
Case Report
Austin J Urol. 2015;2(3): 1028.
Renal Hydatid Cyst Mimicking Renal Cell Carcinoma
Sidani MN, Graziano CE, Soni SD and Smith TG*
Department of Urology, University of Baylor, USA
*Corresponding author: Smith TG, Department of Urology, University of Baylor, 7200 Cambridge Street, Houston, TX, 77030, USA
Received: September 28, 2015; Accepted: October 20, 2015; Published: November 04, 2015
Abstract
Hydatid cyst disease occurs as an isolated infection of the urinary tract in 2-4% of cases. With the rise in immigration from endemic areas into developed nations, reported cases in nations like the United States of America are increasing. Our goal is to shed light on this rare tropical disease in an effort to encourage physicians to include this entity in their differential diagnosis of a renal mass.
A 33-year-old man presented for evaluation of chronic flank pain and frothy urine with fleshy white clots. CT and MRI imaging showed a large multilobular cystic renal mass, which was suspicious for renal cell carcinoma. The patient underwent a radical nephrectomy and a pathologic diagnosis of renal hydatid cyst was reached.
Symptoms of renal hydatid cyst are highly variable and non-specific. Ultra sonography and CT may be helpful to reach a diagnosis and distinguish this disease from renal cell carcinoma, but supplemental blood work-up is often necessary. Therapy entails a combination of surgical and medical approaches.
Renal hydatid disease can be easily confused with the cystic form of renal cell carcinoma Thus, in an effort to shed light on this condition; we have summarized the most distinctive features of renal hydatid cyst disease.
Keywords: Echinococcus; Renal cell; Cyst; Hydatid
Introduction
Hydatid disease is a rare infection caused by the parasite Echinococcus granulose. The parasite is transmitted via the fecaloral route and dogs, the definitive host, become infected after eating the organs of animals infected with hydatid cysts. Humans are an intermediate host who can become infected after the ingestion of food or water contaminated with the fecal matter of an infected dog. The disease is endemic in regions where stockbreeding is a major trade [1]. Infection is initiated when the ingestion of an egg containing an embryo crosses the mucosal lining of the intestine and gains access to the liver via the portal vein. In the liver the embryo becomes arrested and develops into a hepatic hydatid cyst. However, if the embryo enters the pulmonary circulation it can produce pulmonary cysts, and may continue on to the peripheral circulation and to other organs including the kidney or brain. The kidney is the third most common location for cyst formation [2]. Renal hydatid disease may lead to significant morbidity from urinary symptoms and flank pain, and may cause renal deterioration. Furthermore, spontaneous cyst rupture into the peritoneal cavity resulting in death from systemic anaphylaxis has also been reported [3].
With the rise in immigration to the United States over the past two decades (Figure 1), the number of reported cases of echinococcosis in North America has been increasing [4]. Moreover, the World Health Organization has classified the disease as a Neglected Zoonosis subgroup for its 2008– 2015 plan for the control of Neglected Tropical Diseases [5].
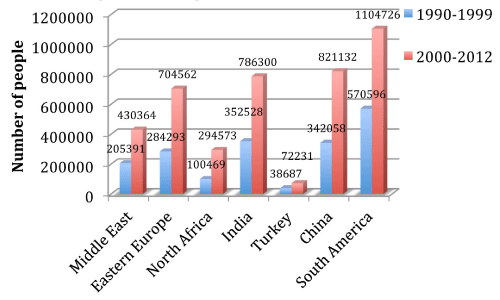
Figure 1: Number of Immigrants obtaining permanent residence in the US
with respect to their region of origin in the past 2 decades.
In the literature, there have been several case reports of urinary tract hydatid cyst disease being misdiagnosed as a urinary tract malignant neoplasm, resulting in unwarranted diagnostic tests and unnecessary surgery with long-term consequences [6]. In reporting this case of primary renal echinococcosis, our goal is to shed light on this rare tropical disease, and to assist in distinguishing renal hydatid disease from malignant renal neoplasms.
Case Presentation
A 33-year-old male with no past medical history presented to our institution for the evaluation of intermittent left flank pain. The pain had been present for 10 years, was dull and achy in nature, and non-radiating. The pain was associated with hematuria, dysuria and intermittent urination of fleshy clots per urethra. He denied fevers or weight loss, but he did report occasional night sweats. He had been a smoker for 15 years. He was originally from Iraq, but had been living in the United States for 5 years. The physical exam was unremarkable
On work-up, laboratory evaluation including WBC, eosinophil counts, and creatinine were unremarkable. A urinalysis showed numerous RBCs and WBCs along with mucus. A non-contrast abdominal Computed Tomography (CT) showed a 7.9 cm multilobular exophytic left renal mass that had a single septation, associated with a mildly dilated left urethra (Figure 2).
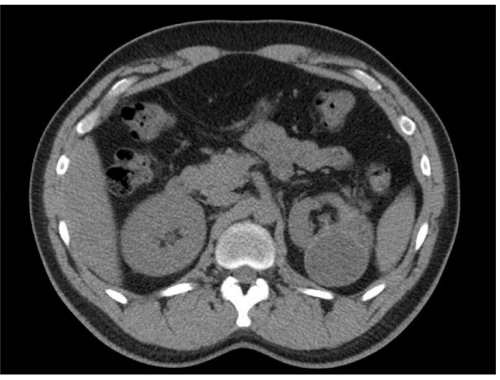
Figure 2: Preoperative CT abdomen showed a large left renal cyst with two
lobes separated by a thin S-shaped membrane.
On MRI the mass appeared as heterogeneous with nonenhancing internal cystic spaces, and was highly concerning for malignancy, specifically for cystic renal cell carcinoma (Figure 3). Differential renal function for the affected kidney was noted to be 5 percent. Based on the above findings, the patient underwent an uneventful left laparoscopic radical nephrectomy. After pathological and microbiological examination, a diagnosis of primary isolated renal echinococcosis, also known as primary renal hydatid cyst, was reached. Grossly, the kidney specimen contained a 7.9 x 6.5 x 4 cm cystic lesion filled with purulent and necrotic thick material and multiple cysts filled with clear fluid The surrounding parenchyma was necrotic secondary to mass effect from the large cyst (Figure 4). No malignant tissue was identified. The patient did well post-operatively, and the patient was started on a course of albendazole 200 mg twice daily for a total of 4 weeks.
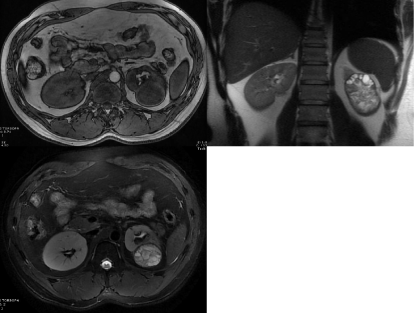
Figure 3: Preoperative MRI, showing a heterogeneous, well circumscribed
mass in the lower pole of the kidney, suspicious for cRCC. A:T2 weighted.
B:T1 weighted. C:T2 weighted.
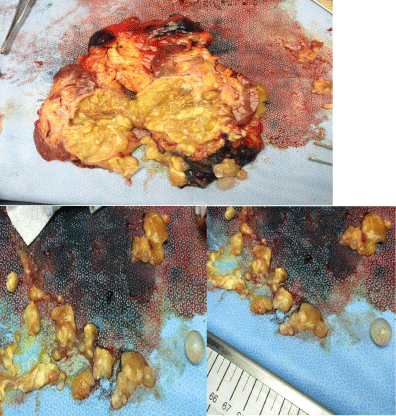
Figure 4: Gross appearance of kidney showing necrotic parenchyma
surrounding several opened and intact daughter cysts expelled from the large
mother cyst.
Discussion
Renal cysts can range from simple to complex, with the more complex cysts often harboring malignant tissue. The Bosniak classification [7] is a useful tool to stratify the internal complexity of renal cysts, but it cannot differentiate cysts of infectious etiology. Unfortunately, it is often quite difficult to distinguish a hydatid cyst from a complex renal cyst by relying solely on imaging techniques. In cases where clinical history and imaging studies fail to provide a definitive diagnosis, other diagnostic modalities such as a complete blood count, urinalysis, and immune histochemistry may be helpful.
Presentation
Renal hydatid cysts have a wide range of presenting signs and symptoms, many of which are nonspecific, such as flank or abdominal pain, hypertension, and a palpable flank mass. In 5-25% of cases of renal hydatid cysts, patients present with hydatiduria, the presence of fleshy, grape-like white to-yellow clots or cysts in the urine [8-10]. In addition, eosinophilia can be detected in 20-50% of patients [11].
Imaging
Several imaging modalities are valuable when differentiating a renal hydatid cyst from a non- infectious complex renal cyst. Excretory urography can demonstrate distortion of the calyceal system secondary to mechanical compression from the cysts [12,13]. In some cases cysts can be released into the collecting system causing obstruction and appearing as well-circumscribed circular or ellipsoid filling defects [14].
Renal ultrasound findings indicative of a hydatid cyst have been separated into multiple categories, representing the different features of the cyst at various maturation stages. The WHO/IWGE classification system is the most widely utilized and categorizes hydatid cysts into five different types based on ultrasound imaging [14-19].
Type I cysts exhibit a double-layered thick wall [14,17].
Type II cysts have a floating, S-shaped, membrane dividing the cyst [17].
Type III cysts are multivesicular cysts with a classic honeycomb appearance [18].
Type IV cysts display a characteristic “spiral sign”, representing fluid trapped between two membranes, surrounding a tumor-like mass [19].
Type V cysts have a distinctive calcified rim surrounding the cyst wall [17].
Typical findings of renal hydatid cyst on CT imaging include: 1) unilocular cysts with a calcified rim occasionally associated with membrane detachment, or 2) multiloculated heterogeneous cysts that occasionally contain daughter cysts of lower density than the maternal matrix [17]. On MRI, type I and II cysts appear as a characteristic low-signal intensity rim surrounding a hyper intense signal on T2-weighted images. On T1-weighted images, the maternal matrix is usually hypo intense.
Differentiating hydatid cyst from renal neoplasm
Many entities can mimic renal neoplasm’s, but some require a thorough investigation with pathological, immunological and microbiological diagnostic tools to reach a diagnosis [20]. Hydatid disease is an infamous imitator of several neoplasm’s, depending on the location that it seeds [6,19,21-23]. The ability to differentiate cystic renal cell carcinoma from hydatid cysts may be further improved using a technique published by Song et al in 2009. Using a combination of Bosniak classification and the degree of enhancement on CT, an enhancement value of = 42 Hounsfield units was determined to provide a 100% NPV for malignancy, whereas enhancement >42 Hounsfield units had a PPV of 96.7% [23].
Immuno histochemistry
Several immuno histochemical tests have been developed to aid in the diagnosis of hydatid cyst disease. The Casoni test, a hypersensitivity skin test involving the intradermal injection of fluid from hydatid cysts, produces positive results in about 25-50% of cases. Alternatively, the Weinberg test, which diagnoses hydatidosis by hem agglutination and complement fixation, provides a reported sensitivity of about 40% [24]. The indirect hem agglutination test is more frequently utilized and has a sensitivity of 90% for liver hydatid disease; however, no clear data has been published about the sensitivity of this test for renal hydatid disease [26]. One of the more recent diagnostic tests, Echinococcus IgG ELISA, detects host antibodies directed against Echinococcal antigen “” [25]. This test is highly sensitive (94%) for liver hydatid disease, but to date no studies have reported the sensitivity of this test for renal hydatid disease [27].
Management
Therapy for renal hydatid cyst disease involves a combination of surgical and medical approaches. Surgical options for renal echinococcosis range from cyst nucleation to partial nephrectomy to radical nephrectomy [21,25,32]. Historically, surgical treatment was performed with an open approach, but with the advent of minimally invasive procedures, percutaneous treatment has become a more common technique when feasible. It is important to note that controversy exists over the safety of the percutaneous approach with regards to spillage of cystic fluid leading to dissemination and anaphylactic shock. However, Neumayr et al showed that the incidence of fatal anaphylaxis was only 0.03%, making it a relatively rare event in the setting of surgical manipulation of a hydatid cyst [28].
The decision to perform a radical nephrectomy for renal hydatid disease involves several factors including an assessment of the differential renal function, amount of residual viable tissue, and the presence of a normally functioning contra lateral kidney. Importantly, recurrence rates following nephron-sparing surgery are in the 10-30% range. Such high recurrence rates provide a strong rationale for implementation of adjuvant medical treatment to ensure complete resolution of the disease [19]. Currently, the most effective medical treatment consists of oral albendazole, given at a dose of 10-15 mg/kg/day in two divided doses [29]. Several authors have implemented albendazole as part of their postoperative protocol to prevent recurrent renal and non-renal hydatid disease [30]; however, to date there is no strong evidence this improves outcomes. Furthermore, these drugs have several significant side effects, which should be weighed against the risk of recurrence or anaphylaxis prior to initiating medical therapy.
Conclusion
Renal hydatid disease is rare, but its prevalence may be increasing due to rising rates of immigration from endemic regions. In the mature form, it can be confused with the cystic form of renal cell carcinoma, both with regards to presentation and appearance on imaging. We have summarized the most distinctive features of this disease in an attempt to assist in diagnosis and treatment.
References
- Shukla A, Saurabh G, Pramod V. A Case of Large Renal Hydatid Cyst. Saudi J Kidney Dis Transpl. 2011; 22: 538-540.
- Huang M, Zheng H. Clinical and Demographic Charactertics of patients Urinary Tract Hydatid disease. Plos One. 2012; 7: 7667.
- Morar S, Dura H, Cristian A, Perju-Dumbrava D, Boicean A, Cernusca-Mitariu M, et al. Hydatid cyst – a rare etiology of sudden death. Case report and literature review. Rom J Leg Med. 2014; 22: 31-34.
- Eckert J, Conraths FJ, Tackmann K. Echinococcosis: an emerging or reemerging zoonosis? Int J Parasitol. 2000; 30: 1283-1294.
- World Health Organization. Global plan to combat neglected tropical diseases. 2008-2015, 2007.
- Sarf I, Meziane A, El Mejjad A, Taha A, Aboutaeib R, Meziane F. Pseudotumoral aspect of the Hydatic Cyst of the Kidney. Scientific World Journal. 2004; 4: 758-759.
- Israel GM, Bosniak MA. An update of the Bosniak renal cyst classification system. Urology. 2005 ; 66: 484-488.
- Afsar H, Yag CF, Meto S, Aybasti N. Hydatid disease of the kidney: evaluation and features of diagnostic procedures. J Urol. 1994; 151: 567.
- Angulo JC, Sanchez-Chapado M, Diego A, Escribano J, Tamayo JC, Martin L. Renal echinococcus: clinical study of 34 cases. J Urol. 1997; 157: 787.
- Ozturk A, Onur K, Ozturk E, Sirmatel O. An unusual complication of renal hydatid disease: Macroscopic hydaturia. Eur. J Radiol Extra. 2005; 54: 3–8.
- Buckley RJ, Smith S, Herschorn S, Comisarow RH, Barkin M. Echinococcal disease of the kidney presenting a renal filling defect. J Urol. 1985; 133: 660-661.
- Gilsanz V, Lozano G, Jimenez J. Renal hydatid cysts: communicating with collecting system. Am J Roentgenol. 1980; 135: 357-361.
- Horchani A, Nouira Y, Kbaier I, Attyaoui F, Zribi AS. Hydatid cyst of the kidney: a report of 147 controlled cases. Eur Urol 2000; 38: 461-467.
- Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radio Graphics. 2003; 23: 475–494.
- Gharbi HA, Hassine W, Brauner MW, Dupuch K. Ultrasound examination of the hydatic liver Radiology. 1981; 39: 459-463.
- Brunetti E, Kern P, Vuitton DA. Writing Panel for the WHO-IWGE Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Tropica. 2010; 114: 1–16.
- Turgut AT, Altin L, Topçu S. Unusual imaging characteristics of complicated hydatid disease. Eur J Radiol. 2007; 63: 84–93.
- Zmerli S1, Ayed M, Horchani A, Chami I, El Ouakdi M, Ben Slama MR. Hydatid cyst of the kidney: diagnosis and treatment. World J Surg. 2001; 25: 68-74.
- Sansot M, LeTrent Y, Burger G, Marlois O, Jouve P. Symptomatology of pseudotumoral forms of hydatid cyst of the liver: apropos of 7 cases [in French]. Ann Radiol (Paris). 1983; 26: 370–375.
- Tynski Z, Gregory T. MacLennan. Renal Pseudo tumors. The Journal of Urology. 2005; 173: 600.
- Ibis C, Albayrak D, Altan A. Primary Hydatid Disease of Pancreas Mimicking Cystic Neoplasm. Southern Medical Journal. 2009; 102: 529-530.
- Diamond HM, Lyon ES, Hui NT. Echinococcal disease of the kidney. J Urol. 1976. 115, 742.
- Song C, Min GE, Song K, Kim JK, Hong B, Kim CS, et al. Differential Diagnosis of Complex Cystic Renal Mass Using Multiphase Computerized Tomography. The Journal of Urology. 2009; 181: 2446-2450.
- Aragona P, Di Candio G, Serretta V, Fiorentini D. Renal hydatid disease: report of 9 cases and discussion of urologic diagnosis procedures. Urol Radiol. 1984; 6: 182-186.
- Razzaghi MR, Mazloomfard MM, Bahrami-Motlagh H, Javanmard B. Isolated renal hydatid cyst: diagnosis and management. Urol J. 2012; 9: 718-720.
- Force L, Torres JM, Carillo A, Busca J. Evaluation of eight serological tests in the diagnosis of humanechinococcosis and follow-up. Clin Infect Dis. 1992; 15: 473-480.
- Smego RA Jr1, Bhatti S, Khaliq AA, Beg MA. Percutaneous aspiration-injection-respiration drainage plus albendazole or mebendazole for hepatic cysticechinococcosis: a meta-analysis. Clin Infect Dis. 2003; 37: 1073-1083.
- Neumayr A, Troia G, de Bernardis C. Justified concern or exaggerated fear: the risk of anaphylaxis in percutaneous treatment of cystic echinococcosis-a systematic literature review. PLoS Negl Trop Dis. 2011; 5: 1154.
- Tryfonas GJ, Avtzoglou PP, Chaidos C, Zioutis J, Gavopoulos S, Limas C. Renal hydatid disease: diagnosis and treatment. J Pediatr Surg. 1993; 28: 228.
- Sielaff TD, Taylor B, Langer B. Recurrence of hydatid disease. World J Surg. 2001; 25: 83-86.