he was again offered surgical intervention. On exploration of his scrotum, abloody but not purulent fluid was drained. Intraoperative cultures of this fluid demonstrated no growth. Surgical drainage failed to improve his WBC, which subsequently increased to 60,000.
Over the next several weeks he continued with a persistent leukocytosis which eventually became greater than 100,000. He continued to have local pain and an indurated, erythematous, left hemiscrotum. After surgical drainage he developed intermittent venous bleeding from the surgical wound. Due to the recurrent bleeding, increasing WBC, the failure of his examination to improve, and the increasing possibility that his condition was not infectious in nature; the patient was offered a left orchiectomy. In anticipation of the necessity of orchiectomy a testosterone level was obtained and was at castrate level. Upon exploration, the testis was completely replaced by an infiltrative process which continued superiorly to the spermatic cord. The cord was dissected to a level above this process and the testis and cord were removed. Pathologic evaluation of the specimen revealed a poorly differentiated urothelial carcinoma (Figure 2).
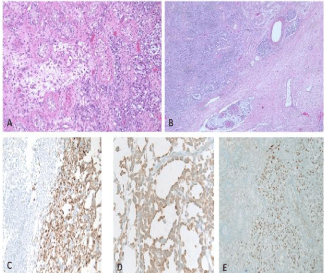
Figure 2: (A,20x) H&E slide demonstrating tumor infiltrating atopic
seminiferous tublules; (B,40X) H&E slide demonstrating lymphatic tumor
emboil; (C,200X) Cytokeratin stain demonstrating epithelial tumor origin and
confirming metastatic carcinoma; Thrombomodulin (D, 200X) and GATA3 (E,
100X) positive IHC phenotype consistent with urothelial origin.
The patient had axial imaging on several occasions throughout the above described course. These studies demonstrated no evidence of retroperitoneal or pelvic lymphadenopathy, liver, lung, or bone lesions with the exception of the suggestion of an infiltrative process in his humerus seen in a post orchiectomy CT chest that incidentally included his upper arms.
Discussion
The metastatic pattern of urothelial carcinoma has recently been described by Shinagare et al. In their review of 150 patients the most common sites of metastasis were lymph nodes, bone, lung, liver, and peritoneum; in order of decreasing frequency. Less than 3% of the patients had metastasis outside of these sites without first having metastatic lesions in one of these five locations [1]. Metastatic lesions to the testis from other primary tumors are also rare. In order of decreasing frequency, tumors of the prostate, lung, gastrointestinal tract, melanoma, and renal parenchymahave been reported to metastasize to the testes [2]. It has been hypothesized that the bloodtestis barrier prevents metastasis as well as possible acting to limit the chemotherapeutic and immune access to the testis [3]. Several case reports have been published of metastatic UC to the testis, and the lesions were thought to be possibly related to an invasive prostatic component. It has been suggested by these authors that it may represent a direct extension of disease that bypassed the blood-testis [4,5].
Independent of possible direct extension, prostatic involvement by TCC in whole-mount sections correlated with lymph node involvement. When the carcinoma was invasive to the prostatic stroma the likelihood of nodal metastasis was greater than no involvement, CIS, or prostatic lamina propria involvement [6].
We present a case of metastatic urothelialcarcinoma to the testiswithout other signs of extravesical disease. The clinical presentation was consistent with epididymo-orchitis and he underwent extensive treatment for this diagnosis. A broad differential must be considered when the clinical response to treatment is incongruent with presumed pathology. This is especially true with a history of invasive UC despite reassuring axial imaging.
Acknowledgement
Special thanks to Dr. Sheldon Bastacky of the UPMC department of pathology for his expertise and assistance in development of the associated figure.
References
- Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. Metastatic Pattern of Bladder Cancer: Correlation with the Characteristics of the Primary Tumor. American Journal of Roentgenology. 2011; 196: 117-122.
- Morgan K, Srinivas S, Freiha F. Synchronous solitary metastasis of transitional cell carcinoma of the bladder to the testis. Urology. 2004; 64: 808-809.
- Bart J, Groen HJ, van der Graaf WT, Hollema H, Hendrikse NH, Vaalburg W, et al. An oncological view on the blood-testis barrier. Lancet Oncology. 2002; 3: 357-363.
- Kozak GN, Field NC. Metastatic transitional cell carcinoma of the bladder to the testis: A Case Report. Case Reports in Urology. 2012.
- Thwaini A, Kaluba J, Shergill I, Kumar R, Lewi H. Testicular metastasis of transitional cell carcinoma of the urinary bladder: An unusual site. International Journal of Urology. 2006; 13: 1136-1137.
- Shen SS, Lerner SP, Muezzinoglu B, Truong LD, Amiel G, Wheeler TM. Prostatic involvement by transitional cell carcinoma in patients with bladder cancer and its prognostic significance. Human Pathology. 2006; 37: 726-734.