
Research Article
Austin J Urol. 2016; 3(2): 1045.
Efficacy and Tolerability of the Histrelin Implant (VANTAS®)
Rudlang TM and Brasso K*
Department of Urology, Copenhagen Prostate Cancer Center, University of Copenhagen, Denmark
*Corresponding author: Brasso K, Department of Urology, Copenhagen Prostate Cancer Center, University of Copenhagen, Denmark
Received: June 17, 2016; Accepted: July 18, 2016; Published: July 22, 2016
Abstract
Introduction: Androgen deprivation is the cornerstone in management of patients with advanced or metastatic prostate cancer. Androgen deprivation can be achieved in a number of different ways, all, leading to lowering of testosterone to castrate range. We present our initial experience with a newly introduced oneyear formulation.
Material and Methods: Consecutive patients managed with Histrelin implants. Main out-come, testosterone levels 3 and 12 months following implantation.
Results: Histrelin implants maintained testosterone levels within castration range in all patients 3 and 12 months following implantation. Major side effects were the expected consequences of androgen deprivation, only few patients had complaints related to the implant or procedure.
Conclusion: Histrelin implants may serve as a valid alternative in patients undergoing either permanent or short-term androgen deprivation therapy
Keywords: Androgen deprivation therapy; Histrelin; Prostate cancer; GnRH agonist; Testosterone
Introduction
Testosterone promotes the cell proliferation and DNA synthesis in the prostate via androgen receptors. Eliminating or blocking the androgen stimulation inhibits proliferation and activates apoptosis in normal prostates and in Prostate Cancer (PC). Androgen Deprivation Therapy (ADT) is the standard care in patients with metastatic prostate cancer [1]. Furthermore, ADT can be used as adjuvant therapy in combination with radiotherapy in patients with localised and locally advanced prostate cancer with curative intent.
Androgen deprivation can be achieved by either bilateral orchiectomy or medical castration using GnRH agonists, GnRH antagonists, or oestrogensaiming to reduce testosterone to castrate levels <1,73 nmol/l. By continuous exposure to GnRH agonists a paradox down regulation in GnRH receptors in the pituitary gland leads to ceased secretion of LH and thereby testosterone suppression as a result.
GnRH agonists are usually administered as subcutaneous or intramuscular injection every 3 to 6 months. Micro-surges in testosterone level, when injections are re-administered, occurs in 4-10% of patients treated with GnRH agonists and may be of significance [2-4].
Histrelin implant (VANTAS®) is a novel subcutaneously administered GnRH agonist. The chemical structure of Histrelin acetate does not differ much from the chemical structure of commonly used GnRH agonists like Goserelin acetate and Leuprolide acetate. However, the potency and receptor affinity of Histrelin is significant higher [5-7]. Recently Histrelin has been introduced as an alternative to other GnRH agonists in the management of advanced PCa. The implant is a permeable hydrogel device measuring 3,5 cm in length and 3,5 mm in diameter and comprise 50 mg Histrelin acetate, providing a continuous daily release of 50 mg Histrelin for a period of 52 weeks, (Figure 1). We report our initial single institution experience with this newly introduced device.
Figure 1: Histrelin implant.
Materials and Methods
A prospective cohort of 26 consecutive PCa patients managed with Histrelin implant (VANTAS®) between January 2012 and February 2013 at the Department of Urology, Rigshospitalet. The objective of the study was to evaluate the efficacy and tolerability of Histrelin implant in our clinic as we introduced the treatment as an alternative to other GnRH agonists.
All patients had histologically confirmed adenocarcinoma of the prostate. All underwent routine diagnostic work-up including bone scan and computerized tomography of the thorax and abdomen before enrolment. Prostate cancer was staged by TNM 2009 classification [8] and D´Amico risk stratification [9]. Patients included in the study were classified as high risk prostate cancer with T stage greater than cT2b, Gleason score greater than 7 or PSA greater than 20 ng/ ml. Histrelin implants were inserted under local anaesthesia in the outpatient clinic. The implants were placed subcutaneously through a 0,5 cm incision in the inner aspect of the non-dominant upper arm using a single use sterile insertion trocar. The incision was closed with Steri-strips. Patients were evaluated at baseline, and 3 and 12 months following Histrelin implantation. After 12 months, the implant was removed through a small incision. If continues ADT was indicated a new implant replaced the one removed. The primary out-come was testosterone suppression. Successful treatment was defined as serum testosterone suppression to castration levels <1,73 nmol/l. The lower limit of detection in testosterone assay used were 0,42nmol/l. Secondary efficacy was assessed by measuring PSA. Tolerability was assessed by normal clinical examination and by interviewing the patients at each visit in the outpatient clinic at baseline and after 3 and 12 month. Local implant tolerability was also evaluated by visual inspection of the insertion site and by active inquiry of the patient regarding any discomfort at the site. The follow-up period was 1 year where disease progression was assessed throughout the study and included evaluation of testosterone and PSA values and clinical observation at baseline and after 3 and 12 month.
Results
A total of 26 patients median age 65 years, range 58 to 73 years were enrolled in the study. Gleason score ranged from 6 to 10, pretreatment PSA from < 0,1 to 109 ng/ml median 0,5 ng/ml. Patients characteristics presented in table 1. The majority – 22 of 26 patients – had ADT as adjuvant therapy in association with external radiation therapy for locally advanced PCa. Three patients had bone-metastasis, six patients had lymph node involvement, of which four participated in a phase 2 trial on combined hormonal and radiation therapy. All except two patients (92%) had had ADT prior to their first Histrelin implant. Of these 14 (54%) patients only had one injection of depot GnRH agonist resulting in 3 month of treatment before insertion of the Histrelin implant.
Median
Range
Age 65,
65
58-73
PSA at baseline
0,5 ng/ml
< 0,1 – 109 ng /ml
Gleason score
Number
(%)
6
2
7,7%
7
15
57,7%
8-10
8
30,8%
Unknown
1
3,8%
TNM classification
T2N0M0
1
T3a/bN0M0
15
T3a/b N1M0
5
T3a/bN1M1
1
T3a/b Nx M0
1
T3 a/b Nx M1
1
T4N0M0
1
TxNxM1
1
Table 1: Patient characteristics.
Due to prior treatment with GnRH agonists 23 (88,5%) of the patients had testosterone levels in castrate range at baseline, figure 2. Despite this, we found a further decrease in testosterone after insertion of the Histrelin implant. At baseline 50 % of the patients had testosterone below the limit of detection < 0,42 nmol/l, 38,5 % had testosterone in castration levels between 0,42 and 1,73 nmol/l, and 11,5 % had testosterone levels > 1,73 nmol/l. After 3 months all patients had testosterone within castrate range, of which 57,7 % had testosterone below the limit of detection. At 12 months all patients were within castration range, and the majority 70,8 % had testosterone below limits of detection, (Figure 2). During the study, four patients had biochemical progression - increasing PSA levels - despite suppression and maintenance of testosterone levels within the castrate range.
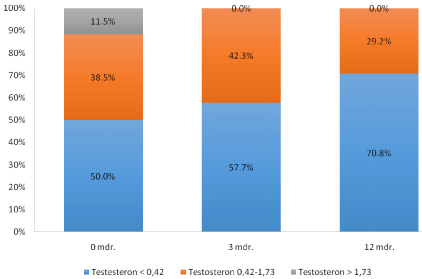
Figure 2: Serum testosterone prior to and on histrelin at 3 and 12 months
following implantation.
The majority – 24 (92 %) patients – did not have any local side effects from the implant. Minor local side effects were encountered in 2 patients, one patient had haematoma after insertion and one patient reported mild pain from time to time at the implant site. Both of these events were without evidence of infection and resolved without treatment. In one patient the implant could not be identified, once local anaesthesia was administered, thus new implants were just inserted without removing the old implant. After additionally 12 month both implants were removed without any problems. The most commonly reported adverse events were the expected systemic side effects from ADT such as hot flashes, fatigue, weight gain, gynecomastia, erectile dysfunction and depression. Most patients did not experience any severe adverse events. However, two patients discontinued ADT one due to severe depression, and the other due to unacceptable side-effects severe hot flashes, weight gain and oedema.
Discussion
Surgical castration is the gold standard for ADT, but the main drawback is that it may have a negative psychological effect because some, especially younger men, consider it to be an unacceptable assault on their manhood.
Alternatively, medical castration using either GnRH agonists or antagonist can be used, they are effective and easy to administer. However, there are some potential disadvantages using repeated injections. They may be associated to discomfort and increased inconvenience because of multiple visits per year, which may negatively affect patient compliance and increase overall health care costs. Furthermore, the drug biodegrades with time, thus the effect cannot be reversed in timely fashion. Several studies have shown that the effect may last beyond the expected duration of the depot preparation [10-13].
Histrelin implant may in many patients be a god alternative to standard depot GnRH agonists. The two modalities have comparable side effects; however, the implant may have several advantages. The implant only has to be changed once yearly, which may improve convenience to both patients and health-care providers by enabling flexible clinic visits according to individual patients needs. In clinical stable patients the number of visits and subsequent injections can be reduced. The risk of testosterone surges may be reduced, and theoretically, this may subsequently lead to better disease control. Since the implant is non-biodegradable, it can easily be removed allowing cessation of the ADT. Previous studies have demonstrated rapidly increase in testosterone levels after removal of Histrelin implant [7,12]. This may be an advantage in patients who has to be taken of ADT due to severe adverse events and patients requiring short-term or intermittent androgen deprivation.
In elderly or more fragile patients with advanced or metastatic PCa bilateral orchiectomy permanently induces ADT, however some patients are unwilling to undergo surgery, and in these patients Histrelin may serve as a valid alternative to standard GnRH.
The Histrelin implant is most cost-effective when the treatment is anticipated to be at least 1 year in duration. The implant is small, soft and flexible permitting maintenance of an active life-style without any limitations. Insertion of the implant is simple and only takes few minutes. The implant can be stored at room temperature as other GnRH agonists. The implantation and removal is simple surgical procedures that can be performed in local anesthesia in the outpatient clinic. The procedure is straightforward, once learned it takes a few minutes and can be performed by residents or nurses.
Conclusion
We found that Histrelin implant maintained castration levels in all patients within the first year of treatment. Besides the expected side effects from castration-based treatment, only minor local side effects related to the implant were encountered. Histrelin implant can be removed and reinserted under local anaesthesia. Treatment with Histrelin may reduce the number of outpatient visits in patients with stable disease and might increase compliance and lead to less discomfort due to fewer injections.
References
- Helgstrand JT, Berg KD, Lippert S, Brasso K, Roder MA. Does androgen deprivation therapy prolong survival in patients with prostate cancer? In press Scand Jour Urol. 2016.
- Tombal B, Berges R. How good do current LHRH agonists control testosterone? Can this be improved with Eligard? Eur Urol Suppl. 2005; 4: 30-36.
- Berges R, Bello U. Effect of a new leuprorelin formulation on testosterone levels in patients with advanced prostate cancer. Curr Med Res Opin. 2006; 22: 649-655.
- Zinner NR, Bidair M, Centeno A, Tomera K. Similar frequency of testosterone surge after repeat injections of goserelin (Zoladex) 3.6 mg and 10.8 mg: results of a randomized open-label trial. Urology. 2004; 64: 1177-1181.
- Conn PM, Crowley WF. Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991; 324: 93-103.
- Sundaram K, Wang N, Bardin C, Zatuchini J, Shelton J, Sciarra J. LHRH peptides as female and male contraceptives; Contraception. Philadelphia, Harper and Row. 1981.
- Schlegel PN, Kuzma P, Frick J. Effective long-term androgen supression in men with prostate cancer using a hydrogel implant with the GnRH agonist histrelin. Urology. 2001; 58: 578-582.
- Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of malignant tumours, International union against cancer. Wiley-Liss. 2009.
- D Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280: 969–974.
- Oefelein MG. Time to normalization of serum testosterone after 3-month luteinizing hormone-releasing hormone agonist administered in the neoadjuvant setting: implications for dosing schedule and neoadjuvant study consideration. J Urol. 1998; 160: 1685-1688.
- Hall MC, Fritzsch RJ, Sagalowsky AI, Petty B, Roehrborn CG. Prospective determination of the hormonal response after cessation of luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Urology. 1999; 53: 898-902.
- Fridmans A, Chertin B, Koulikov D, et al. Reversibility of androgen deprivation in prostate cancer. J Urol 2005; 173: 784-789.
- Deeks ED. Histrelin. Adis drug profile. 2010; 70: 623-630.