
Special Article: Dialysis
Austin J Urol. 2023; 9(1): 1079.
Chronic Hyperkalaemia with Patiromer in Haemodialysis: A Single-Center, Prospective Observational Study in the Clinical Practice
Vicent Esteve Simó*; Irati Tapia González; Ursula Vadillo Vidal; Claudia Guzmán Rubiano; Fátima Moreno Guzmán; Diana Oleas Vega; Verónica Duarte Gallego; Mónica Pou Potau; Anna Saurina Solé; Manel Ramírez de Arellano Serna
Department of Nephrology, Hospital de Terrassa Consorci Sanitari Terrassa (CST), Barcelona, Spain
*Corresponding author: Vicent Esteve Simó Department of Nephrology, Hospital de Terrassa. Consorci Sanitari Terrassa (CST), Crta Torrebonica s/n 08227 Terrassa (BCN), Barcelona, Spain. Tel: +34937310007 Email: vesteve@cst.cat
Received: August 18, 2023 Accepted: September 30, 2023 Published: October 07, 2023
Abstract
Introduction: Chronic Kidney Disease (CKD) patients on Hemodialysis (HD) experience increased risk of hyperkalemia, a serious potential fatal electrolyte disorder. Although novel effective strategies for managing hyperkalaemia are available, experience in routine clinical practice is still insufficient. Here we report chronic hyperkalemia prevalence and analyze the effects of different treatments on potassium management, adherence ratio and gastrointestinal symptoms in HD population.
Methods: 12-week, prospective, single-center study in HD patients with chronic hyperkalaemia (>5.5 mmol/l). Three study phases were established: Phase 1, Dietary Advice (DA); Phase 2, Calcium Polystyrene Sulfonate Resins (CPSRs); and Phase 3, patiromer. Sociodemographic and biochemical data, treatment adherence and compliance (Simplified Medication Adherence Questionnaire), gastrointestinal symptoms (Gastrointestinal Symptom Rating Scale, GSRS), HD characteristics and usual medical treatment were analyzed in each phase.
Results: Serum potassium values decreased significantly (p<0.05) only in phase 3 (–0.75 mmol/l), with a higher patient percentage reaching optimal K range. Compared with CPSRs, patiromer yielded significantly better overall GSRS scores: abdominal pain (3.7 versus 2.5), constipation (7.1 versus 5.3), indigestion (6.2 versus 5.6); and better treatment compliance. No significant changes were found in other biochemical data, HD characteristics or usual medication over the course of the study.
Conclusions: Chronic hyperkalemia is a highly prevalent disorder on our HD unit. Compared to DA and traditional potassium binders; patiromer was effective in managing chronic hyperkalemia, improving gastrointestinal symptoms and treatment adherence with no associated severe adverse effects. Therefore, patiromer can be considered a first-line treatment for chronic hyperkalemia in patients with HD.
Keywords: Chronic hyperkalemia; Hemodialysis; Patiromer; Potassium binding polymer; Efficacy
Introduction
Hyperkalemia is an electrolyte disturbance characterized by elevated serum potassium levels to values greater than 5 mmol/l [1,2]. Several homeostatic mechanisms keep extracellular potassium concentrations in a narrow physiologic range that simultaneously control both internal potassium redistribution and excretion. The first one is caused by the directional effects of acidemia and alkalemia between the intracellular and extracellular compartments [3]. Conversely, potassium is passively secreted into the lumen of the distal nephron in a process dependent of the concentration gradient across the luminal membrane, the lumen negative electrical gradient, and permeability of the luminal membrane to potassium [1]. When either or both processes are disturbed, a rise of extracellular potassium concentration is developed, which leads to hyperkalemia. While this condition is usually linked to the appearance of potentially fatal cardiac dysrhythmia, there are other severe consequences such as peripheral neuropathy, renal tubular acidosis or even death [4]. A retrospective cohort study of hemodialysis patients reported that the prevalence of hyperkalemia (=5.5 mEq/L) was 16.3 to 16.8 events per 100-patient months with the risk being approximately twice after the long interdialytic interval [5]. Indeed, significant alterations in potassium levels are common among patients undergoing hemodialysis, making it a well-documented condition [6]. In addition, an increased frequency has been observed among patients with Chronic Kidney Disease (CKD), diabetes, heart failure, and prescribed with certain medications like Renin Angiotensin Aldosterone System (RAAS) inhibitors and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) [7-9].
Treatment of chronic hyperkalemia is focused on the identification and correction of the disturbances in potassium homeostasis, starting with the removal of high potassium intake diet, hyperkalemia-inducing therapies, or metabolic acidosis [10]. Nevertheless, these interventions are not usually effective to treat the condition, so treatment with potassium binding resins such as sodium-polystyrene sulfonate or calcium polystyrene sulfonate are usually initiated. However, concerns about their effectiveness and safety have arisen, due to the treatment efficacy being attributed to the co-administered sorbitol or the appearance of severe gastrointestinal injuries [11,12]. Despite this, the use of these compounds is widely common due to the lack of alternatives [13]. Nonetheless, during the last years, a novel non-absorbable polymer which binds potassium in exchange for calcium, patiromer, has demonstrated its efficacy in several studies as an alternative for the treatment of chronic hyperkalemia [14-18]. However, until date, there are currently no published studies about the use of novel potassium binders such as patiromer in the management of chronic hyperkalemia in hemodialysis patients in daily clinical practice.
The aim of this study was to report the prevalence of chronic hyperkalemia and analyze the effects of different treatment strategies on potassium management in clinical practice, ratio of adherence and gastrointestinal symptoms in our HD population.
Materials and Methods
Study Design
This was a single-center, prospective, observational study that included patients with hyperkalemia (>5.5 mEq/l) on a periodic hemodialysis program from of our hospital, approved by the Ethics Committee of the Consorci Sanitari de Terrassa (Barcelona, Spain) and conducted in accordance with the standards of the Helsinki Declaration. This trial consisted of three phases spanned over a 12-week period, with duration of three weeks for each one and a one-week bleaching period between the different stages. Three study phases were established: phase 1, Dietary Advice (DA); phase 2, calcium polystyrene sulfonate resins (CPSRs); and phase 3, patiromer. During the second and third phases, participants were administered 15g CPSR (Resincalcio®) every eight hours and 8.4g patiromer (Veltassa®) every 24 hours, respectively, in line with the approved posology.
The inclusion criteria were HD patients with proven hyperkalemia (>5.5 mEq/l potassium) after two consecutive measurements, treated with Renin-Angiotensin-Aldosterone System Inhibitors (RAASi) according to their clinical condition, being capable of understanding dietary recommendations, had enrolled in our hemodialysis program for over 2 months and signed the informed consent. On the other hand, exclusion criteria were not giving informed consent and a serum potassium level <5.5 nmol/l after two repetitive measurements at the beginning of the study.
In each phase, we analyzed sociodemographic data, related biochemical data, treatment adherence and compliance (Simplified Medication Adherence Questionnaire, SMAQ), gastrointestinal symptoms (Gastrointestinal Symptom Rating Scale, GSRS), HD characteristics and usual medical treatment.
The following data were collected for all patients included in the study: main sociodemographic variables and variables associated with renal disease (sex, age, etiology of kidney failure, time on HD) at baseline; biochemical parameters related (serum levels of calcium [Ca], phosphorus [P], sodium [Na], magnesium [Mg], hemoglobin, platelet ate each phase and hemodialysis characteristics [KTV] according to 2nd-generation Daugirdas formula), dialysate calcium and potassium concentration, HD duration, dry weight and interdialytic weight gain at baseline as well as the end of the study.
Treatment adherence and compliance, and gastrointestinal symptoms were collected at each phase. Gastrointestinal symptomatology was measured through the Gastrointestinal Symptom Rating Scale (GSRS). It consists of 15 items grouped into five blocks according to the different symptoms (reflux, abdominal pain, diarrhea, indigestion and constipation), with a 7-point Likert-like scale where a rate of 1 and 5 represent the most positive and negative option respectively [19]. Finally, treatment adherence was measured through the Simplified Medication Adherence Questionnaire (SMAQ). This is a short and simple questionnaire comprised of six questions posed directly to the patient regarding medication-taking habits, which was originally used to validate adherence of patients to anti-retroviral treatments [20]. Any patient who responds to any of the items with a non-adherence answer was considered as non-compliant.
In reference to the standard medical treatment, data were collected on the type of phosphorus binders (calcium-based binders, non-calcium-based binders, binders with added magnesium and ferric chelators), traditional cardiovascular treatment (betablockers, Angiotensin Converting Enzyme Inhibitors [ACEI] or Angiotensin II Receptor Blockers [ARB]) as well as proton pump inhibitors and antacids.
Study Endpoints
The primary endpoint was to describe the prevalence, clinical characteristics and associated factors of chronic hyperkalemia in hemodialysis patients from the medical center. Secondary endpoints included the analysis of the control of serum potassium levels, treatment adherence, gastrointestinal symptomatology, safety profile and satisfaction with the different therapeutic options (dietetic measures, calcium polystyrene sulfonate resins and patiromer).
Statistical Analysis
Statistical analysis was carried out using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed using the mean and standard deviation. The qualitative variables were expressed as a percentage. The comparison of quantitative data was carried out using the Wilcoxon test for non-parametric related variables, and the qualitative data was compared using the McNemar test; statistical significance for any comparison was set at a value of p<0.05.
Results
Patient Characteristics
Out of 65 identified patients, only 27 participants had a serum potassium level higher than 5.5mEq/l. A confirmatory analysis included 19 patients, with two and four of them either being excluded or discontinued the trial (three due to hospital admissions and one because of acute confusional syndrome), respectively. Therefore, a total of 13 patients, with a mean age of 63.8±14.1 years and 46.4±41.6 months in hemodialysis, took part in the study. Chronic tubulointerstitial nephritis was the most common cause of kidney failure (n=4, 30.8%), followed by nephroangiosclerosis, diabetic nephropathy and hepatorenal polycystic kidney disease (all n=2, 15.4%). Regarding comorbidities, all participants had arterial hypertension, with most of them having dyslipidemia (n=7, 53.8%), cardiopulmonary insufficiency (n=6, 46.2%) and/or smoking (n=6, 46.2%), respectively. Most of the participants underwent hemofiltration (n=11, 84.6%) and had an arteriovenous fistula (n=10, 76.9%; Table 1). At baseline, all the patients were treated with renin angiotensin aldosterone system inhibitors (RAASi) during the study (69.2% ACEI, 30.8% ARB) according to their clinical condition. No patient received mineralpcorticoids receptor antagonist and 22.5% received betablockers. In reference to the percentage and type of phosphorus binders, a 38.4%, 53.8%, 15.4% and 7.6% received calcium-based binders, non-calcium-based binders, binders with added magnesium and ferric chelators, respectively. A total of 69.2% received proton pump inhibitors and 15.4% antiacids. All the patients had 1.5 mEq/l potassium and 3.0 mmol/l calcium dialysate.
n=13
Mean age, years (SD)
63.8(14.1)
Time in hemodialysis, months (SD)
46.4(41.6)
Charlson index, mean (SD)
7.5(2.3)
Non-compliant (SMAQ), n(%)
4(30.8)
Kidney failure, n(%)
Chronic tubulointerstitial nephritis
4(30.8)
Nephroangiosclerosis
2(15.4)
Diabetic nephropathy
2(15.4)
Hepatorenal polycystic kidney disease
2(15.4)
Glomerular
1(7.7)
Not registered
1(7.7)
Others
1(7.7)
Comorbidities, n (%)
Arterial hypertension
13(100.0)
Dyslipidemia
7(53.8)
Cardiopulmonary insufficiency
6(46.2)
Tobacco use
6(46.2)
Stroke
4(30.8)
Diabetes mellitus
3(23.1)
Type of hemodialysis, n(%)
Hemofiltration
11(84.6)
Online
2(15.4)
Vascular access, n(%)
Arteriovenous fistula
10(76.9)
Central venous catheter
2(15.4)
Polytetrafluoroethylene (PTFE)
1(7.7)
Note: simplified medication adherence questionnaire, SMAQ.
Table 1: Demographic and clinical characteristics of participants.
Reduction of Serum Potassium Levels
Overall serum potassium levels remained stable during the diet (6.3 mEq/l) and resin (6.2 mEq/l) phases compared to the baseline values (6.3 mEq/l). However, a statistically significant reduction was observed in the patiromer phase (5.6 mEq/l, p<0.001; Figure 1A). In addition, it was observed that while potassium levels remained stable among participants with a low treatment adherence during the whole cycle, in those patients which followed the treatment prescription patiromer managed to significantly decrease even further these levels (from 6.2 mEq/l at baseline to 6.0 mEq/l at the diet phase, 6.2 mEq/l at the resin phase and 5.2 mEq/l at patiromer phase; p<0.001; Figure 1B). Due to this reduction, 53.8% and 71.2% of patients had their potassium levels in a 3.5-5.5 and 3.5-6.0 range, respectively, in the patiromer phase (Figure 1C). When only adherent participants were considered, these ratios increased to 71.4% and 100.0% (Figure 1D). All these values were significantly higher than those reported both at baseline and in the diet and resin phases.
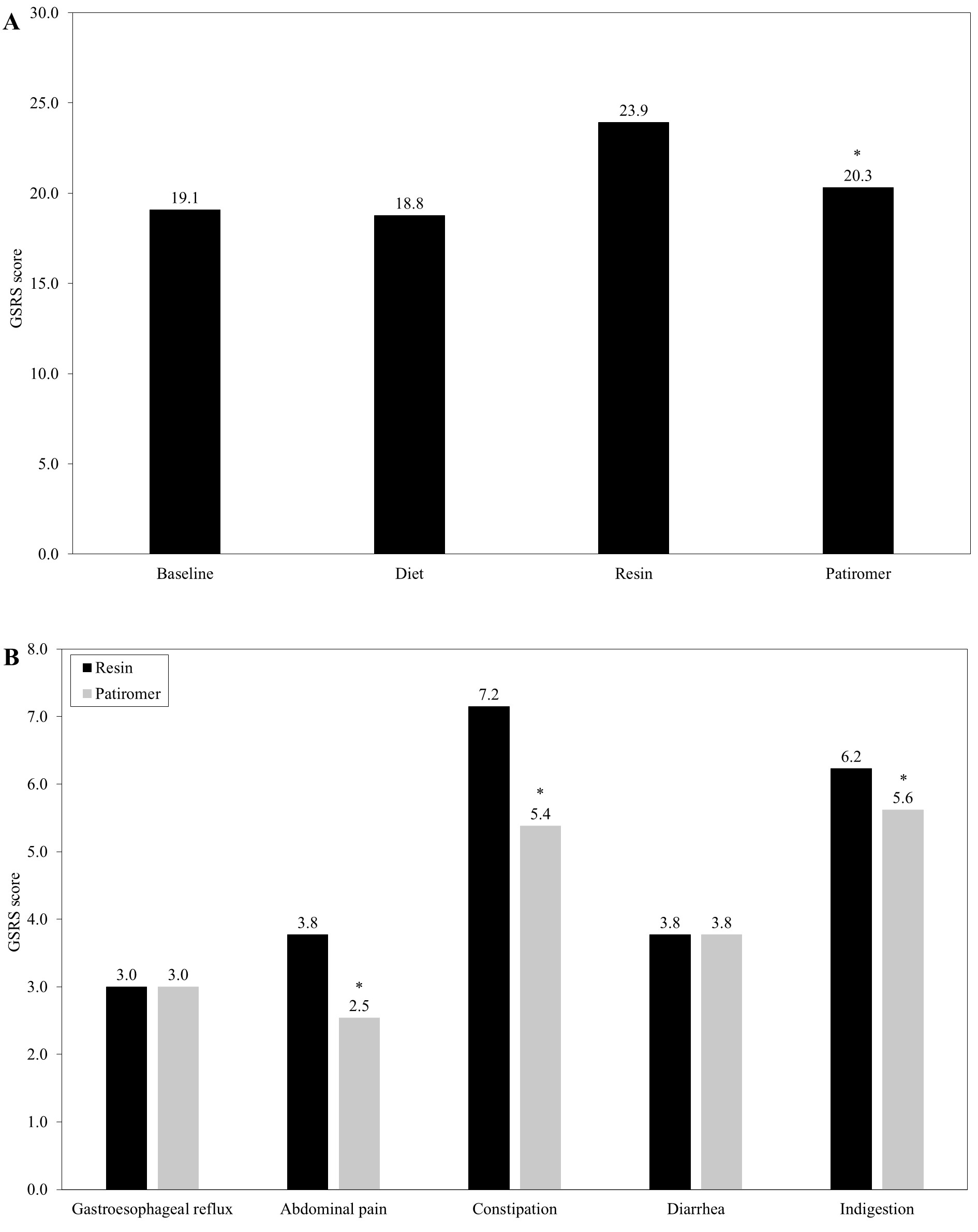
Figure 1: Serum potassium levels throughout the treatment period. In the figure, potassium levels for the whole sample (A), comparison between the adherent and non-adherent patients (B), and the percentage of patients that managed to get to the 3.5-5.5 and 3.5-6.0 mEq/l potassium ranges, for both all (C) and adherent participants (D), during each of the phases are depicted. Asterisk (*) represents a statistical significance of p<0.001.
Treatment Safety Profile
Regarding the gastrointestinal tract, patients reported comparable GSRS scores during the baseline (19.1) and diet (18.8) stages. During the resin phase the values significantly worsened (23.9, p<0.001), subsequently improving during the patiromer stage (20.3; Figure 2A). When the different blocks of the GSRS scale were compared between the resin and patiromer phases, no differences were observed with regards to the gastroesophageal reflux (3.0 for both) and diarrhea (3.8 for both). However, disparities were reported respecting abdominal pain (3.8 vs 2.5, p<0.001), constipation (7.2 vs 5.4, p<0.001) and indigestion (6.2 vs 5.6, p<0.001), with scores in the patiromer phase being lower in these cases (Figure 2B).
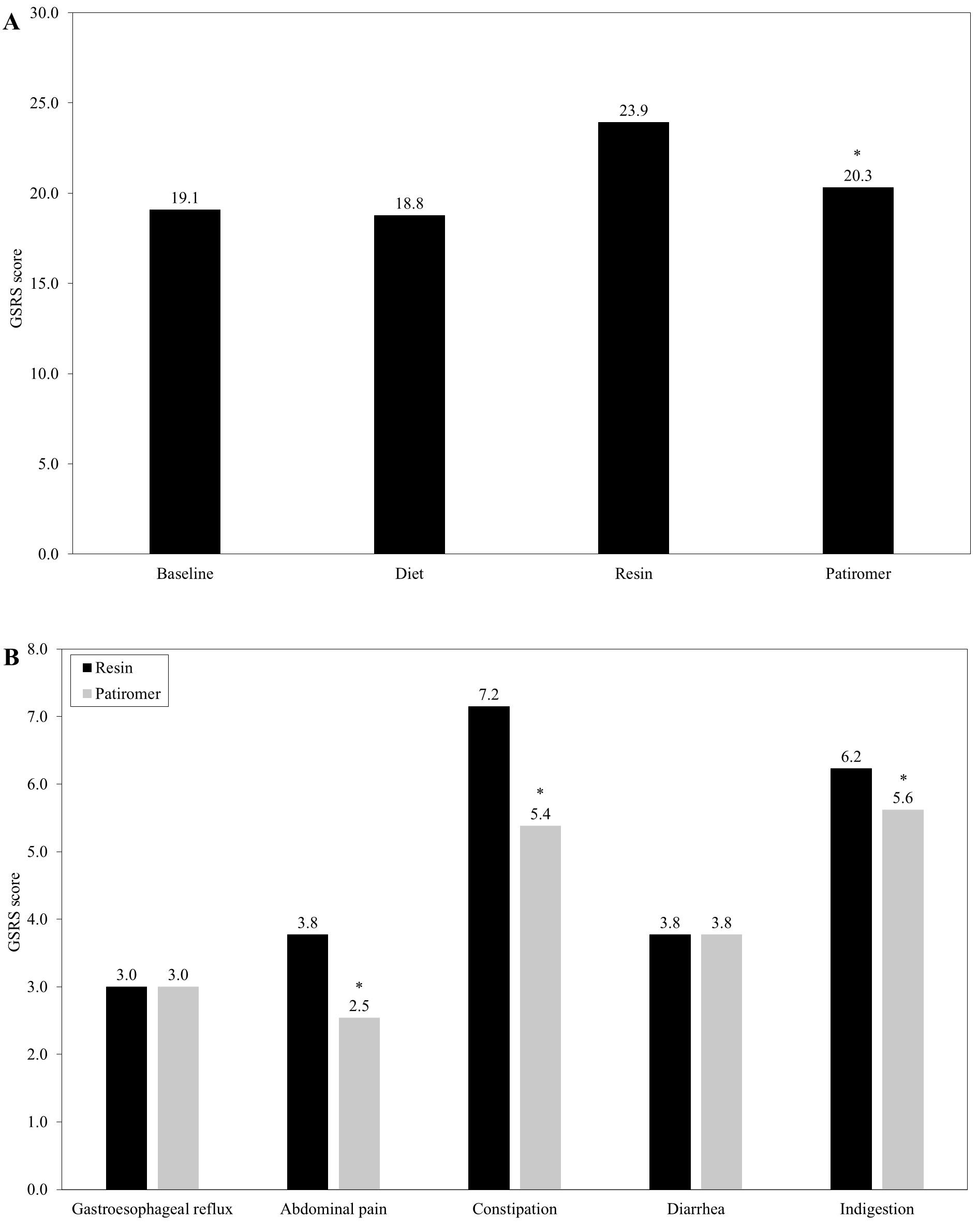
Figure 2: Obtained scores in the Gastrointestinal Symptom Rating Scale (GSRS). In the figure, values for the whole sample throughout the study (A) and scores obtained for the different blocks of the GSRS scale during both the resin and patiromer phases (B) are represented. Asterisk (*) represents a statistical significance of p<0.001.
Additionally, no statistically significant differences were observed in the different analyzed biochemical parameters among the several phases, with calcium, potassium, sodium, magnesium, albumin, hemoglobin, and platelet values remaining stable throughout the treatment (Table 2). Simultaneously, no significant distortions in the different parameters of the hemodialysis were observed (Table 3). No relevant changes were observed in reference to the type of phosphorus binders, traditional cardiovascular treatment as well as proton pump inhibitors and antacids during the study.
Baseline
Diet
Resin
Patiromer
Ca (mmol/l)
2.1±0.2
2.1±0.2
2.1±0.2
2.1±0.2
P (mmol/l)
1.6±0.6
1.6±0.7
1.8±0.8
1.7±0.7
Na (mmol/l)
138.2±2.5
139.5±2.9
138.4±2.7
137.7±2.3
Mg(mmol/l)
0.9±0.1
0.9±0.3
1.0±0.2
0.9±0.1
Albumin (g/dl)
38.9±5.2
39.4±19.2
39.7±4.5
38.9±4.5
Hemoglobin (g/dl)
11.4±1.5
11.7±1.4
11.6±1.2
11.5±1.7
Platelet (x109/L)
193.0±52.5
184.2±51.6
189.6±69.3
198.1±71.5
Table 2: Baseline biochemical data for each treatment phase. Values are represented as mean±SD.
Baseline
Final
p
Theoretical weight, mean kg (SD)
64.1(13.1)
64.4(13.3)
0.680
2nd generation Daugirdas Kt/V, mean (SD)
1.6(0.5)
1.7(0.4)
0.295
Urea Reduction Percentage (PRU), mean % (SD)
70.3(21.9)
75.9(6.5)
0.322
Radiocephalic vascular access, mean % (SD)
4.7(2.6)
5.1(2.4)
0.597
Blood flow, mean Qb ml/min (SD)
335.8(22.2)
346.2(25.4)
0.113
Dialysate flow, mean Qb ml/min (SD)
523.1(83.2)
523.1(83.2)
0.999
Hemodialysis time, mean min (SD)
212.3(31.3)
216.9(30.8)
0.924
Table 3: Hemodialysis characterization at baseline and at the end of the treatment. Values are represented as mean±SD.
Therapeutic Adherence
Among all stages, the resin phase was the one which reported the highest discontinuation rate (92.3%), followed by patiromer (46.1%), diet (38.4%) and baseline (30.7%; Figure 3A). While therapeutic adherence for resins was leaning towards low rates, the trend in the case of patiromer was towards high rates (Figure 3B).
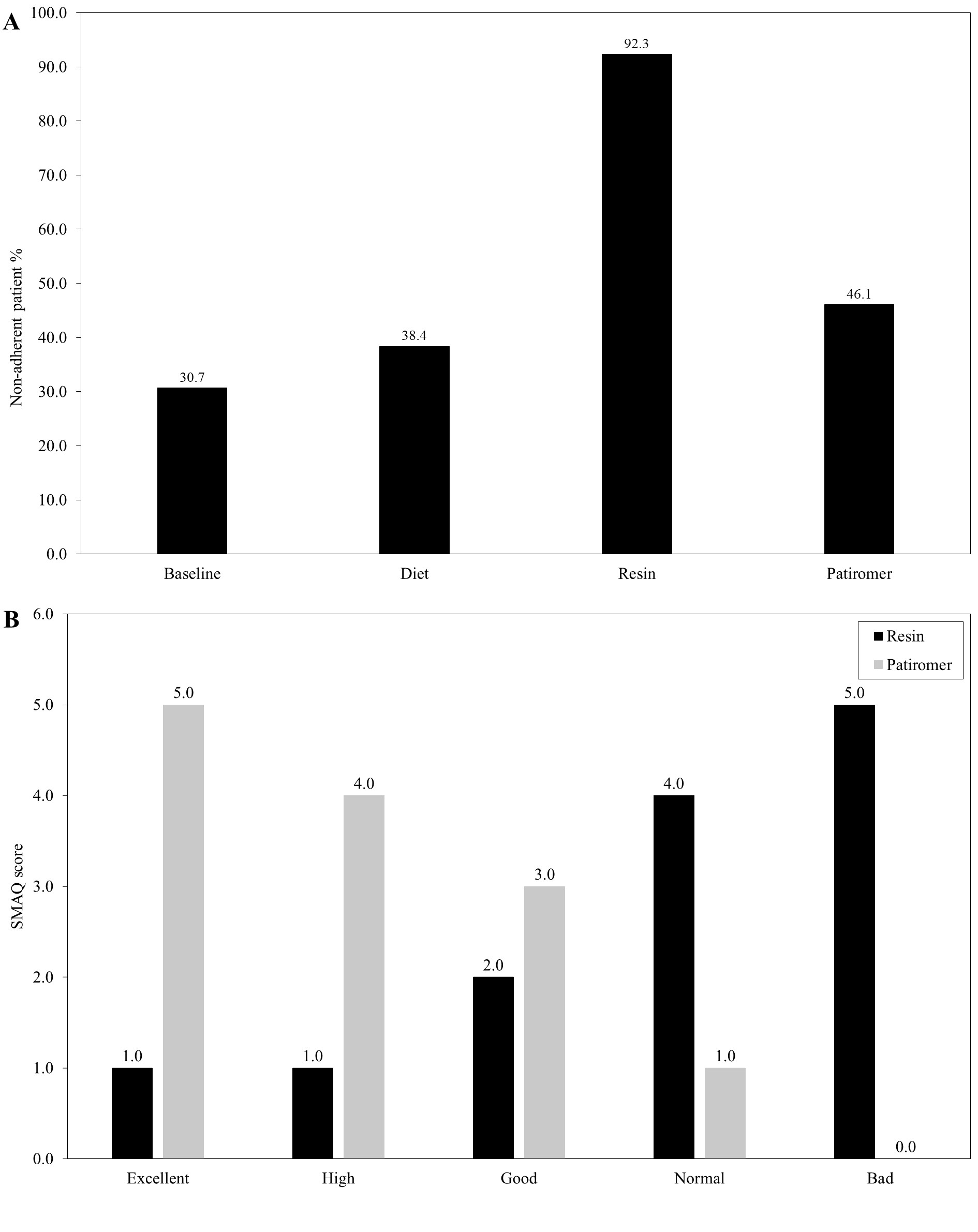
Figure 3: Therapeutic adherence. In the figure, percentages of non-adherent patients (A) and the SMQA score for the resin and patiromer phases (B) are depicted.
Discussion
Hyperkalemia is an acute or chronic disorder usually presented in patients with cardiorenal syndrome due to a reduced kidney function. In severe cases, it is usually accompanied by muscle weakness, cardiac arrhythmias, and ventricular fibrillation, which might lead to sudden cardiac death, with reported in-hospital mortality rates as high as 30% [21]. The outcomes in these patients are highly dependent of their clinical characteristics and associated comorbidities. In our study, the most frequent causes of kidney failure were chronic tubulointerstitial nephritis, nephroangiosclerosis, diabetic nephropathy and hepatorenal polycystic kidney disease. These values are in line with those reported by other studies. Recently, Seidel et al [22] have described that diabetic nephropathy was the most common cause (26.8%) among five hemodialysis outpatient centers in Germany, followed by nephrosclerosis (19.6%), glomerulonephritis (12.5%), and cardiorenal syndrome (10.7%). Regarding comorbidities, all participants included in our study had arterial hypertension, with most of them having primarily dyslipidemia, cardiopulmonary insufficiency and/or smoking. In comparison, Moradi et al [23] reported that hypertension was the most common comorbidity (79%) among 33109 studied patients in maintenance hemodialysis, followed by congestive heart failure (27%), atherosclerotic heart disease (21%) and peripheral vascular disease (11%). In the German study, most participants (76.8%) had hypertension, diabetes (44.6%) and/or coronary artery disease (37.5%) [22].
The increased potassium levels characteristic of hyperkalemia are usually related to the administration of RAAS inhibitors in these patients, since they interfere with renal potassium excretion because of a reduction of either the levels or activity of aldosterone [24]. Therefore, clinical practice guidelines recommend these patients to switch to a low potassium diet in addition to a prescription of a non-potassium-sparing diuretic, increasing its dose if it is already being administered [25]. Simultaneously, both potassium supplements and medications that compromise kidney functions, like NSAIDs, or increase potassium levels (such as RAAS inhibitors, especially mineralocorticoid receptor antagonists) must be interrupted [26]. However, discontinuation of these treatments is related to higher rates of post-discharge mortality and hospital readmission within the following 30 days [27]. So, physicians are usually in that difficult situation in which they have to continue or interrupt the treatments. Novel potassium binders such as patiromer might be a response to this problem in the decision making.
Patiromer is synthesized as a 100-mm bead through a polymerization-hydrolysis process. It exchanges calcium for potassium in the gastrointestinal tract, preferentially binding potassium in the colon, where its concentration is higher than that of sodium, calcium and magnesium [28,29]. With this process, this compound lowers potassium levels and maintains normokalemia, allowing the treatment continuation in cardiorenal syndrome patients at high risk of hyperkalemia. The results presented in this study have shown that patiromer is effective and safe in hemodialysis patients. This compound allowed to significantly decreasing serum potassium levels compared to the values obtained at baseline and with both diet and resins. In addition, the treatment was safe, since no changes in the biochemical and no hemodialysis parameters or adverse events in the gastrointestinal tract, measured through the GSRS scores, were observed in the patiromer phase. Finally, a higher treatment adherence was reported with this compound compared to the resin administration, with these values being similar with baseline and diet.
These results are in line with those reported in several clinical trials. The first one, the PEARL-HF study [14], it was a multicenter, double-blind, randomized trial that included patients with hyperkalemia with a history of withdrawal of RAAS inhibitors due to the appearance of hyperkalemia or an estimated glomerular filtration rate lower than 60 ml/min/1.73m2. Compared to placebo, patiromer significantly decreased serum potassium levels (0.45 mEq/l, p<0.001), with a higher percentage of participants that were able to remain with previous treatment (91% vs 74%; p=0.019). In the AMETHYST-DN study, a long-term multicenter, open-label, dose-ranging randomized trial in diabetes mellitus patients with mild to moderate hyperkalemia and chronic kidney disease at stages 3 or 4, a twice daily 4.2-16.8g patiromer significantly decreased potassium levels and hyperkalemia recurrence from week four to week 52. In addition, systolic and diastolic blood pressures were reduced, which lead to a decrease in aldosterone levels. Regarding adverse events, most of them were reported during the long-term maintenance phase, with hypomagnesemia being the most common one (7.2%), although no severe cases were described [15]. In the OPAL-HK study, a two-phase, multicenter, phase III trial that included 243 patients receiving RAAS inhibitors with either stage 3 or 4 chronic kidney disease and serum potassium levels between 5.1 and 6.5 mmol/l, these levels decreased significantly (-1.01±0.03 mmol/l; p<0.001) during the first single-blind phase, consisting of a prescription of 4.2 or 8.4 mg patiromer twice each day for four weeks [16]. This decline was higher the greater baseline levels were. In addition, after the second eight-week randomized withdrawal phase, in which 107 participants with potassium levels between 3.8 and 5.1 mmol/l after the first phase either continued with the treatment or switched to placebo, a statistical difference was observed between the patiromer and control arms (0.72 mmol/l, 95% confidence interval 0.46-0.99; p<0.001). Moreover, 94% of the patients with potassium levels greater than 5.5 mmol/l at the beginning of the study could continue with the treatment with RAAS inhibitors. This percentage was higher than the one observed in the placebo group (44%). Finally, the only reported adverse event was constipation, with an incidence lower than 3%. In a subanalysis of patients aged >65 years, a -1.01±0.05 mEq/l (p<0.001) mean change in serum potassium levels was reported during the first phase, with participants in the placebo arm experiencing a higher serum potassium increase (p<0.001) and incidence of recurrent hyperkalemia (92% vs 30%) than those taking patiromer in the second stage of the trial [17]. Recently, a meta-analysis by Shrestha et al [18] described that, in comparison with the standard of care, patiromer had lower rates of hyperkalemia (OR: 0.44, 95% confidence interval: 0.22-0.89), in addition to no differences regarding adverse events and treatment discontinuation between both groups. Regarding hemodialysis patients, a recent retrospective cohort study by Kovesdy et al [30] described a mean serum potassium reduction of approximately -0.5 mEq/l post-patiromer initiation, with 48% and 22% of pre-patiromer and post-patiromer patients, respectively, having levels greater than 6.0 mEq/l (p<0.001).
The low number of patients and the short-term administration of the treatment are among the limitations of this study. However, strengths of this study included the design and methodology of the trial, being the only study performed in routine clinical practice to date, and gathering data about both treatment adherence and intestinal tolerance, which remain as the main problems regarding potassium binding polymers.
In conclusion, hyperkalemia is a common, severe and potentially fatal disorder, with an increased incidence that is linked with age, chronic kidney disease and cardiovascular comorbidities. The results reported in this study shows that patiromer, a novel potassium binding polymer, was effective in managing chronic hyperkalemia compared to dietary advice and traditional potassium binders, improving gastrointestinal symptoms and treatment adherence without associated severe adverse effects. Therefore, patiromer can be considered a first-line treatment for this disease in patients undergoing hemodialysis.
Author Statements
Conflict of Interest
The authors declare no conflict of interest
Disclosure of Grants or Other Funding
The authors received no grants or other funding for this publication, research and authorship.
References
- Evans KJ, Greenberg A. Hyperkalemia: a review. J Intensive Care Med. 2005; 20: 272-90.
- Montford JR, Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017; 28: 3155-65.
- Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011; 22: 1981-9.
- Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019; 34: iii2-iii11.
- Yusuf AA, Hu Y, Singh B, Menoyo JA, Wetmore JB. Serum potassium levels and mortality in hemodialysis patients: a retrospective cohort study. Am J Nephrol. 2016; 44: 179-86.
- Pirklbauer M. Hemodialysis treatment in patients with severe electrolyte disorders: management of hyperkalemia and hyponatremia. Hemodial Int. 2020; 24: 282-9.
- Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009; 169: 1156-62.
- Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010; 5: 531-48.
- Lafrance JP, Miller DR. Dispensed selective and nonselective nonsteroidal anti-inflammatory drugs and the risk of moderate to severe hyperkalemia: a nested case-control study. Am J Kidney Dis. 2012; 60: 82-9.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004; 351: 585-92.
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010; 21: 733-5.
- Kamel KS, Schreiber M. Asking the question again: are cation exchange resins effective for the treatment of hyperkalemia? Nephrol Dial Transplant. 2012; 27: 4294-7.
- Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci. 2014; 347: 93-100.
- Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011; 32: 820-8.
- Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015; 314: 151-61.
- Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015; 372: 211-21.
- Weir MR, Bushinsky DA, Benton WW, Woods SD, Mayo MR, Arthur SP, et al. Effect of patiromer on hyperkalemia recurrence in older chronic kidney disease patients taking RAAS inhibitors. Am J Med. 2018; 131: 555-564.e3.
- Shrestha DB, Budhathoki P, Sedhai YR, Baniya R, Cable CA, Kashiouris MG, et al. Patiromer and sodium zirconium cyclosilicate in treatment of hyperkalemia: a systematic review and meta-analysis. Curr Ther Res Clin Exp. 2021; 95: 100635.
- Revicki DA, Wood M, Wiklund I, Crawley J. Reliability and validity of the gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual Life Res. 1998; 7: 75-83.
- Knobel H, Alonso J, Casado JL, Collazos J, González J, Ruiz I, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS. 2002; 16: 605-13.
- An JN, Lee JP, Jeon HJ, Kim DH, Oh YK, Kim YS, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012; 16: R225.
- Seidel M, Hölzer B, Appel H, Babel N, Westhoff TH, COVID Dialysis Working Group. Impact of renal disease and comorbidities on mortality in hemodialysis patients with COVID-19: a multicenter experience from Germany. J Nephrol. 2020; 33: 871-4.
- Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015; 10: 98-109.
- Bridgeman MB, Shah M, Foote E. Potassium-lowering agents for the treatment of nonemergent hyperkalemia: pharmacology, dosing and comparative efficacy. Nephrol Dial Transplant. 2019; 34: iii45-50.
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004; 43: S1-290.
- Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013; 158: 825-30.
- Albert NM, Yancy CW, Liang L, Zhao X, Hernandez AF, Peterson ED, et al. Use of aldosterone antagonists in heart failure. JAMA. 2009; 302: 1658-65.
- Li L, Harrison SD, Cope MJ, Park C, Lee L, Salaymeh F, et al. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther. 2016; 21: 456-65.
- Bushinsky DA, Spiegel DM, Gross C, Benton WW, Fogli J, Hill Gallant KM, et al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol. 2016; 11: 1769-76.
- Kovesdy CP, Rowan CG, Conrad A, Spiegel DM, Fogli J, Oestreicher N, et al. Real-world evaluation of patiromer for the treatment of hyperkalemia in hemodialysis patients. Kidney Int Rep. 2019; 4: 301-9.