
Case Report
Austin J Urol. 2022; 8(1): 1075.
Inflammatory Myofibroblastic Tumor of the Bladder in an 8-Year-Old Girl: About a Case with Review of the Literature
El Kebir A¹, Zongo PE²*, Alomri Z³, Chaabi S¹, Alzemmouri M³, Sahraoui S², Guebbissi NB¹ and Karkouri M¹
¹Department of Pathology, University Hospital Center Ibn Rochd, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
²Department of Radiation Oncology, University Hospital Center Ibn Rochd, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
³Department of Pediatric Surgery, University Hospital Center Ibn Rochd, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
*Corresponding author: Pawendtaoré Esdras Zongo, Department of Radiation Oncology, Faculty of Medicine and Pharmacy, Hassan II University 19 Rue Tariq BnouZiad, Mers Sultan BP: 9167 Casablanca, Marocco
Received: May 30, 2022; Accepted: June 27, 2022; Published: July 04, 2022
Abstract
Introduction: The inflammatory myofibroblastic tumor is a rare tumor. It most often occurs in the bladder in the genitourinary sphere. This benign and aggressive spindle cell tumor affects children and frequently girls. The objective of this work is to describe from a clinical case, the clinical, radiological, and histological characteristics of an inflammatory myofibroblastic tumor in an 8 years old girl.
Case Presentation: An 8-year-old girl was followed for an inflammatory myofibroblastic tumor of the bladder revealed by micturition burns complicated by hematuria. She underwent an ultrasound and radiological workup demonstrating an anterior bladder parietal mass reaching the rectus abdomen is muscle. Subsequently, an excisional biopsy was performed and histological and immunohistochemical examination revealed an inflammatory myofibroblastic tumor.
Discussion: Inflammatory myofibroblastic tumors of the bladder are benign tumors with aggressive features usually revealed by voiding symptoms, pelvic pain, and pelvic mass. Treatment is based on Trans urethral resection of the bladder. Anatomical pathology examination shows spindle-shaped myofibroblast and fibroblast cells, a collagenous or myxoid matrix, and inflammatory cells composed of plasma cells, lymphocytes, and eosinophils. The diagnosis is confirmed by immunohistochemistry with an expression of CAM5.2, desmin, aSMA, and vimentin and especially of Anaplasic Lymphoma Kinase (ALK).
Conclusion: Rare tumor process, whose malignant potential remains unknown. Management is by conservative surgery of the bladder.
Keywords: Inflammatory; Myofibroblastic; Tumor; Bladder
Introduction
An inflammatory myofibroblastic tumor (IMT) is a rare neoplasm composed of spindle cells with an associated inflammatory cell infiltrate [1]. The first case of inflammatory myofibroblastic tumor (IMT) was described by Brunn [2] in two cases of "lung myoma" in 1939. The location of such a lesion in the genitourinary tract was then described by Roth [2] in 1980. IMT has been called plasma cell granuloma, inflammatory pseudotumor, pseudomalignant spindle cell proliferation, or pseudosarcomatous myofibroblastic tumor [3]. Confusion regarding nomenclature may have contributed to the difficulty of establishing standard therapeutic procedures for this disease [4]. An absolute definition of the malignant potential of these tumors is not possible at this time [5]. In adults, it may be associated with urinary tract instrumentation, but its etiology in children remains unknown [1]. Although rare (less than 1% of all bladder tumors), the bladder is the most frequently affected site in the genitourinary system [3]
Clinical Observation
An 8-year-old female child without any particular pathological history consulted us for micturition burns complicated by macroscopic hematuria, all evolving in a context of apyrexia and alteration of the general state. A radiological workup was performed with abdominopelvic ultrasound and an abdominopelvic CT scan. Abdominal and pelvic ultrasound revealed a bladder with regular content and a heterogeneous pedunculated anterior parietal mass protruding into the bladder lumen and measuring 30 x 23 mm. The abdominopelvic CT scan showed a right-lateralized anterior bladder parietal mass, fairly well limited, hypodense, heterogeneously enhanced after injection of contrast medium, measuring 34 x 33 mm, extended over 40 mm in height. It arrives anteriorly in contact with the rectus abdomen is muscle which is displaced with the loss of the fatty separation line (Appendix 2).
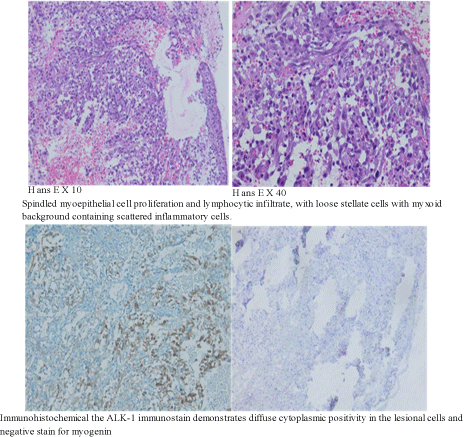
Appendix 1: Histological and immunohistochemical appearance of the inflammatory myofibroblastic tumor.
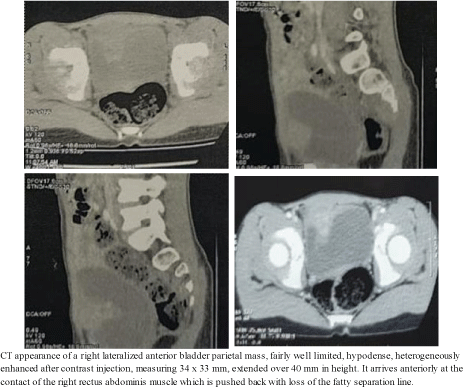
Appendix 2: CT image showing the bladder tumor process.
It underwent surgical biopsy (Appendix 3) and a pathology study noted an inflammatory myofibroblastic tumor of the bladder (Appendix 1).
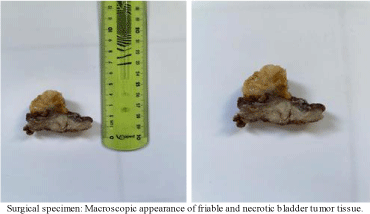
Appendix 3: Image of the resection specimen of the inflammatory myofibroblastic tumor of the bladder.
Discussion
Inflammatory pseudotumor is a general term used to describe a wide range of reactive or neoplastic lesions sharing common histologic appearances [2]. Many different terminologies, including pseudosarcomatous myofibroblastic proliferation, inflammatory myofibrohistiocytic proliferation, and atypical fibromyxoid tumor, have been described previously [2]. This varied nomenclature may have contributed to the difficulty of accumulating clinical data to analyze the histology and clinical course of the disease [4]. IMT is a rare, benign, spindle cell tumor with locally aggressive behavior [6]. In other words, it is characterized by an atypical proliferation of spindle cells and inflammatory cellular infiltrates involving mainly lymphocytes and plasma cells [7].
The pathogenesis of IMT is controversial, and the question of whether it is a post-inflammatory process or a true neoplasm is still unresolved [6]. Since IMT is characterized histologically by an inflammatory infiltrate and various microbes have been isolated from the lesions (such as mycobacteria, corynebacteria, Ebstein-Barr virus, and human herpesvirus), the infection has long been suspected to play an important role in the pathogenesis of IMT [6]. A history of surgery, trauma, and steroid use has also been associated with IMT [6]. In this case, there was no definite evidence of an infection, traumatic episode, or prior steroid use [6].
Historically, IMT and similar tumors were first described in the lungs and published by Brunn et al in 1939 [5]. It occurs in pulmonary and extrapulmonary sites, including the abdomen, retroperitoneum, head, neck, brain, and extremities. In the genitourinary system, IMT has been reported in the kidney, urethra, prostate, ureter, and testis, but is most commonly seen in the bladder [6]. IMT of the bladder was first reported by Roth in 1980 [6]. Most reported cases occurred in young adults and there is a predilection for females (female to male ratio, 2:1) [6].
A systematic review by Teoh et al [3] in 2014 evaluated 182 cases of urinary bladder IMT in the literature [3]. Another review of the literature by Song et al in 2019 revealed 16 additional patients, for a total of 198 cases of urinary bladder IMT to date [3]. In children, the literature review by Houben et al [8] identified 35 cases of IMT of the bladder in children [3]. Of these 35 patients, 20 were girls, and the median age was 7 years (range 2-16) [3].
The clinical presentation varies depending on the anatomical location of the lesion [6]. In bladder IMT, painless hematuria with or without clots from exophytic and ulcerated lesions is the most common initial manifestation and may result in anemia [6]. Other signs that may accompany hematuria include voiding symptoms, abdominal pain, or a palpable abdominal mass detected by physical examination [5]. Symptoms, such as fever and weight loss, were reported [5]. Radiologically, Liang et al [3] described a certain appearance of urinary bladder IMT on scans. He described it as lesions appearing mainly on the anterior wall of the bladder, with ring enhancement on contrast-enhanced CT [3]. Although this was evident in our case, being nonspecific and inconclusive, radiological examinations, including CT with contrast, are insufficient to establish a diagnosis [3]. Cystoscopic examinations may reveal a single polypoid intraluminal mass [6].
The main diagnostic tool is tissue biopsy by transurethral resection of bladder tumor (TURBT) and histopathologic examination. The histologic appearance varies but typically includes a spindle cell element of myofibroblasts and fibroblasts, a collagenous or myxoid matrix, and inflammatory cells consisting of plasma cells, lymphocytes, and eosinophils [1]. It must be differentiated from benign lesions, such as leiomyoma or solitary fibrous tumors, and malignant lesions, such as leiomyosarcoma, sarcomatoid carcinoma, or embryonalrhabdomyosarcoma [5]. These entities have common histopathological findings. To differentiate them well, immunochemistry is essential. Immunohistochemistry shows cells positive for CAM5.2, desmin, SMA, and vimentin [4,5]. Recently, genetic fusions involving ALK on chromosome 2p23 have been described in IMT [5]. ALK (Anaplastic lymphoma kinase) has been suggested as a good marker of IMT with a level that varies between 30 and 60% [6,8]. In this case, ALK immunohistochemistry was positive and useful for a definitive final diagnosis [7]. Translocation of the ALK gene in IMT has also been reported, for which detection by fluorescence in situ hybridization (FISH) is useful [7]. IMT is also influenced by mutations in p53 and the expression of the MDM2 homolog, which appear to play a major role in its pathogenesis [6]. Cytokeratin is more likely to be expressed in bladder lesions than in lesions at other sites [6]. Thus, almost all cases of IMT are diffusely positive for actin-associated proteins (e.g., smooth muscle actin, muscle-specific actin, calponin). Furthermore, the expression of COX2 and VEGF has been detected in IMTs by immunohistochemistry [7].
Therapeutically, surgical resection is initially recommended. In a 2014 systematic review, most patients underwent transurethral resection of the bladder tumor (TURBT) (60.8%); the remainder underwent partial (29.2%) and radical (9.2%) cystectomy and 5 patients had local recurrence [7]. Complete surgical resection is important to avoid local recurrence [7]. While partial or radical cystectomy ensures complete resection of IMT, TURBT is also a considerable choice, given the benign course of an IMT. In children, Transurethral resection of IMT of the bladder is frequently followed by conversion or partial cystectomy at a later stage. There is little evidence regarding chemotherapy for IMT, and most data are from pediatric populations [6]. Radiation therapy and even immunomodulation have not been reported to be consistently effective against aggressive IMT [6]. Recurrence of bladder IMT in adults is rare after complete surgical removal, but recurrence rates up to 25% have been reported [1].
COX2 and VEGF expression have been detected and are thought to be therapeutic targets [7]. Anti-inflammatory drugs and COX2 inhibitors used for unresectable IMT have been frequently reported [7]. An ALK inhibitor, crizotinib, has also been used in the treatment of IMT [7] In case of recurrence when the tumor is unresectable, drug therapy (targeted therapy) may become a viable treatment option [7].
Conclusion
IMT is a rare neoplasm with unknown malignant potential and multiple differential diagnoses. In clinical practice, conservative bladder surgery (transurethral resection of the bladder tumor or partial cystectomy) is recommended, associated with close clinical follow-up.
References
- Lecuona AT, Wyk ACV, Smit SG, Zarrabi AD, Heyns CF. Inflammatory myofibroblastic tumor of the bladder in a 3-year-old boy. Urology. 2012; 79(1): 215-218. doi:10.1016/j.urology.2011.04.052.
- Teoh JYC, Chan N, Cheung H, Hou SSM, Ng C. Inflammatory myofibroblastic tumors of the urinary bladder: a systematic review. Urology. 2014; 84(3): 503- 508. doi:10.1016/j.urology.2014.05.039.
- Fadaak K, Al-Otaibi A, Al-Zahrani A, Alhaam A, Al-Dandan O, Kussaibi H, et al. Transurethral Resection for the Treatment of an Inflammatory Myofibroblastic Tumor of the Urinary Bladder: A Case Report. Case Reports in Oncology. 2019; 12(2): 344-353. doi:10.1159/000500503.
- Kato M, Masui S, Kanda H, Arima K, Shiraishi T, Sugimura Y. Successful Preservation of the Bladder in a Case of Inflammatory Myofibroblastic Tumor with the Diagnostic Efficacy of ALK/p80 Immunohistochemistry and FISH Analysis: Case Report and Review of the Literature. Urology Case Reports. 2017; 11: 19-21. doi:10.1016/j.eucr.2016.11.018.
- Süer E, Gülpinar, Mermerkaya M, Burgu B, Celepli P, Sertçelik A, et al. Inflammatory myofibroblastic tumor of the bladder in a 10-year-old girl. Urology. 2012; 80(5): 1138-1140. doi:10.1016/j.urology.2012.07.023.
- Kim HW, Choi YH, Kang SM, Ku JY, Ahn JH, Kim JM, et al. Malignant Inflammatory Myofibroblastic Tumor of the Bladder with Rapid Progression. Korean Journal of Urology. 2012; 53(9): 657. doi:10.4111/kju.2012.53.9.657.
- Etani T, Naiki T, Nagai T, Iida K, Ando R, Naiki-Ito A, et al. Inflammatory Myofibroblastic Tumor of the Urinary Bladder: A Case Report. Case Reports in Oncology. 2016; 9(2): 464-469. doi:10.1159/000448550.
- Houben CH, Chan A, Lee KH, Tam YH, To KF, Cheng W, et al. Inflammatory myofibroblastic tumor of the bladder in children: what can be expected?. Pediatric Surgery International. 2007; 23(8): 815-819. doi:10.1007/s00383- 007-1885-y.