
Research Article
Austin J Urol. 2022; 8(1): 1076.
The Alarming Situation of Hospital Acquired Multi-drug Resistant Urinary Tract Infections in Pediatric Population in Developing World
Kakakhel MK, Arshad and Khan MK*
Institute of Kidney Diseases, Hayatabad Medical Complex, Pakisthan
*Corresponding author: Muhammad Kamran Khan, Assistant Professor Pediatric Urology, Institute of kidney diseases, Hayat Abad Medical Complex, Peshawar, Pakistan
Received: July 13, 2022; Accepted: August 06, 2022; Published: August 13, 2022
Abstract
Objectives: The aim of this study was to find the magnitude of multi drug resistant UTI in paediatric urology, showing different uropathogenic bacteria, sensitivity pattern, associated urologic pathologies and surgical procedures.
Material and Methods: All Paediatric patients with some urologic procedure in this institute who have multi or extensive or pan drug resistant UTI were included, the record retrospectively collected from hospital record system and analysed with SPSS.
Results: We total 54 patients included having mean age of 5.4 years and predominantly male patients. The three most common MDR uropathogens were Pseudomonas aueroginosa (63%), E.coli (18.5%) and Kliebsiella spp (9.3%). Other MDR organisms were Enterococcus, Providencia, Serratia marcescens and Staphylococcus. Overall most sensitive drug was colistin (64.8%), followed by Fosfomycin (18.5%), Carbapenems (16.6%), Aminoglycosides (12.9%), Tigecycline (11.1%), Nitrofurantoin (7.4%), Cephalosporin (5.5%), Vancomycin (5.5%), Linzolid ( 5.5%), Septran (3.7%), while Teicoplanin, Fusidic acid and chloramphenicol all 1.8%. We found two culture reports of Pseudomonas aueroginosa as pan drug resistant. The most common urologic pathologies associated with MDR UTI was urinary stone disease, neurogenic bladder and posterior urethral valves. The prevalent surgical procedures were those, underwent for stone diseases. A substantial increase in hospitalization time noted in these patients.
Conclusion: The emergence of multi drug resistance is reaching an alarming level in Paeds urology. Pseudomonas aueroginosa causing UTI replacing E.coli with some pan drug resistant species is worrisome. The wide spread use of broad spectrum antibiotics at primary healthcare level and even without culture reports in the tertiary care level needs revision. This has significant impact on the child morbidity, length of hospital stay and finally financiall loss.
Keywords: Multi drug resistant; Urinary tract infection; Pseudomonas; E.coli; Kliebsiellaspp; Colistin; Carbapenems
Abbreviation and Acronyms
UTI: Urinary tract infection; MDR: Multi Drug Resistant; XDR: Extensive Drug Resistant; PDR: Pan Drug Resistant; CFU: Colony Forming Units; PCNL: Percutaneous Nephrolithotomy; CU: Cystourethroscopy; DJS: Double J Stent; URS: Ureterorenoscopy; ICL: Intra Corporeal Lithotripsy; CIC: Clean Intermittent Catheterization; PUV: Posterior Urethral Valves; RIRS: Retrograde Intra Renal Surgery; RPG: Retrograde Pyelogram
Introduction
Urinary tract infection is defined by the presence of 105 CFU/ml of pathogenic bacteria in a clean catch urine specimen or 104 CFU/ ml for catheter specimen, which may present as frequency, urgency, dysuria, cloudy urine, fever and vomiting [1]. Bacterial UTI is one of the most common infections in paediatric patients [2]. Among infants presenting with fever, other unwell children and older children who manifest urinary symptoms, 6-8 %will have UTI [3]. The high prevalence of UTI result in almost 8.3 million physician visits, one lac hospitalization per year, 1 million emergency visits and drive significant use of antibiotics around the world which cost $1 billion per annum in the United States [4,5]. Most children have other illnesses in addition to UTI and the urinary symptoms are usually not prominent thus the urine is not tested for infection. This causes UTIs to go un-noticed and not accounted for the childhood morbidity.
In 2010 the international consensus defined Multi-Drug Resistance (MDR) as non-susceptibility to at least one antimicrobial in three or more classes, based on laboratory testing. Extensive Drug Resistance (XDR) is defined as sensitivity to only one or two antimicrobials while resistant to all other categories. Pan Drug Resistance (PDR) is defined as resistance to all classes of antimicrobials [6]. Antimicrobials are the mainstay of treatment option for bacterial UTI, but appropriate selection of antibiotics is necessary to improve treatment outcome and prevent the emergence of antimicrobial resistance. As their consumption is considered to be the main risk factor for the drug resistance [7]. Unfortunately because of extensive, improper and un-necessary use of antibiotics the antimicrobial resistance in uropathogens reached currently up to an alarming level [8]. Now the antimicrobial resistance is considered an international public health problem. The primary health care got now a significant contribution to this issue, because this is place for almost 80% of the total antibiotics use. Subsequently the multi-drug resistant infections cause increased morbidity, mortality, financial burden on patient and healthcare system [9].
This study is aimed at highlighting the burden of the problem, prevalence of different organisms, antibiotic resistance pattern and different Paediatric diseases and urological procedures which may contribute as risk factor for developing UTI.
Materials and Methods
After approval from research ethics board of the institute of kidney diseases and transplant, this observational study included all patients up to age 16 years having multi, extensive or pan drug resistant UTI. The data retrieved from hospital record system from June 2017 to December 2020. The data detailed the type of organism with its antimicrobial sensitivity and resistance, disease and type of surgical procedure underwent. The data analysed with IBM® SPSS®, version 20.0.
Results
We included 54 patients who developed multi-drug resistant UTI, with mean age of 5.4 years, including 38 (70.3%) male and 16 (29.6%) female patients. Mean hospital stay was 9.28 days, with minimum 2 and maximum 26 days. The multi-drug resistant organisms profile is detailed in Table 1.The antimicrobial sensitivity to colistin was (n=35, 64.8%), Fosfomycin (n=10, 18.5%), Carbapenems (n=9, 16.6%), Aminoglycosides (n=7, 12.9%), Tigecycline (n=6, 11.1%), Nitrofurantoin (n=4, 7.4%), Cephalosporin (n=3, 5.5%), Vancomycin (n=3, 5.5%), Linzolid (n=3, 5.5%), Septran (n=2, 3.7%), while Teicoplanin, Fusidic acid and chloramphenicol all has sensitivity of1.8%. We found two culture reports of Pseudomonas aueroginosa resistant to all available antibiotics. About individual sensitivity, Pseudomonas aueroginosa was sensitive to colistin in 79.3%, Fosfomycin in 17.2%, Carbapenems in 10.3% and aminoglycosides in 3.4%. E.coli was sensitive to colistin in 54%, Carbapenems in 45% and Fosfomycin in 9%. Kliebsiellaspp was sensitive to colistin in 66%, Fosfomycin in 33.3%, Carbapenems and Nitrofurantoin 16.6% each. The urologic diseases in these patients were urinary stone disease (n=26, 48.14%), followed by neurogenic bladder (n=5, 9.2%), posterior urethral valves (n=4, 7.4%), pelvi-ureteral junction obstruction (n=3, 5.6%), isolated UTI (n=3, 5.6%), vesicoureteral reflux (n=3, 5.6%), ureterocele (n=2, 3.7%), pyelonephritis (n=1, 1.9%), bladder tumor (n=1, 1.9%), psoas abscess (n=1, 1.9%) and post ureteric re-implantation (n=1, 1.9%). The urologic procedures done are shown in (Figure 1).
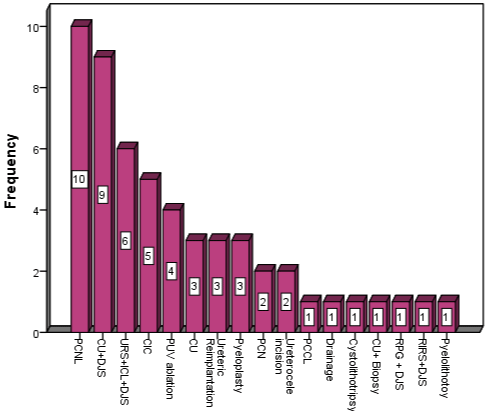
Figure 1: Type of urologic procedures associated with MDR uti.
Organisms
Frequency
Percent
Pseudomonas auerogenosa
34
63
E.coli
10
18.5
Kliebsiellaspp
5
9.3
Enterobacter
1
1.9
Enterococcus
1
1.9
Providencia
1
1.9
Serratia marecescens
1
1.9
Staphylococcus
1
1.9
Total
54
100
Table 1: Frequency of Multi drug resistant organisms.
Discussion
The major difference noted here is, that Pseudomonas aueroginosa is the predominant organism that’s 63% rather than E.coli which accounted for 18.5% cases, followed by Kliebsiellaspp (9.3%). Samanci S et al found E.coli the leading cause in 85.8%, Kliebsiellaspp in 5.3%, while Pseudomonas aueroginosa in 0.6% [10]. In the study by Yoon JE et al E.coli accounted for 81.4%, Kliebsiellaspp for 7.7% and Pseudomonas aueroginosa only for 0.7% [11]. Vazouras K et al found E.coli in 79.2%, Kliebsiellaspp in 7.2% and Pseudomonas aueroginosa in 4.7% [12]. MI khan found E. coli is the predominant isolate accounting for 69.7% isolates, Kliebsiellaspp in 21.21% and Pseudomonas aueroginosa in 9.1% [13]. Duicu C et al, in central Romania identified E.coli in 72.2%, Kliebsiellaspp in 8.15% and Pseudomonas aueroginosa in 5.75% [14], In Nepal, study by Shrestha LB et al, E. coli caused MDR UTI in 53%, Kliebsiellaspp in 7% and Pseudomonas aueroginosa in 2% [15]. The possible explanation for this difference could be, the nosocomial pattern, as all patients underwent some surgical procedure in this hospital (persistence of Pseudomonas aueroginosa in the hospital environment). Second is there may be good sensitivity of the E.coliin this region, which makes it second as a drug resistant uropathogen. Kliebsiellaspp remains third as cause of UTI in our study, this similar finding as shown in other studies.
Pseudomonas aueroginosa was sensitive to colistin in 79.3% cases, while in a study conducted in china Zhang H et al, found a sensitivity of 94.6% [16] and Ramalakshmi et al detected sensitivity of 83% [17]. Somily AM found 93 % Pseudomonas aueroginosa sensitive to colistin [18]. This shows we have relatively a lower sensitivity of Pseudomonas aueroginosa to colistin. Pseudomonas aueroginosa sensitivity to Carbapenems was much lower than colistin (10.3%), in study of Ramalakshmi discussed previously, the sensitivity was 67% [17]. The possible explanation is the wide spread use of Carbapenems on empirical basis which led to high resistance.
We found much lower sensitivity of E.coli to Carbapenems (45%). In a study in India by Niranjan V et al, susceptibility to Carbapenems was 98.9% [19], while in study by Kulkarni et al 96.7% of E.coliwere sensitive to Carbapenems [20]. Zhang, H. et al in identified Susceptibilities of Escherichia coli in UTI strains to Carbapenems of > 90% [21]. In a Pakistanian study by Zubair KU et al, that’s 100% [22].
We found sensitivity of Kliebsiellaspp to Carbapenems, only 16.6 %, which is significantly lower than previous literature showed. In study conducted in India by N G et al, the identified almost all sensitive to Meropenem (100%) [23]. In a study in Iraq by Naqid IA et al, they found more than 95% Kliebsiellaspp species sensitive to Meropenem and Ertapenem [24]. In another Indian study Ashiskumarsaha found Kliebsiellaspp sensitive to Meropenem in 75.3%, Imipenem in 88.9%, and Ertapenem in 75% [25]. Varghese et al found sensitivity to Imipenem (74.3%) and Meropenem (65.7%) [26].
About half of the patients with MDR-UTI were having urinary stone disease. Other studies confirm this association. In a study in Nepal by pratima shah et al, 50% of patient with stone disease had UTI [27]. In study done in Taiwan by Huang WY et al, 34% of urinary stone disease had associated UTI [28]. In the study of Cetin N et al, 30.2% of urinary stone disease patients had UTI [29]. The study done in Baghdad by Qadeer DS et al, 24.3% [30] while Hsiao et al found 17.1% [31] and Kasew D et al, in Ethopia 16.3% [32] of urinary stone disease patient with UTI.
The prevalent population was male (70.3%) in our study which is similar with the findings of Shrestha LB et al [15]. But most of the positive cultures obtained in other studies are from female population like in study by Samanci S et al (80%) [10], in the study of MI Khan et al (72%) [13], and in study of Ashiskumarsaha females were 69.71% [25]. The difference may reflect the influence of social and cultural factors which result is less number of female patient’s visits to tertiary care centers in this region.
The mean hospital stay was almost 3 time the normal, as all these patients received parenteral antibiotics. Most commonly sensitive among them was colistin, which is an expensive drug, thus un affordability was a contributory factor. This shows how the resistant uropathogens increase health care cost both in part of patient and healthcare system.
Conclusion
The drug resistance in the Pediatric urology patients is increasing. The Pseudomonas aeruginosa causing MDR/PDR UTI is taking over E.coli as the most prevalent organism in these patients. The resistance pattern with sensitivity to highly potent antibiotics like colistin and Carbapenems instead of commonly prescribed and previously considered drugs of choice is showing the seriousness of the issue. The culture of injudicious and empirical broad-spectrum use of antibiotics should be controlled and regular monitoring of antibiotics sensitivity required. The urinary stone disease and other common pathologies with high rate of subsequent MDR UTI need careful management. This has impact on the child morbidity, length of hospital stay and finally a burden on the health system.
References
- Shaikh N, Morone NE, Lopez J, Chianese J, SangvaiS, D’Amico F, et al. Does this child have a urinary tract infec-tion? JAMA. 2007; 298: 2895-904.
- Korbel L, Howell M, Spencer JD. The clinical diagnosis and management of urinary tract infections in children and adolescents. Paediatrics and International Child Health. 2017; 37: 273-279.
- O'Brien K, Edwards A, Hood K, Butler CC. Prevalence of urinary tract infection in acutely unwell children in general practice: a prospective study with systematic urine sampling. The British journal of general practice: the journal of the Royal College of General Practitioners. 2013; 63: e156-e164.
- Allen UD, MacDonald N, Fuite L, Chan F, Stephens D. Risk factors for resistance to "first-line" antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 1999; 160: 1436-40.
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. The American journal of medicine. 2002; 113: 5-13.
- Magiorakos A, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012; 18: 268-281.
- Erb A, Stürmer T, Marre R, Brenner H. Prevalence of antibiotic resistance in Escherichia coli: overview of geographical, temporal, and methodological variations. European Journal of Clinical Microbiology & Infectious Diseases. 2006; 26: 83-90.
- Mazzariol A, Bazaj A, Cornaglia G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: a review. Journal of Chemotherapy. 2017; 29: 2-9.
- Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. The BMJ. 2016; 352: i939.
- Samanci S, Çelik M, Kösker M. Antibiotic resistance in childhood urinary tract infections: A single-center experience. Turkish Archives of Pediatrics/Türk Pediatri Arsivi. 2020; 55: 386-392.
- Yoon JE, Kim WK, Lee JS, Shin K, Ha T. Antibiotic susceptibility and imaging findings of the causative microorganisms responsible for acute urinary tract infection in children: a five-year single center study. Korean Journal of Pediatrics. 2011; 54: 79.
- Vazouras K, Velali K, Tassiou I, Anastasiou-Katsiardani A, Athanasopoulou K, Barbouni A, et al. Treatment and Antimicrobial Resistance in Children with Urinary Tract Infections. Journal of global antimicrobial resistance. 2019; 20: 4-10.
- Muhammad Imran Khan, Surui Xu, Malik Mubashar Ali, Rizwan Ali, AhsanKazmi, et al. Assessment of multidrugresistance in bacterial isolates from urinary tract-infected patients. Journal of Radiation Researchand Applied Sciences. 2020; 13: 267-275.
- Duicu C, Cozea I, Delean D, Aldea AA, Aldea C. Antibiotic resistance patterns of urinary tract pathogens in children from Central Romania. Experimental and therapeutic medicine. 2021; 22: 748.
- Shrestha LB, Baral R, Poudel P, Khanal B. Clinical, etiological and antimicrobial susceptibility profile of pediatric urinary tract infections in a tertiary care hospital of Nepal. BMC Pediatrics. 2019; 19.
- Zhang H, Zhang G, Zhang J, Duan S, Kang Y, Yang Q, et al. Antimicrobial Activity of Colistin Against Contemporary (2015 – 2017) P. aeruginosa and A. baumannii Isolates From a Chinese Surveillance Program. Frontiers in Microbiology. 2020; 11.
- Ramalakshmi K, Apparao P, Kamala P, Himabindu V. Study of Colistin Sensitivity Pattern of Pseudomonas Aeruginosa in a Tertiary Care Hospital. International Journal of Contemporary Medical Research [IJCMR]. 2019; 6.
- Somily AM, Absar MM, Arshad MZ, Aska AIA, Shakoor ZA, Fatani AJ, et al. Antimicrobial susceptibility patterns of multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii against carbapenems, colistin, and tigecycline. Saudi medical journal. 2012; 33: 750-5.
- Niranjan V, Malini A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. The Indian Journal of Medical Research. 2014; 139: 945 - 948.
- Kulkarni SR, Peerapur BV, Sailesh KS. Isolation and Antibiotic Susceptibility Pattern of Escherichia coli from Urinary Tract Infections in a Tertiary Care Hospital of North Eastern Karnataka. Journal of Natural Science, Biology, and Medicine. 2017; 8: 176.
- Zhang H, Kong H, Yu Y, Wu A, Duan Q, Jiang X, et al. Carbapenem susceptibilities of Gram-negative pathogens in intra-abdominal and urinary tract infections: updated report of SMART 2015 in China. BMC Infectious Diseases. 2018; 18.
- Zubair KU, Shah AH, Fawwad A, Sabir R, Butt A. Frequency of urinary tract infection and antibiotic sensitivity of uropathogens in patients with diabetes. Pakistan Journal of Medical Sciences. 2019; 35: 1664-1668.
- G MN, Math GC, Nagshetty K, Patil SA, Gaddad SM, Shivannavar CT. Antibiotic Susceptibility Pattern of ESβL Producing Klebsiella pneumoniae Isolated from Urine Samples of Pregnant Women in Karnataka. Journal of clinical and diagnostic research: JCDR. 2014; 8: DC08-11.
- Naqid I A , Hussein N R, Balatay A A, Saeed K A, Ahmed H A. The Antimicrobial Resistance Pattern of Klebsiella pneumonia Isolated from the Clinical Specimens in Duhok City in Kurdistan Region of Iraq, J Kermanshah Univ Med Sci. 2020; 24: e106135.
- Ashis Kumar Saha. Pattern of antimicrobial susceptibility of klebsiella pneumoniae isolated from urinary samples in urinary tract infection in a tertiary care hospital, Kishanganj, Bihar, 5 years’ experience. International Journal of Contemporary Medical Research. 2019; 6: L25-L28.
- Varghese A, George S, Gopalakrishnan R, Mathew A. Antibiotic susceptibility pattern of Klebsiella pneumoniae isolated from cases of urinary tract infection in a tertiary care setup. Journal of Evolution of Medical and Dental Sciences. 2016; 5.
- Shah P, Baral R, Agrawal CS, Lamsal M, Baral D, Khanal B. Urinary Calculi: A Microbiological and Biochemical Analysis at a Tertiary Care Hospital in Eastern Nepal. International Journal of Microbiology. 2020; 2020: 1-9.
- Huang W, Chen Y, Chen S, Lee Y, Lan C, Huang K. Pediatric urolithiasis in Taiwan: a nationwide study, 1997-2006. Urology. 2012; 79: 1355-1359.
- Cetin N, Gencler A, Tufan AK. Risk factors for development of urinary tract infection in children with nephrolithiasis. Journal of Paediatrics and Child Health. 2019; 56: 76-80.
- Qaader DS, Yousif SY, Mahdi LK. Prevalence and etiology of urinary stones in hospitalized patients in Baghdad. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah lisharq al-mutawassit. 2006; 12: 853-61.
- Hsiao C, Chen T, Lee Y, Hsiao M, Hung P, Chen Y, et al. Urolithiasis Is a Risk Factor for Uroseptic Shock and Acute Kidney Injury in Patients With Urinary Tract Infection. Frontiers in Medicine. 2019; 6.
- Kasew D, Eshetie S, Diress A, Tegegne Z, Moges F. Multiple drug resistance bacterial isolates and associated factors among urinary stone patients at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Urology. 2021; 21.