
Perspective
Austin J Urol. 2022; 8(1): 1077.
Equivalence of Immunological Response to COVID-19 Vaccination in Prostate Cancer Patients
Gunge N¹, Miyazaki T¹, Okabe Y¹, Nakamura N¹, Nabeshima S² and Haga N¹*
¹Department of Urology, Fukuoka University Faculty of Medicine, Fukuoka, Japan
²General Medicine, Fukuoka University Hospital, Fukuoka, Japan
*Corresponding author: Haga N, Department of Urology, Fukuoka University Faculty of Medicine, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan
Received: September 23, 2022; Accepted: October 08, 2022; Published: October 15, 2022
Abstract
Objectives: To elucidate antibody responses to COVID-19 (coronavirus disease 2019) vaccination among patients with Prostate cancer (Pca), group comparisons between patients with Pca and healthy controls were conducted at several times points after COVID-19 vaccination.
Methods: Blood samples from 19 consecutive patients and 12 healthy controls were prospectively collected before first vaccination, and at 1, 3, and 6 months after first vaccination. BNT 162b2 (Pfizer/BioNTech) vaccines were administered for all patients with Pca. Antibody titers were determined by enzyme-linked immunosorbent assay using recombinant protein of the SARSCoV- 2 spike-protein receptor-binding domain as an antigen.
Results: Mean age was significantly higher for patients (73.6±9.7 years) than for controls (63.2±3.4 years; P<0.01). Mean initial PSA for the overall cohort was 330±615 ng/mL. Ten patients had localized Pca and 9 patients had metastatic Pca. ADT was administered for the 84% (16/19) patients. Treatment with ADT and/or Androgen Receptor-Axis-Targeted (ARAT) agent had been continuously performed in 68% (13/19) patients since the first vaccination. After the first vaccination, antibody titer was significantly increased at 1 month after vaccination and then gradually declined over time in each group. Regarding the comparison between the two groups, mean antibody titer was not significantly different in patients during the observational period.
Conclusions: This is a first report from an Eastern country to find that immunological response to first COVID-19 vaccination was equivalent in patients with Pca compared with healthy controls.
Perspective
Infection with COVID-19 (coronavirus disease 2019) for patients with prostate cancer (Pca) significantly increases the mortality rate and length of hospitalization [1]. Protection against COVID-19 by vaccinations would be important for patients with Pca. Although antibody response to COVID-19 vaccination is reportedly decreased in immunosuppressed patients, such as those with hematological cancer or end-stage renal disease [2,3], antibody response to COVID-19 vaccination for patients with Pca has not been clarified to date. In the present study, to elucidate antibody responses to COVID-19 vaccination among patients with Pca, group comparisons between patient’s with Pca and healthy controls were conducted at several times points after COVID-19 vaccination.
To reach the above-mentioned objectives, blood samples from 19 consecutive patients and 12 healthy controls were prospectively collected before first vaccination, and at 1, 3, and 6 months after first vaccination. BNT 162b2 (Pfizer/BioNTech) vaccines were administered for all patients with Pca. Antibody titers were determined by enzyme-linked immunosorbent assay using recombinant protein of the SARS-CoV-2 spike-protein receptor-binding domain as an antigen. The investigation protocols were approved by the ethics committee at our institution (IRB registration no. H21-263).
Mean age was significantly higher for patients (73.6±9.7 years) than for controls (63.2±3.4 years; P<0.01). Other patient characteristics are listed in supplementary table 1. Mean initial PSAfor the overall cohort was 330±615 ng/mL. Ten patients had localized Pca and 9 patients had metastatic Pca. ADT was administered for the 84% (16/19) patients. Treatment with ADT and/or androgen receptoraxis- targeted (ARAT) agent had been continuously performed in 68% (13/19) patients since the first vaccination.
Before the first vaccination, no patients showed positive results for antibody. After the first vaccination, antibody titer was significantly increased at 1 month after vaccination and then gradually declined over time in each group (Figures 1A and 1B). Regarding the comparison between the two groups, mean antibody titer was not significantly different in patients during the observational period (Figure 1C). After second vaccination, antibody titers immediately increased in all patients with Pca (data not shown).
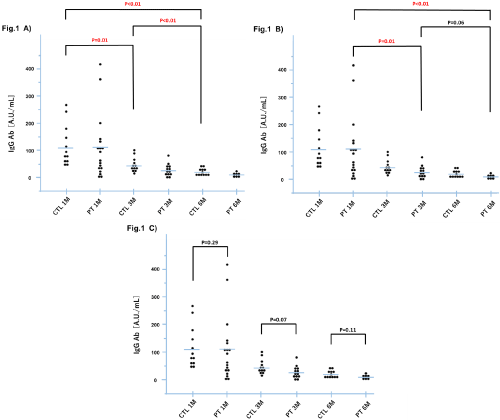
Figure 1: Comparison of antibody titers for COVID-19 SARS-CoV-2 spike-protein receptor-binding domain between patients with prostate cancer and healthy
controls.
a) Time course of change of antibody titer in the control
b) Time course change of antibody titer in the patients with prostate cancer
c) Group comparisons of antibody titer between the patients with prostate cancer and the controls
CTL; Healthy control; PT; Prostate cancer.
Immune responses to vaccination are known to be diverse among patients with cancers [4]. In the present study, a significant decrease in antibody titer was not observed among patients with Pca compared with controls. Although aging was involved in lowering the antibody titer [5], significant higher age in the patients did not induce the decrease of antibody titer compared with the control. These results were consistent with those reported by Liontos et al [6]. They demonstrated that antibody titers in 25 patients with Pca who were treated using ARAT agent were similar to those in healthy controls.
Several limitations must be considered in the present study. First, the sample size was small, although the number of patients in the study by Liontos et al. was almost identical [6]. The reason why the sample size was small in the present study was that the present study had started after the first vaccination in Japan. Thus, the first vaccination had been administered for almost the patients with Pca. As a result, we could not collect the many samples. Second, the significant difference of the age has emerged between the patients with Pca and the control. Because the control group consisted of the healthy cohort belonging to our institution, maximum age of the control group was 70 years old. Thus, although we selected the preferably high age objects as the healthy control, significant difference of age has emerged. At last, little data have been accumulated regarding the safety and actual efficacy of vaccination against COVID-19. In conclusion, this is a first report from an Eastern country to find that immunological response to first COVID-19 vaccination was equivalent in patients with Pca compared with healthy controls.
Acknowledgement
We have no conflict of Interest.
References
- Chakravarty D, Ratnani P, Sobotka S, Lundon D, Wiklund P, et al.: Increased Hospitalization and Mortality from COVID-19 in Prostate Cancer Patients. Cancers (Basel). 2021; 13: 1630.
- Moreno NF, McAdams R, Goss JA, Galvan NTN. COVID-19 Vaccine Efficacy and Immunogenicity in End-Stage Renal Disease Patients and Kidney Transplant Recipients. Curr Transplant Rep. 2022; 9: 174-184.
- Herishanu Y, Rahav G, Levi S, Braester A, Itchaki G, et al. Efficacy of a third BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL who failed standard 2-dose vaccination. Blood. 2022; 139: 678-685.
- Griffiths EA, Segal BH. Immune responses to COVID-19 vaccines in patients with cancer: Promising results and a note of caution. Cancer Cell. 2021; 39: 1045-1047.
- Sakamoto A, Yoshimura M, Itoh R, Ozuru R, Ishii K, et al. Longitudinal Dynamics of SARS-CoV-2 IgG Antibody Responses after the Two-Dose Regimen of BNT162b2 Vaccination and the Effect of a Third Dose on Healthcare Workers in Japan. Vaccines. 2022; 10: 830.
- Liontos M, Terpos E, Kunadis E, Zagouri F, Briasoulis A, et al. Treatment with abiraterone or enzalutamide does not impair immunological response to COVID-19 vaccination in prostate cancer patients. Prostate cancer and prostatic diseases. 2022; 25: 117-118.