
Review Article
Austin J Urol. 2019; 6(1): 1062.
An Evaluation of the Role of fMRI in Patients with Lower Urinary Tract Dysfunction
Ghanem MA1,2*, Adawi EA1, Hendi AH3 and Ghanem AA4
¹Department of Urology, Jazan University, KSA
²Department of Urology, Menoufiya University, Egypt
³Radiology Department, Jazan University, KSA
4Gynecology and Obstetric Department, Mansoura University, Egypt
*Corresponding author: Ghanem MA, Department of Urology, Jazan University and Menoufiya University, KSA and Egypt
Received: September 09, 2018; Accepted: February 15, 2019; Published: February 22, 2019
Abstract
Lower urinary tract dysfunction, including urinary incontinence and Overactive Bladder (OAB) syndrome, have detrimental effects on health-related quality of the life and also exert a significant economic burden on health care systems. The role of imaging in managing urinary incontinence is diagnostic and reserved only for situations based on clinical and/or neurophysiological test findings.
Recently, the field of fMRI has grown explosively and is fast becoming a regular feature in the news media. fMRI emerges a useful research tool for voiding dysfunction studies. With this modern brain imaging, it is now possible to identify the role of the supraspinal control system and brain areas in the living human brain involved in voluntary control of the micturition reflex.
So, the combination of anatomical and functional information by fMRI enabled us in the diagnosis of a wide range of diseases which may cause urinary dysfunction and to develop individual accepted recommendations for all patients. So, it assisted in the decision-making processes between invasive and conservative management for voiding dysfunction patients.
Keywords: Urinary dysfunction- OAB; Urinary urgency- fMRI
Introduction
Patients with Lower Urinary Tract Symptoms (LUTS), including urinary incontinence and Overactive Bladder Syndrome (OAB) constitute a considerable part of urology patients. The prevalence of storage LUTS (men, 51%; women, 59%) was greater than that for voiding (men, 26%; women, 20%). The prevalence of OAB was 12% which was more prevalent than all types of urinary incontinence combined (9%) [1]. Also, OAB affects approximately 15% of urgency women and prevalence rates are higher in women than men [2].
The cause of the urinary dysfunction is usually multifactorial. Although urinary dysfunction may entirely be non-neurogenic, it may also present with an overt or occult neurogenic abnormality underneath [3]. Many clinically relevant methods have been put forward to allow an early diagnosis and identification of urinary dysfunction. A detailed patient's history, physical examination, and the determination of underlying pathophysiology are considered essential components of the initial evaluation of urinary dysfunction [4].
Beyond these assessments, there are no universally accepted imaging recommendations for urinary dysfunction and, to date, imaging is indicated only if pelvic pathology is suspected; Videourodynamics (VUDE) and cystourethrography are considered optional diagnostic tests and continue to be refined; fMRI is considered an important research tool in evaluating lower urinary tract disorders, but at present its clinical role remains investigational [5].
Neurophysiology and Pathophysiology of Micturition: Normal Volunteers
Peripheral and central processes control Micturition, in the conscious and unconsciousness states. Peripheral innervations arise from caudal segments of the sacral spinal cord, passes through the cauda equina to the sacral plexus, and via pelvic and pudendal nerves to the bladder and sphincter. The parasympathetic nervous system controls bladder contraction and micturition, and the sympathetic system becomes especially active during the last part of urine storage when micturition is actively postponed [6,7].
It is generally accepted that the brain plays an important role in normal micturition [8]. Depending upon the stage of storage and voiding process, a network involving subcortical and cortical structures was identified. A part of the pons and the Periaqueductal Gray (PAG) showed activity as the bladder filled and during emptying. Further activation was observed in some regions of the cerebral cortex, i.e. frontal lobe, insula and anterior cingulated gyrus, as well as in the cerebellum, putamen, thalamus, and hypothalamus. The activation of the insular cortex is triggered by sympathetic stimulation of the urethral sphincter [9,10].
Furthermore, the PAG plays a crucial role in regulating the micturition reflex. The PAG directly receives information about the bladder filling and if they exceed a certain threshold, the reflex is triggered and a signal is sent to an area in the dorsomedial pontine tegmentum, referred to as the Pontine Micturition Center (PMC) called Barrington’s area or M–region. PMC excitation will produce complete synergic micturition via long descending motor efferents pathways to the parasympathetic bladder motor neurons and to sacral GABA-ergic (gamma amino butyric acid) and glycinergic premotor interneurons that inhibit motorneurons in Onuf’s nucleus which would lead to sphincter and pelvic diaphragm relaxation. In addition, the excitatory parasympathetic pathway leads to detrusor contraction [9,11]. PAG is also known as an area responsible for emotional response, which explain the urge to void during anxiety and emotional stress [5,12]. However, the circuitry controlling micturition from the higher regions of the brain remained unclear.
Since the introduction of fMRI as an imaging modality, there has been a growing interest in its use as an investigative tool in the evaluation of patients with voiding dysfunction. Despite the increasing number of animal and human experimental fMRI studies, its application concerning central micturition control network and the voiding control mechanism in suprapontine structures is rare. To our knowledge little information has been reported on followup fMRI in these patients and to what extent this new modality may affect its management.
Basic role of fMRI in brain scanning
FMRI methods are used as a powerful tool for the noninvasive mapping of brain’s functional localization and connectivity. In functional brain MRI, the term may refer to congnitive function, while the actual brain function occurs in neuronal activity; fMRI is adept in characterizing the associated haemodynamic responses.
Based on Blood Oxygen Level Dependent (BOLD) contrast, fMRI takes advantage of the changes in signal intensity, which arise due to alterations in the local transverse relaxation times, associated with regional changes in cerebral deoxyhemoglobin concentration. FMRI quantifies paramagnetic properties of oxygenated and deoxygenated hemoglobin, which correlate with neuronal activity related changes in blood flow [13].
In noninvasive neuroimaging, neural activity is inferred from fluctuations in deoxyhemoglobin. FMRI BOLD signal demonstrated that there is a strong coupling between local field potentials and changes in tissue oxygen concentrations in the absence of spikes. These results imply that the BOLD signal is more closely coupled to the synaptic activity. So, neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Due to the small signal activity change which results, the correlation between the task activity and fMRI response identified in a statistical manner, using series of images acquired during alternating periods of activity triggered paradigm experiment or any trigger e.g. by pelvic floor muscle contraction in alternation with periods of rest. After this holding for a suitable level of statistical significance, the T-map of the correlation is displayed as a color-coded image in which the color is indirectly related to neuronal activation [14].
Furthermore, resting-state fMRI can elucidate and monitor multiple brain activity regions during voiding in normal persons and correlate with one another at rest. By using this method, better understanding of normal voiding under normal physiological conditions as well as changes that may occur in voiding dysfunction were studied [15]. Furthermore, Diffusion Tensor Imaging (DTI) enables visualization of brain tissue microstructure, which is extremely helpful in understanding various neuropathologies and neurodegenerative disorders. In DTI, the white matter tracts are analyzed by inference from the local diffusion of water molecules. So, the DTI method helps to identify the deficits in white matter that appear normal by conventional radiological imaging methods. Deficits of this region are more prevalent in younger patients and might lead to urgency [16].
Arterial Spin Labeling (ASL) is another type of fMRI technique that allows quantitative measurement of absolute cerebral perfusion [17]. Unlike PET scans that require the administration of radioactive intravenous contrast to measure cerebral perfusion, ASL uses inverted proton spins of magnetically labeled water in the blood as a tracer. ASL fMRI can non-invasively quantify perfusion changes in the brain using a non-catheterized oral fluid challenge paradigm that does not require repeated filling and emptying the bladder, unlike BOLD fMRI [17,18]. The advantage of an oral fill protocol is that it prevents false positive afferent signals induced by placement of a catheter, especially in conditions such as OAB that are characterized by increased afferent signaling. The disadvantage of this protocol is that it does not allow bladder filling volumes to be precisely controlled. The type of fill protocol used during imaging studies depend on the goals of the project [18].
Role of fMRI in studying brain- bladder control of micturition
Recent function imaging studies fMRI reveals a network of brain regions that responds to different signals provoked by bladder filling in response to water intake or furosemide. In the early stages of fMRI studies, urodynamic recordings were not possible in the magnetic field of the scanner. However, a recent study has adapted urodynamic methods to the fMRI environment, so as to monitor bladder pressure and brain activity in the scanner simultaneously during bladder filling [13].
Functional studies have shown that control of bladder is a complex process. The process of the central control mechanisms of bladder function involves spinal reflexes and interactions between the higher brain centers as well as many other brain regions [19]. The role of spurapontine brain structures in the voluntary control of voiding by fMRI based on the pelvic floor muscle function. The idea is that repeated pelvic floor muscle contractions during full bladder induce a stronger contrast of bladder sensation, desire to void, and the effect of this contraction was thought to be activation of the continence areas, and inhibition of the micturition reflex triggering, when the subjects were asked not to urinate. Activation maps calculated by contrast of subtracting the two different conditions were applied to reveal these brain areas that are involved during the inhibition of the micturition reflex [20].
Response differs in subjects with good or with poor bladder control. Among those with poor control, cortical responses were exaggerated at larger bladder volumes and are consistent with symptoms of urgency and frequency. These abnormalities are associated with adequate response to bladder filling in a specific bilateral region of the orbitofrontal cortex, an area known from clinical lesion studies to be crucial to voluntary bladder control. Among subjects with good control, this strengthening of response was prominent in the orbitofrontal cortex. This suggests a similar neurophysiological basis in dysfunction of the orbitofrontal cortex for poor bladder control in absence of neurological lesion [13].
FMRI detected activation of many brain regions involved in bladder control, including PAG, thalamus, insula, cingulate gyrus, ventromedial cerebellum, PMC and preoptic hypothalamus (Figure 1).
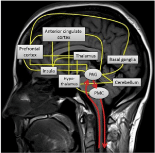
Figure 1:
fMRI localization of anatomical areas involved in brainbladder control of micturition
Primary motor cortex: fMRI showed activation in the primary motor cortex during pelvic floor muscle contraction and relaxation. The activation in the primary motor cortex was modulated by attention and also, showing absence of correlation between counting and movement, in contrast, a negative interaction was found in the sensorimotor areas (SMA, cingulated cortex and post-central gyrus). This suggests that this area mediates motor function differently [13].
Parietal cortex: fMRI showed that the right side of parietal cortex seems more dominant during micturition and bladder filling. The inferior parietal cortex activation reflects the transfer of visual commands into the resulting muscle contraction, rather being specific for the paradigm used than for the micturition process itself [7].
The cerebellum: The activity was shown in the cerebellum during both relaxation and contraction of the pelvic floor muscles. The process of increasing bladder filling was followed by bilateral cerebellar activation [9]. However, the activity of vermis was found during a full bladder but not during an empty contraction which was stronger during contraction than relaxation. It seems that right cerebellar hemisphere is more dominant than left hemisphere during pelvic muscle contractions. It is proved that cerebellar influences visceral functions in children who suffered from urinary and fecal incontinence. This indicates that global cerebellar dysfunction also affects bladder function [20].
PFC (Prefrontal cortex): Sakakibara et al. [21] reported PFC regions activation with fMRI study during bladder filling.
Cingulated gyrus: The entire cingulated gyrus is a part of cingulated cortex, which lies immediately above the corpus callosum. The activity in anterior portion of the Cingulated Cortex (ACC), which is the frontal part of the cingulate cortex surrounding the frontal part of the corpus callosum, increased as bladder volume increased. In contrast, to the decreased activation of the mid-portion as urge to void. The location of activation in the ACC is widely distributed, and depends on the type of task to be carried out. Besides its role in the emotional and motivational aspects of micturition, it is also involved in certain higher-level of functions, such as attention allocation, reward anticipation, decision-making, ethics and emotion [22-26]. Therefore, cingulated cortex might present as a complex role of the control of micturition.
Insula: Effective connectivity between insular and ACC has been observed in almost functional brain imaging studies. Whereas, functional connectivity analyses of resting state fMRI BOLD have shown that the anterior insula is connected functionally with the anterior and mid-cingulate cortex; and the mid- and posterior regions of the insula are connected only with the posterior region of the mid-cingulate cortex. Furthermore, the insula is engaged in the integration of the variety of limbic system function and autonomic responses [27,28].
The role of the basal ganglia: Bilateral activation during states of relaxation and contraction of pelvic floor muscle with a higher activation level during pelvic floor muscle contraction was found by fMRI [20]. The present findings substantiate that the basal ganglia play an important role in the modulation of micturition reflex but they are not responsible for the reflex itself [19,23].
Amygdala/hippocampal region: This region was activated in bladder voiding control; this finding extends the evidence that it is highly involved in visceral sensation like irritable bowel syndromes [28] and in emotional processes, especially fear emotions [29]. The amygdala is involved in the normal response to bladder filling, and in suppression of urgency provoked by bladder filling in OAB patients [29-31]. The amygdala/hippocampal region becomes less active after treatment of urge incontinence by sacral neuromodulation. Studies using dynamic causal modeling of fMRI in healthy subjects have observed a robust increase in effective connectivity from the amygdala to the hippocampus [29-31]. However, it is largely unknown how these two brain structures functionally interact.
The thalamus: The activation of the thalamus was found during bladder filling [19,32].
L-region: A few fMRI studies showed a localized activity that corresponds to the pontine L-region or continence center [7,23,33]. This L-region is under the strong control of PAG and higher regions, but no information exists regarding suprapontine control of the L-region. However, not all these studies recognized the L-region as such and still need further investigation [26].
The PMC: fMRI showed activation in the PMC in the medial aspect of the pons during the initiation of voluntary voiding pelvic [34,35]. No laterlization was found during pelvic floor muscle activity in pons [23].
Role of fMRI in evaluation factors which influence voiding mechanism
Psychological factor: Psychological factor can influence bladder awareness by self-distraction which used as a management strategy for “Latchkey incontinence” (Incontinence of urine as one approaches a known toilet) e.g., reciting a poem. An fMRI study on painful conditions show changes in activity in the prefrontal, cingulate PAG, and thalamic regions, demonstrated decreased pain scores with distraction [36].
Self- control inhibition: fMRI study combined with MMPI (Minnestoa Multiphasic Personality Inventory) suggested that self inhibition is associated with Supplementary Motor Area (SMA) activation during voluntary voiding control. So, SMA interpreted as higher inhibition area suppressing voiding desire during full bladder [37].
Performance of cognitive and motor task: fMRI experiments with simultaneous ECG during performance of cognitive and motor task provide evidence for a direct link, neuropsychological and physiological observations between dorsal Anterior Cingulated Gyrus (dACC) activity and modulation of cardiac and voiding function via sympathetic output. However, continuous physiological monitoring during fMRI has enabled the exploration of integrative neural mechanisms facilitating behavior that necessarily include modulation and feedback of bodily arousal states [38].
Role of fMRI in localizing brain- bladder pathological conditions
fMRI of the brain proved to elucidate and confirm brain regions involved in pathophysiology of voiding dysfunction caused by certain neurological disease.
Overactive Bladder (OAB) syndrome and urinary urgency: OAB, a functional disorder of bladder storage characterized by the symptoms of urinary urgency with or without urge incontinence, usually associated with frequency and nocturia. Urinary urgency, which is the main symptom of OAB, is defined as a sudden compelling desire to urinate that is difficult to control [39].
Brain imaging studies, using BOLD fMRI, reported that urinary urgency is associated with activation of several cortical and subcortical regions that comprise the limbic system, that is, region of the brain that processes emotional response to pain and discomfort, such as ACC, insula, and Prefrontal Cortex (PFC). Measuring brain activation during urinary urgency rather than with cystometric capacity has the advantage that it may help to elucidate mechanisms of a functional syndrome that is defined based on symptoms such as urinary urgency rather than objective findings such as cystometric capacity [32,40].
Using ASL in women with OAB, urinary urgency is associated with 10-14% increase in absolute regional CBF in the limbic system. The using of SPM (Statistical Parametric Mapping) supports these findings and identified additional areas of activation in the dorsolateral PFC and the pons/midbrain area. Those findings suggest that, in women with OAB, urinary urgency is associated with abnormal processing of sensory input in the limbic cortex. Also, those findings advance understanding of the pathogenesis of urinary urgency that substantiates existing conceptual models of sensory processing in the brain during urgency [41]. So, urgency may represent an emotional response to discomfort and potentially explains the success of techniques such as distraction in preventing leakage. The activation in the pons/midbrain likely represents the Periaqueductal Gray (PAG) where sensory information from the bladder is integrated [32].
Interstitial cystitis: Using continuous ASL in women with interstitial cystitis, Deutsch et al. [18] reported quantitative increases in absolute rCBF in several regions of the brain involved in the pain matrix such as the supplementary motor area, motor and sensory cortex, insula, hippocampus, and middle and posterior cingulate areas during bladder filling.
Brain dysmaturation: fMRI is the imaging of choice to evaluate brain myelination maturation and the consequences on brain maturation of the different urinary dysfunction pathologies encountered after birth [42].
Nocturnal enuresis (NE): fMRI found that the sensory threshold of the urethra in NE children was significantly higher, indicating that bladder and urethra are controlled by separate neuronal pathways. NE is considered an inhibitory micturition control disorder from premotor cortex [20].
Parkinson Disease (PD): In a meta-analysis of rs-fMRI studies, connectivity changes in the right posterior Inferior Parietal Cortex (IPC) represent the most consistent finding in PD [43] and may disrupt activity of the precuneus, Posterior Cingulate Cortex (PCC), mid-cingulate cortex, middle and superior frontal gyrus, and orbitofrontal gyrus
Idiopathic focal epilepsies: The combination of EEG-fMRI is a powerful tool to describe the irratative zone. This combination was able to discriminate the spike onset zone from propagated epileptiform sources activity [44].
Multiple Sclerosis (MS): Patients with MS who had demonstrable neurogenic detrusor overactivity and Detrusor-Sphincter Dyssynergia (DSD) showed a trend toward distinct brain (supraspinal) activation at full urge and at initiation of voiding respectively, which are consistent with the observation in other patients with overactive bladder [45]. Such brain activation was studied using a concurrent fMRI/urodynamic testing platform during the entire micturition cycle (storage and voiding phase) in female patients with neurogenic lower urinary tract dysfunction and multiple sclerosis [46,47].
Role of fMRI in diagnosis and therapy of urinary dysfunction
Based on fMRI studies of OAB patients, a third backup continence mechanism (paralimbic circuits) has proposed by a Pittsburgh group. According to their theory, information of bladder filling ascending from the PAG is relayed not only to the thalamus, but also to the dorsal ACC and the SMA, which presumably evokes the “urgency” [48]. The SMA is activated during contraction of the pelvic floor muscles, and provides protection against incontinence [16,23,33]. Furthermore, ACC and SMA might bypass the brainstem switch, which is concerned with parasympathetic control. So, bladder filling provokes deactivations in parahippocampal complex areas, posterior cortex and perhaps hypothalamus, which may be concerned with basic safety of voiding. So, 3 neural continence circuits have been identified which maintain continence by suppressing the spinobulbospinal voiding reflex at its terminus in the PAG. Circuit 1 involves the mPFC, and its afferent and efferent pathways, possibly including the insula, while circuit 2 involves the dACC (midcingulate) and the adjacent SMA, and circuit 3 may involve subcortical regions such as the parahippocampal complex (Figure 2). In normal subjects, circuits 1 and 2 are not significantly activated during storage but circuit 1 (mPFC) is activated during voluntary voiding. fMRI study in urge urinary incontinence patients have identified abnormalities in brain activation provoked by bladder filling and these abnormalities predict the response to pelvic floor muscle training therapy [33,49].
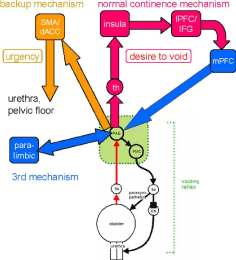
Figure 2:
fMRI studies confirm the importance of neuroplasticity, refers to the phenomenon of neurons and neural networks modifying their connections and/or behavior in response to new information, sensory stimulation, development, damage, or dysfunction. The cortical neuroplastic changes were observed after pelvic floor muscle training and biofeedback therapy for stress urinary incontinence. These phenomena show that the therapeutic neurorehabilitation that modulates some regions in the brain can change the whole network problem. In the field of neurorehabilitation, however, assessing neuroplasticity using task t-fMRI presents a significant challenge. Direct measures of such changes are beneficial to understanding how and when recovery from brain injury takes place and ultimately may provide a novel rehabilitative treatment [50].
The quantitative measurement of cerebral perfusion by ASL fMRI could be used for the diagnosis of both complex cases of bladder pain syndrome and complex patients of OAB, beside clinical symptoms [18]. As, women with overlapping symptoms of OAB and bladder pain are in need for both a diagnostic and therapeutic challenge. fMRI is an imaging modality that can provide a quantitative biomarker for both the underlying pathogenesis and more specific diagnosis in complex cases of OAB [51].
Objective measurement of cerebral perfusion could also be used to develop novel treatments for OAB such as hypnosis and transcranial magnetic stimulation that target cerebral perfusion in specific regions of interest and have been used in the treatment of depression [52]. Furthermore, the effectivity of anti-OAB drugs by fMRI was studied in OAB patients in experimental trials [50,52].
BOLD fMRI also has higher temporal resolution, making it more suited to event-related designs, for example, in a language task, and is technically easier to implement since it does not usually require any additional programming of the radiofrequency and gradient pulses provided by the manufacturer [53]. Several neuroimaging studies using functional MRI have advanced the understanding of how afferent signals from the bladder are processed in the brain. Of these, BOLD fMRI and ASL have gained wide applications because both use endogenous tracers and are therefore noninvasive.
fMRI provides preoperative cortical localization of speech, motor, and sensory cortex in patients with supratentorial arteriovenous malformations and recurrent parasagittal meningioma. So, stereotactic radiosurgical dose plans were safely applied that preserve these cortical areas [54].
Future prospective studies for urinary dysfunction
The applied voiding dysfunction animals' model provides a tool that might be applicable to insert the fMRI in the noninvasive diagnostic assessment of disorders of the central bladder control due to different brain diseases. This will lead to the utility of fMRI translational research from preclinical models to humans; making it useful for longitudinal noninvasive studies. However, the additional information on the status of the urethra sphincter and supporting ligament provided by fluoroscopic dynamic MR imaging obtained with the patient at rest and during contraction, combined with the available fMRI data may contribute to the accurate diagnosis of urinary dysfunction [55].
An accurate noninvasive method for providing information about brain lesions would be of great benefit toward reducing potential operative/postoperative morbidity. One of the promises of fMRI in the clinical arena has been in pre-surgical mapping in patients with brain lesions. This type of information can be applied by integrating functional and anatomic information on a neurosurgical navigation workstation. Where, vital cortical areas such as for motor function must be precisely identified to avoid significant postoperative morbidity resection of brain tumors. The gold standard for this evaluation remains invasive, where cortical stimulation performed at the time of surgery. Previous studies comparing the localization accuracy of fMRI to intra-operative localization have shown good correlation. These studies, however, have relied on subjective comparison of operative photographs to make this assessment and need further evaluation [56]. Also, fMRI provides preoperative cortical localization of speech, motor, and sensory cortex in patients with supratentorial arteriovenous malformations and recurrent parasagittal meningioma. So, stereotactic radiosurgical dose plans were safely applied that preserve these cortical areas [54].
The need for more accurate, noninvasive imaging of the fetal CNS has been increased for assessing fetal CNS anatomy and intrauterine therapy. Fetal MRI can support the diagnosis of aqueductal stenosis which can differentiate between the good and bad candidates for Endoscopic Third Ventriculostomy (ETV) which is based on the presence and shape of the floor of the 3rd ventricle together with the presence of an abnormality of the aqueduct or midbrain [57]. In fetal surgery centers, MRI can be performed to evaluate the lesions, to provide the inclusion criteria for fetal surgery, and to analyze the postoperative outcome [58]. MRI help to diagnose idiopathic normal pressure hydrocephalus by measurement of CSF flow (>18ml/min) at the level of the cerebral aqueduct. With further advances, it seems fetal fMRI/MRI is an effective, noninvasive means of assessing fetal CNS anatomy and pathophysiology. It is conceivable that fMRI will replace sonography in the evaluation of the fetus. So, clinical correlation of findings on prenatal ultrasound and fetal fMRI is vital to accurately characterize abnormalities [59].
The concept of fMRI VUDE may give us some insight into the role of various higher centers in controlling the micturition process. The idea was based on some interesting correlated observations of MRI with videourodynamic findings in localized brain infarction and multiple sclerosis. However, it brings new possibilities for the study of voiding dysfunction and represents a useful tool for further studies [60,61].
The longitudinal nature of the patients with voiding dysfunction must be followed for the metabolic abnormalities. MRI is an imaging modality that measures the mobility of water within tissues and, as such, may function with fMRI as a surrogate biomarker for both tissue cellularity and response to treatment e.g., Antibodies against aquaporin-4 in Neuromyelitis Optica (NMO) [62,63].
By analyzing the frequencies present in the MR signal, the investigator can identify the metabolites in the tissue and estimate their concentration. This procedure forms the basis of MR spectroscopy [64]. MR spectroscopy was found as promising tool in assessment of spinal dysraphism surgical outcomes. Where high levels of lactate, alanine, acetate, glycerophosphorylcholine and choline were observed in the CSF of patients with spinal dysraphism and cord retethering before surgery; after surgery these levels normalized. The persistence of deranged anaerobic metabolism after surgery may be indicative of the presence of untreatable lesioning spinal cord [65]. With this respect to this, the correlation between fMRI and MR spectroscopy analysis of CSF would provide several possible biochemical markers and quantitative parameters that can be used to postulate a prognosis e.g., urinary excretion of L-2 hydroxyglutaric acid [66]. Also, Abnormal MRI in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) showed greatly enlarged nerve roots filling the lumbosacral spinal canal. This indicates a breakdown of the blood-nerve barrier. This appeared to be the cause of bladder dysfunction as evidenced by voiding difficulty and urinary areflexia which appeared 10 years after onset of CIDP [67]. So, the correlation between fMRI/MRI can be used as a tool for providing additional diagnostic pathophysiology information about CIPD, thereby improving prognosis owing to early diagnosis.
We suggest further studies with raised quality of the imaging and assess pharmacological treatment, which may bring information about the bladder dynamics. Where, an fMRI scanning demonstrated that after antidepressant sertraline treatment, a functional connectivity between the ACC and limbic regions increased while limbic activation in response to emotional stimuli decreased [68]. Also, MRI can be used in predicting response for alpha –adrenergic blockade in patients with BPH. Since androgen deprivation causes atrophy of epithelial tissue and a reduction in prostate volume. MRI has the ability of estimating the area density of smooth muscle cells in pelvic organ which was determined using immunostaining with antiactin antibody [69]. So, certain pharmacotherapeutic products may become treatment for urinary dysfunction with help of fMRI/ MRI imaging.
The combination of fMRI/MRI may play an important role in assessing voiding dysfunction before and after minimally invasive surgical treatment modalities. The safety of MRI in patients with pelvic floor dysfunction treated by neuromodulation was demonstrated, where, there was no change in perception of the stimulation after re-programming of the neurostimulator. Also, no change in bladder functions was reported after MRI examinations [70]. This combination is, also, will be of great importance in follow up patients in whom transperineal BTX-A injections under MRI control are used to relieve spasticity of muscles and lower urinary tract obstruction due to DSD [71]. Also, MRI demonstrated distinct anatomic abnormalities in the levator plate and diminished the levator ani muscle tissue density in patient with urinary incontinence [72]. Where, the fibrosis at the external urethral sphincter (EUS), detected by MRI, has a negative effect on EUS, which plays a role in the development of postprostatectomy incontinence after TUR. These findings may play a role in selecting patients who will benefit from this, minimally invasive treatment modalities [73,74].
The applications of fMRI have been primarily used by diagnostic utility in the radiological community. So, their results have to be correlated with the clinical, pathological, immunohistochemistry parameters for urinary dysfunction studies with the aid of metaanalysis. So, more accurate data can be provided than structural imaging data alone [66].
It has been difficult to compare brain activation across studies because of the variety of different operators and presentations modalities used. In order to avoid invalid generalizations of the involved brain areas, the future experiment must examine fMRIBOLD activity in participants. For future studies, multi planner fMRI analysis and a faster, single shot sequences imaging slices would be needed for sufficient temporal resolution to characterize the shape of the BOLD response and potential temporal regional differences [29]. Smart exogenous contrast agents that are turned under specific conditions like the presence of specific enzyme and can allow for further refinements in efficient tissue characterization paradigms will be one of the next revolutions in MRI. Real time MRI considered to be one of the most important tools for future clinical MRI.
Conclusion
In conclusion, this modern non-invasive imaging technology fMRI is gaining widespread acceptance as an anatomical or functional imaging tool for voiding dysfunction and can be used in understanding physiology and pathophysiology that is important in biology, and pharmacology. This may result in improved sensitivity and /or specificity of diagnostic evaluations of voiding dysfunction, and more importantly, accurate diagnosis at early and more treatable course of the disease and should allow for the evaluation of novel therapeutic interventions and preventive maneuvers.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003; 61: 37-49.
- Reynolds WS, Fowke J, Dmochowski R. “The Burden of Overactive Bladder on US Public Health,” Current Bladder Dysfunction Reports. 2016; 11: 8-13.
- Goldman HB, Zimmern PE. The treatment of female bladder outlet obstruction. BJU Int. 2006; 98: 359-366.
- Artibani W, Cerruto MA. The role of imaging in urinary incontinence. BJU Int. 2005; 95: 699-703.
- Sakakibara R, Fowler CJ, Hattori T. Voiding and MRI analysis of the brain. Int Urogynecol J Pelvic Floor Dysfunct. 1999; 10: 192-199.
- Fowler C. Neurological disorders of micturition and their treatment. Brain. 1999; 122: 1213-1231.
- Athwal BS, Berkley KJ, Hussain I, Brennan A, Graggs M, Sakakibara R, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001; 124: 369-377.
- Blok BF, Holstege G. The central nervous system control of micturition in cats and humans. Behav Brain Res. 1998; 92: 119-125.
- Mastuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T. Huamn brain region response to discussion or cold stimulation of the bladder: a positron emission tomography study. J Urol. 2002; 168: 2035-2039.
- Meyer S, Strittmatter M, Fischer C, Georg T, Schmitz B. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport. 2004; 15: 357-361.
- Holstege G. Micturition and the soul. J Comp Neurol. 2005; 493: 15-20.
- Liu Z, Sakakibara R, Nakazawa K, Uchiyama T, Yamamoto T, Ito T, et al. Micturition-related neuronal firing in the periaqueductal gray area in cats. Neuroscience. 2004; 126: 1075-1082
- Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol. 2005; 174: 1862-1867.
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci. 2007; 10: 1308- 1312.
- Nardos R, Gregory WT, Krisky C, Newell A, Nardos B, Schlaqqar B, et al. Examining mechanisms of brain control of bladder function with resting state functional connectivity MRI. Neurourol Urodyn. 2014; 33: 493-501.
- Assaf Y, Pasternak O. Diffusion Tensor Imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008; 34: 51-61.
- Detre JA, Wang J, Wang Z, Rao H. “Arterial spin-labeled perfusion MRI in basic and clinical neuroscience,” Current Opinion in Neurology. 2009; 22: 348-355.
- Deutsch G, Deshpande H, Fr ¨ olich MA, Lai HH, Ness TJ. “Bladder Distension Increases Blood Flow in Pain Related Brain Structures in Subjects with Interstitial Cystitis,” J Urol. 2016; 196: 902-910.
- Kavia RBC, Dasgupta R, Fowler CJ. Functional imaging and the central control of the bladder. J Comp Neurol. 2005; 493: 27-32
- Zhang H, Retiz A, Kollias S, Summers P, Curt A, Schurch B. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contracton. Neuroimage. 2005; 24: 174-180.
- Sakakibara R, Tsunoyama K, Takahashi O, Sugiyama M, Kishi M, Oqawa E, et al. Real-time measurement of oxyhemoglobin concentration changes in the frontal micturition area: an fNIRS study. Neurourol Urodyn. 2010; 29: 757-764
- Griffths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007; 37: 1-7.
- Seseke S, Baudewig J, Kallenberg K, Ringert RH, Seseke F, Dechent P. Voluntary pelvic floor muscle control-an fMRI study. Neuroimage. 2006; 31: 1399-1407.
- Mehnert U, Boy S, Svensson J, Resnick NM. Brain activation in response to bladder filling and simultaneous stimulation of the dorsal clitoral nerve--an fMRI study in healthy women. Neuroimage. 2008; 41: 682-689.
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011; 56: 2157-2172.
- Kitta T, Mitsui T, Kanno Y, Chiba H, Moriya K, Shinohara N. Brain-bladder control network: The unsolved 21st century urological mystery. Int J Urol. 2015; 22: 342-348.
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004; 7: 189-195.
- Komesu YM, Ketai LH, Mayer AR, Teshiba TM, Rogers RG. Functional MRI of the brain in women with overactive bladder: brain activation during urinary urgency. Female Pelvic Med Reconstr Surg. 2011; 17: 50-54.
- Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003; 103: 99-110.
- Tadic SD, Griffiths D, Schaefer W, Resnick NM. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. Neuroimage. 2008; 39: 1647-1653.
- Tadic SD, Griffiths D, Schaefer W, Cheng CI, Resnick NM. Brain activity measured by functional magnetic resonance imaging is related to patient reported urgency urinary incontinence severity. J Urol. 2010; 183: 221-228.
- Griffiths DJ, Fowler CJ. The micturition switch and its forebrain influences. Acta Physiol. (Oxf.) 2013; 207: 93-109.
- Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, et al. Cortical representation of the urge to void: a functional magnetic resonance imaging study. J Urol. 2005; 174: 1477-1481.
- Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997; 120: 111-121.
- Nour S, Svarer C, Kristensen JK, Paulson OB, Law I. Cerebral activation during micturition in normal men. Brain. 2000; 123: 781-789.
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. Distraction modulates connectivity of the cingulofrontal cortex and the midbrain during pain-an fMRI analysis. Pain. 2004; 109: 399-408
- Matsui M, Yoneyama E, Sumiyoshi T, Noguchi K, Nohara S, Suzuki M, et al. Lack of self-control as assessed by a personality inventory is related to reduced volume of supplementary motor area. Psychiatry Res. 2002; 116: 53-61.
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003; 126: 2139-2152.
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. International Urogynecological Association; International Continence Society “An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction,”. Neurourol Urodyn. 2010; 29: 4-20.
- Arya NG, Weissbart SJ, Xu S, Rao H. "Brain activation in response to bladder filling in healthy adults: An activation likelihood estimation meta-analysis of neuroimaging studies," Neurourol Urodyn. 2017; 36: 960-965.
- Fowler CJ and Griffiths DJ. "A decade of functional brain imaging applied to bladder control," Neurourol Urodyn. 2010; 29: 49-55.
- Girard N, Confort-Gouny S, Schneider J, Barberet M, Chapon F, Viola A, et al. MR imaging of brain maturation. J Neuroradiol. 2007; 34: 290-310.
- Tahmasian M, Eickhoff SB, Giehl K, Schwartz F, Herz DM, Drzezga A, et al. Resting-state functional reorganization in Parkinson's disease: An activation likelihood estimation meta-analysis. Cortex. 2017; 92: 119-138.
- Boor R, Jacobs J, Hinzmann A, Bauermann T, Scherg M, Boor S, et al. Combined spike-related functional MRI and Multiple source analysis in the non-invasive spike localization of benign rolandic epilepsy. Clin Neurophysiol. 2007; 118: 901-909.
- Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008; 27: 466- 474.
- Shy M, Fung S, Boone TB, Christof K, Sophie G, Fletcher S. Functional magnetic resonance imaging during urodynamic testing identifies brain structures initiating micturition. J Urol. 2014; 192: 1149-1154.
- Khavari R, Karmonik C, Shy M, Fletcher S, Boone T. Functional Magnetic resonance imaging with concurrent urodynamic testing identifies brain structures involved in micturition cycle in patients with multiple sclerosis. J Urol. 2017; 197: 438-444.
- Tadic SD, Griffiths D, Schaefer W, Murrin A, Clarkson B, Resnick NM. Brain activity Underlying impaired continence control in older women with overactive. Neurourol Urodyn. 2012; 31: 652-658.
- Griffiths D, Clarkson B, Tadic D, Resnick M. Brain mechanisms underlying urge incontinence and its response to pelvic floor muscle training. J Urol. 2015; 194: 708-715.
- Huang Y, Lane H, Lin C. "New treatment strategies of depression: Based on mechanisms related to neuroplasticity," Neural Plast. 2017; 2017: 4605971
- Minaglia S, Ozel B, Bizhang R, Mishell DR Jr. Increased prevalence of interstitial cystitis in women with detrusor overactivity refractory to anticholinergic therapy. Urology. 2005; 66: 702-706.
- Di Gangi Herms AM, Veit R, Reisenauer C, Grodd W, Enck P, Stenzl A, et al. Functional imaging of stress urinary incontinence. Neuroimage. 2006; 29: 267-275.
- Detre JA, Wang J. Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol. 2002; 113: 621-634.
- Witt TC, Kondziolka D, Baumann SB, Noll DC, Small SL, Lunsford LD. Preoperative cortical localization with functional MRI for use in stereotactic radiosurgery. Stereotact Funct Neurosurg. 1996; 66: 24-29.
- Boemers TM, Ludwikowski B, Forstner R, Schimke C, Ardelean MA. Dynamic magnetic resonance imaging of the pelvic floor in children and adolescents with vesical and anorectal malformations. J Pediatr Surg. 2006; 41: 1267- 1271.
- Joseph AM, Michael S, Wen-Ching L, In-Ki M, David H, Raj M, et al. Intraoperative functional MRI using a real-time neurosurgical navigation system. J Comput Assisted Tomc. 1997; 21: 910-912.
- Miller E, Ben-Sira L, Constantini S, Beni-Adani L. Impact of prenatal magnetic resonance imaging on postnatal neurosurgical treatment. J Neurosurg. 2006; 105: 203-209.
- Appasamy M, Roberts D, Pilling D, Buxton N. Antenatal ultrasound and magnetic resonance imaging in localizing the level of lesion in spina bifida and correlation with postnatal outcome. Ultrasound Obstet Gynecol. 2006; 27: 530-536.
- Aaronson OS, Hernanz-Schulman M, Bruner JP, Reed GW, Tulipan NB. Myelomeningocele: Prenatal evaluation-comparison between transabdominal US and MR imaging. Radiology. 2003; 227: 839-843.
- Kim YH, Goodman C, Omessi E, Rivera V, Kattan MW, Boone TB. The correlation of urodynamic findings with cranial magnetic resonance imaging findings in multiple sclerosis. J Urol. 1998; 159: 972-976.
- Borghesi G, Simonetti R, Goldman SM, Szejnfeld J, Srougi M, Ortiz V, et al. Magnetic resonance imaging urodynamics. Technique development and preliminary results. Int Braz J Urol. 2006; 32: 336-341.
- Poser CM, Brinar VV. Disseminated encephalomyelitis and multiple sclerosis: two different diseases a critical review. Acta Neurol Scand. 2007; 116: 201- 206.
- Hamstra DA, Rehemtulla A, Ross BD. Diffuse magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007; 25: 4104- 4109.
- Geitung JT. Magnetic resonance imaging of the pelvic floor. Possibilities and present status. Scand J Urol Nephrol Suppl. 2001; 207: 92-93.
- Sharama U, Pal K, Pratap A, Gupta DK, Jagannathan NR. Potential of proton magnetic resonance spectroscopy in the evaluation of patients with tethered cord syndrome following surgery. J Neurosurg. 2006; 105: 396-402.
- Prasad PV. Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Renal Physiol. 2006; 290: F958-F974.
- Ishii K, Tamaoka A, Fujita Y, Shoji S. Bladder and bowel dysfunction in chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Intern Med. 2005; 16: 211-213.
- Anand A, Li Y, Wang Y, Gardner K, Lowe M. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an fMRI study. J Neuropsychiatry Clin Neurosci. 2007; 19: 274-282.
- Mimata H, Nomura Y, Kasagi Y, Satoh F, Emoto A, Li W, et al. Prediction of alpha-blockers response in men with benign prostatic hyperplasia by magnetic resonance imaging. Urology. 1999; 54: 829-833.
- Elkelini MS, Hassouna MM. Safety of MRI at 1.5 tesla in patients with implanted sacral nerve neurostimulator. Eur Urol. 2006; 50: 311-316.
- Schurch B, Hodler J, Rodic B. Botulinum A toxin as a treatment of detrusorsphincter dyssynergia in patients with spinal cord injury: MRI controlled transperineal injections. J Neurol Neurosurg Psychiatry. 1997; 63: 474-476.
- Eguare EI, Neary P, Crosbie J, Johnston SM, Beddy P, Mc Govern B, et al. Dynamic magnetic resonance imaging of the pelvic floor in patients with idiopathic combined fecal and urinary incontinence. J Gastrointest Surg. 2004; 8: 73-82.
- Tuygun C, Imamoglu A, Keyik B, Alisir I, Yorubulut M. Significance of fibrosis around and/or at external urinary sphincter on pelvic magnetic resonance imaging in patients with postprostatectomy incontinence. Urology. 2006; 68: 1308-1312.
- Linemann A, Anthuber C, Baron A, Reuser M. Diagnostic enteroceles using dynamic magnetic resonance imaging. Dis Colon Rectum. 2000; 43: 205- 212.