
Case Report
Austin J Urol. 2016; 3(1): 1040.
A Case of Bowel Perforation during Temsirolimus Treatment for Metastatic Renal Cell Carcinoma
Matsuzawa Y¹, Nishimatsu H¹*, Hagiwara K¹, Murayama S¹, Kaneko T¹, Hirano Y¹, Kawamura T¹ and Homma Y²
¹Department of Urology, The Fraternity Memorial Hospital, Japan
²Department of Urology, University of Tokyo, Japan
*Corresponding author: Nishimatsu H, Department of Urology, The Fraternity Memorial Hospital, 2-1-11 Ryogoku, Sumida-ku, Tokyo, Japan
Received: January 13, 2016; Accepted: January 22,2016; Published: January 25, 2016
Abstract
Treatment outcomes for metastatic Renal Cell Carcinoma (mRCC) have improved with the use of molecular-targeted drugs. However, we should manage several kinds of Adverse Event (AE) more carefully. We report a case of bowel perforation during temsirolimus treatment for metastatic Renal Cell Carcinoma (RCC). A 59-year-old male with left RCC and left supraclavicular lymph node and lung metastases underwent radical nephrectomy in February 2014. Temsirolimus was started after surgery, and Computed Tomography (CT) 3 months later showed shrinkage of the metastases. However, after fourteen courses of temsirolimus treatment, the patient suffered from severe right lower abdominal pain, and CT showed ascites and free air in the anterior surface of the liver. He was diagnosed with bowel perforation, and emergent surgery was performed. After the surgery and antibiotic treatment, the patient recovered. We have to keep in mind the risk of bowel perforation during temsirolimus treatment.
Keywords: Bowel perforation; Temsirolimus; Renal cell carcinoma
Introduction
The treatment of mRCC has markedly changed with the recent introduction of molecular-targeted drugs. Such drugs are offering clinical benefits over immunotherapy when AEs are managed appropriately. Temsirolimus is one of the molecular-targeted drugs, a novel inhibitor of the mammalian Target of Rapamycin (mTOR). MTOR is a serine-threonine kinase of the phosphatidylinositol 3-kinase (PI3K/Akt) signaling pathway, which has a central role in the control of cell growth, proliferation, and survival. Furthermore, mTOR prevents tumor cell proliferation and angiogenesis through inhibition of the hypoxia inducible factor (HIF-1a) / vascular endothelial growth factor VEGF pathway [1].
AEs with mTOR inhibitors are generally milder than those with Tyrosine Kinase Inhibitors (TKIs), but serious AEs such as interstitial pneumonitis have been reported [2]. We report a case of bowel perforation during temsirolimus treatment for renal cell carcinoma.
Case Presentation
A 59-year-old Japanese male was referred to our hospital for the evaluation of a renal tumor. CT showed left RCC, of 16 cm in diameter. In February 2014, left nephrectomy was performed. The tumor size was 16*12*9cm (Figure 1). Histopathology showed RCC, unclassified, with sarcomatoid change, Fuhrman grade 4 (Figure 2). Cancer cells invaded through the renal capsule. Lymphovascular invasion was detected. Imaging studies showed left cervical lymph node and multiple lung metastases. Temsirolimus treatment (25 mg weekly) was started in April 2014, and 1 month later, CT showed the shrinkage of the left cervical lymph nodes. However, after fourteen courses of temsirolimus treatment, the patient complained of anorexia and severe pain in the right lower abdomen. Physical examination showed peritoneal irritation sign and silent bowel sounds. Laboratory data supported systemic inflammation (leukocyte count: 8.8×103/μ L, C-reactive protein: 30.91 mg/dL). Abdominopelvic CT (Figure 3) showed a small amount of ascites and free air in the anterior surface of the liver, and bowel perforation was diagnosed. Emergent surgery was performed, revealing perforation in the ascending colon. An omental flap was harvested to the part of perforation in ascending colon, and ileostomy was performed. After the operation, the patient recovered with antibiotic therapy.
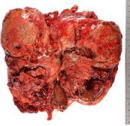
Figure 1: The brownish-yellow tumor, 16*12*9cm, occupied the whole of the
left kidney. And the ureter was extended because of the invasion of the tumor.
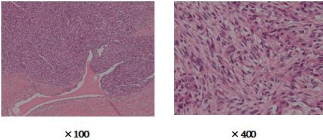
Figure 2: Histopathology showed spindle cell components ( sarcomatoid like
change). And there were a number of vein and lymph invasions.
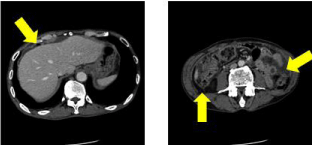
Figure 3: Abdominopelvic CT showed a small amount of ascites, free air in
the anterior surface of the liver and thickening the wall of ascending colon.
TABLECREATED
Table 1: Demographic data.
Discussion
Temsirolimus is an mTOR inhibitor that has been recommended as a first-line treatment for poor risk patients based on the Memorial Sloan-Kettering Cancer Center (MSKCC) risk classification and non-clear cell RCC patients. In a phase 3 trial, patients who received temsirolimus alone showed a longer overall survival and progressionfree survival than patients who received interferon alone (hazard ratio: 0.73, p=0.008). Overall survival in the combination-therapy group did not differ significantly from that in the interferon group (hazard ratio: 0.96, p=0.7). Median overall survival times in the interferon, temsirolimus, and combination-therapy groups were 7.3, 10.9, and 8.4 months, respectively. In addition, in a subgroup analysis for non-clear cell RCC, median overall survival times with temsirolimus alone and interferon alone were 11.6 and 4.3 months, respectively. Temsirolimus thus improved survival regardless of the tissue type [3].
Our patient was classified as intermediate risk based on the MSKCC classification, but histopathology showed an unclassified type with a sarcomatoid change, non-clear cell RCC, therefore, we selected temsirolimus.
The most common non-laboratory related adverse events on temsirolimus treatment were asthenia (51%), rash (47%), and mucositis (41%). The most common clinically important laboratory abnormalities on temsirolimus treatment were anemia (94%), hyperglycemia (89%), hyperlipidemia (87%), and hypertriglyceridemia (83%). Rare serious adverse events, in some cases resulting in death, included interstitial lung disease and acute renal failure [4].
The mechanism of temsirolimus-associated bowel perforation is a matter of debate, but inhibition of HIF-1a/VEGF pathway may be associated with this AE. HIF-1a controls the mechanism of glycolysis, the principal form of energy metabolism of most cancer cells, and angiogenesis through induction of the over expression of VEGF. Bowel perforation associated with bevacizumab (a targeted VEGF inhibitor) is well-known. Several potential of mechanisms for bevacizumab-induced bowel perforation could be useful when considering temsirolimus. Firstly, the inhibition of VEGF can reduce the intestinal capillary density and contribute to the development of micro-perforation [5]. Secondly, VEGF inhibition may lead to Cholesterol Emboli Syndrome (CES) and mesenteric ischemia, resulting in bowel perforation [6]. Thirdly, metastatic intestinal tumor necrosis may be a cause of bowel perforation [7]. Also, mTOR inhibitor itself inhibits intestinal cell migration and repair of the intestine, which may result in bowel perforation [8].
Other patient factors involved in bowel perforation may include enteritis, diverticulitis, ulcers, previous intestinal obstruction, radiotherapy, or multiple previous surgeries. Long-term NSAID use may also be involved [9,10] our patient had a history of gastroenteritis about 1 month before developing bowel perforation.
In bowel perforation, particularly large intestine perforation, peritonitis due to fecal material, septic shock, and multiorgan failure may occur. Bowel perforation is a life-threatening disease, and associated mortality rates of 6-34% have been reported. The sigmoid colon is a common site of perforation [11], However, our patient showed perforation of the ascending colon. Generally, emergent surgery, including decontamination and colostomy, should be performed.
During molecular-targeted treatment, clinicians should keep in mind the risk of bowel perforation not only with TKIs, but also with mTOR inhibitors.
References
- Fasolo A, Sessa C. Targeting mTOR pathways in human malignancies. Curr Pharm Des. 2012; 18: 2766-2777.
- Maroto JP, Hudes G, Dutcher JP, Logan TF, White CS, Krygowski M, et al. Drug-related pneumonitis in patients with advanced renal cell carcinoma treated with temsirolimus. J Clin Oncol. 2011; 29: 1750-1756.
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007; 356: 2271-2281.
- Kwitkowski VE, Prowell TM, Ibrahim A, Farrell AT, Justice R, Mitchell SS, et al. FDA approval summary: temsirolimus as treatment for advanced renal cell carcinoma. Oncologist. 2010; 15: 428-435.
- Asmis TR, Chung KY, Teitcher JB, Kelsen DP, Shah MA. Pneumatosis intestinalis: a variant of bevacizumab related perforation possibly associated with chemotherapy related GI toxicity. Invest New Drugs. 2008; 26: 95-96.
- Mir O, Mouthon L, Alexandre J, Mallion JM, Deray G, Guillevin L, et al. Bevacizumab-induced cardiovascular events: a consequence of cholesterol emboli syndrome? J Natl Cancer Inst. 2007; 99: 85-86.
- Flaig TW, Kim FJ, La Rosa FG, Breaker K, Schoen J, Russ PD. Colonic pneumatosis and intestinal perforations with sunitinib treatment for renal cell carcinoma. Invest New Drugs. 2009; 27: 83-87.
- Rhoads JM, Niu X, Odle J, Graves LM. Role of mTOR signaling in intestinal cell migration. Am J Physiol Gastrointest Liver Physiol. 2006; 291: 510-517.
- Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009; 6: 465-477.
- Kobayashi H, Ashikari A, Namitome R, Yagi Y, Kohno Y, Nishiyama T, et al. [Two cases of bowel perforation in patients with metastatic renal cancer treated with a molecularly targeted drug]. Nihon Hinyokika Gakkai Zasshi. 2012; 103: 660-664.
- Kobayashi H, Ashikari A, Namitome R, Yagi Y, Kohno Y, Nishiyama T, et al. [Two cases of bowel perforation in patients with metastatic renal cancer treated with a molecularly targeted drug]. Nihon Hinyokika Gakkai Zasshi. 2012; 103: 660-664.