Research Article
Austin J Vaccines & Immunother. 2014;1(1): 6.
Plasmodium Axenically Developed Exo-erythrocytic Forms Immunization Confer Strong Protection against Infectious Sporozoite Challenge
Kaiser K1,2, Camargo N1,3, Kappe SHI1,3 and Singh AP1,4*
1Department of Pathology, New York University, USA
2Université Claude Bernard-Lyon I Laboratoire de Parsitologie, France
3Seattle Biomedical Research Institute, USA
4Infectious Diseases Laboratory, National Institute of Immunology, India
*Corresponding author: Singh AP, Infectious Diseases Laboratory, National Institute of Immunology, New Delhi, 110067, India
Received: July 09, 2014; Accepted: August 25, 2014; Published: September 01, 2014
Abstract
Malaria causes nearly a million deaths every year and nearly 50% of the world population is at risk. Irradiated sporozoite vaccination is a proven and successful strategy but difficult to implement. An attenuated whole parasite vaccine is an achievable goal in spite of difficulties. Here we explore the possibility of using exo-erythrocytic forms (EEF) immunization as an attenuated whole parasite vaccine. As proof of principle we used in vitro derived EEF from Plasmodium yoelii (a mouse malaria parasite) for immunization of mice and that these forms confer strong protection against infective sporozoite challenge. Antibodies generated were species specific and not the strain specific. We also show that antibody response is mounted against few antigens. This shall help in narrowing important antigens of liver stage.
Keywords: Plasmodium; Malaria; Liver stage; Vaccine; Exo-Erythrocytic Forms; Attenuated parasite immunization
Abbreviations
CAS: Chemically Attenuated Sporozoites; CTL: Cytotoxic T Lymphocyte; EEF: Exo-Erythrocytic Forms or liver stage parasite; ITV: Infection Treatment Vaccination; GAS/GAP: Genetically Attenuated Sporozoite/parasite; RAS: Radiation Attenuated Sporozoites; WPI: Whole Parasite Immunization
Introduction
Malaria remains serious public health problem for roughly 50% of the world’s population. Morbidity associated with malaria is ~ 0.63 million each year [1]. There is limited repertoire of available drugs that can cure malaria. The problem is further aggravated due to emergence of drug resistant parasite [2]. An urgent need for the effective vaccine is greater than ever. Vaccine candidates tried till-date are mostly based on single antigens [3] except few combinations [4,5] and targeted at individual stages of the malaria life cycle. Limited success was obtained with the vaccines tested to-date [6-9]. The only proven vaccination strategy that provides sterile protection is through the Radiation Attenuated Sporozoites (RAS) vaccine [10-12]. Recently, alternative to RAS was created in the form of Genetically Attenuated Sporozoites (GAS) and successfully tested in small animals [13-15] as well as in humans with limited success [16]. A vaccine targeting the naïve traveler or newcomer to malaria endemic area would require complete protection and this may be achieved using vaccine against pre-erythrocyte stages [10].
Most successful vaccines (smallpox, measles, polio etc.) were empirically developed using the attenuated or inactivated whole pathogens or material derived directly from the infectious agent [17,18]. Except a few [example Hepatitis B Vaccine] [19], recombinant protein vaccines currently are not very successful [6,7,20]. A successful recombinant protein vaccine for malaria is currently unavailable [21]. Under these circumstances to protect the travelers and infant’s naïve to malaria antigens, a non-replicating pre-erythrocytic whole organism vaccine needed. There are several hurdles in obtaining such a vaccine and they must be addressed [22]. The practical questions that need to be answered are: a) Can one produce enough material for large-scale application, which is cost effective and practically feasible. b) Can one administer such a vaccine by the route that is clinically acceptable. c) Can one produce such a vaccine that meets the regulatory norms [22].
Proof of principle of the live attenuated vaccine first time came in 1967, when Nussenz weig and colleagues reported, that immunizing mice with radiation attenuated Plasmodium berghei (P. berghei) sporozoites protected them against challenge with fully infectious sporozoite [12]. Protection was also demonstrated in humans in 1973 [23]. Recent studies in humans immunized with purified, radiation attenuated sporozoite, introduced by intravenous route further confirmed the previous findings [24]. Irradiated sporozoites are able to invade liver cells and transform into exo-erythocytic forms (EEFs) but fail to develop further [25]. A lot has been done to understand how the irradiated sporozoite vaccine works. Nussenzweig [26] and others [27,28] have shown that optimal dose of irradiation is required to achieve protection. A higher dose of irradiation lead to reduction in the number of early EEFs, a prerequisite for protection, while lower dose of irradiation leads to full growth of EEFs and subsequent development of blood stages leading to disease. Treatment with Primaquine a drug that kills the EEFs also abrogates the protection. Important point that emerged from the aforementioned studies was species specific but not strain specific immunity [10]. In the RAS immunization multiple effect or mechanisms, including antibodies, helper cells, lymphocytes, CD4+, CD8+ cytotoxic T lymphocytes (CTLs) [29-31] are involved. Immunity against EEFs, developing inside the liver hepatocytes, arise from the Interferon gamma (IFN-γ) produced by effecter cells [32,33]. IFN-γ based EEF killing is mediated by nitric oxide (NO) [34,35]. A study with normal sporozoite immunization combined with Chloroquine treatment [Infection treatment vaccination, ITV] provided further evidence of EEF antigens importance in inducing the protective immune response [35].
Liver stages were neglected for long time owing to practical difficulties in obtaining pure EEFs from host cells. Since these EEFs are intracellular, they are not direct targets of humoral responses. This has hindered the studies on their antigenic composition unlike to sporozoite stage proteins. The protection offered by RAS immunization is mainly due to T-cells. Availability of methodology for cell free development of EEFs [36] has opened the doors to study the immune response elicited by the partially developed EEFs produced after whole parasite immunization [WPI].
Materials and Methods
Parasite and axenic EEFs
Four to five day old female Anopheles stephensi mosquitoes were blood fed on anesthetized BALB/c mice infected with wild type P. yoelii yoelii 17XNL strain. After the blood meal, mosquitoes were maintained at 23°C and 83% humidity. Between the days fourteen to seventeen post-feeding, mosquitoes were washed in 70% ethanol for five minute and rinsed twice with sterile medium. Salivary glands were dissected and sporozoite were recovered in sterile DMEM medium containing 2x antibiotic-antimycotic (Invitrogen). Transformation was done essentially as described by Kaiser et al. [36]. Characterization of axenic EEFs was done as described previously [36]. In brief, one million sporozoite per well were put in twenty-four well culture plates along with one ml of medium and incubated for twenty-four hours, at 37°C. Transformed parasites were harvested and washed twice with cold PBS before they were administered to mice. Transformation efficiency for each batch was determined by IFA.
Animals and immunization
Female BALB/c mice aged 6-8 weeks were immunized subcutaneously. Immunizations were done with or without adjuvant. In the case of adjuvant, Freund’s complete adjuvant for priming and Freund’s incomplete adjuvant for boosts were used. We used four doses of EEF immunization and for each dose group five animals were used. The control group received equivalent amount of uninfected salivary glands in PBS or adjuvant. The following schedule for immunizations were used, priming on day one, first boost on day fourteen and second boost on day twenty-one. On day thirty-one livers and sera were collected. For CD4/CD8 cells depletion experiments mice were immunized with 30,000 axenic EEFs on the schedule described above, followed by injection of 0.2 mg of anti- CD4 (monoclonal GK1.5) or anti-CD8 (monoclonal YTS169) on days 26, 27, 28 and 29. In all the experiments mice were challenged with 10,000 sporozoite on day 29.
Sporozoite challenge and quantitation of parasite burden
Seven days after the second boost, animals were intravenously injected with 10, 000 P. yoelii 17XNL infectious sporozoites. Forty-four hours post challenge livers of the mice were collected, RNA extracted, c DNA prepared and real-time RT-PCR performed as described previously [37], to determine liver stage burden. There was a difference from previous protocol that we used SYBR green-I dye in place of fluorescent probe. The liver stage burden was determined by estimating the parasite 18S r RNA copy numbers. Parasite 18S r RNA copy numbers were normalized with murine GAPDH copy numbers in the corresponding reactions. The normalized values for each group were compared to the control group to get percentage inhibition. The inhibition obtained with RAS immunization was considered as 100 percent and those with EEF immunization were compared with respect to the 100 percent of RAS immunization. In this study a 90% inhibition of liver stage burden means 90% with respect to RAS immunization (100%, sterile protection) and not with respect to control immunized group.
Indirect fluorescence antibody Test (IFAT)
EEF transformation efficiencies were checked by IFAT using antibody against parasite HSP70 [38] and Myosin A tail domain interacting protein (MTIP). To check the reactivity of anti-EEF sera against in vitro or in vivo transformed parasite we used mouse anti EEF sera together with a rabbit polyclonal antibody against MTIP that recognize an inner membrane complex associated protein [39]. Secondary antibodies against mouse or rabbit IgG was coupled to Alexa 488 or Alexa 594 and used at 1:250 dilutions in 1% BSA/PBS. Nuclei were stained with DAPI (6 amidino 2 phenyllindole).
Western analysis of EEF lysate
P. yoelii yoelii 17XNL 2×105 transformed (~10% efficiency) or untransformed sporozoite were lyses in lysis buffer [150 mM NaCl, 50 mM Tris pH 8.0, Protease inhibitor cocktail (Roche) 1x, and 1% Triton × 100], heated at 95°C for 10 minute and separated on 10% SDS-PAGE (Bio-Rad). Separated proteins were transferred to a PVDF membrane and blocked in 3% BSA/PBS. Membrane was cut in three parts and one part each incubated with anti-EEF sera, anti- CS (monoclonal 2F6) or anti HSP70 (monoclonal 2E6). Membranes washed three times with PBS/0.05% Tween-20 followed by detection with ECL kit (Amersham Biosciences, USA).
ELISPOT
ELISPOT method used here has previously been described [40]. We followed the sections 6.1, 6.2.1, 6.2.2, 6.2.4, and 6.2.6 as described in the study by Carvalho et al.
Results
Immunization with axenic-EEFs protect from infective sporozoite challenge
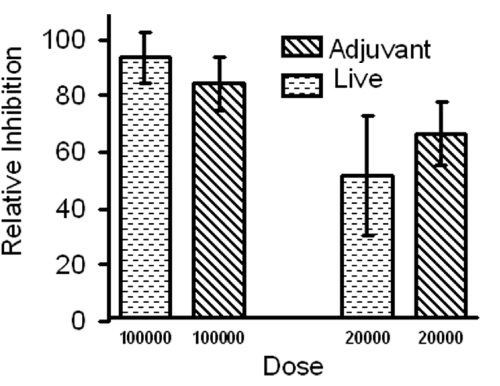
Immunization with irradiated sporozoite confers complete protection and the development of EEFs is essential. Availability of in vitro method to culture the early liver stage parasites lead us to examine whether axenic EEFs were equally potent, to RAS immunization, in inducing protective immune responses. Animals were immunized subcutaneously with live axenic EEFs without any adjuvant or as a crude antigen emulsion made in adjuvant (dead). Both immunizations lead to highly protective immune response (Table-1, Figure 1) despite the low antibody titers (IFA titers <500). The protection was dose dependent and live parasite immunization gave slightly better protection compared to dead. At highest dose tested (equivalent to 100,000 axenic EEF) live parasite gave 93 + 9.02% while dead (equal number emulsified) gave 84 + 9.30% reduction in parasite burden when compared to RAS immunization that we consider as 100% (Figure 1). Both immunizations regimens either emulsified in Freunds adjuvant or not, gave comparable protection indicating that EEFs alone in absence of any adjuvant can mount strong immune response.
Axenic EEF immunization confers protection against infectious sporozoite challenge.
Comparison between live and dead (emulsified with adjuvant) EEF immunization. Similar levels of protections were observed. Any difference in protection between live or dead EEF immunizations is not significant (P= >0.05). Bars represent mean of five mice per group. Data is from one of the two experiments with similar results. P values were calculated by students t-test.
Figure 1:Axenic EEF immunization confers protection against infectious sporozoite challenge.
Comparison between live and dead (emulsified with adjuvant) EEF immunization. Similar levels of protections were observed. Any difference in protection between live or dead EEF immunizations is not significant (P= >0.05). Bars represent mean of five mice per group. Data is from one of the two experiments with similar results. P values were calculated by students t-test.
Immunization
Dose
Immunized with
Relative % protection *
(EEF compared to RAS as 100%)
[$] 100,000
[$] Axenic EEF
93
20,000
Axenic EEF
51
4,000
Axenic EEF
30
8,00
Axenic EEF
5
30,000
Radiation attenuated
Sporozoite (RAS)
100 @
Negative
Control
Un-infected salivary gland tissue culture spent media #
0
Table 1: Axenic EEF immunization confers strong protection against sporozoite challenge.
Antibody response was mainly directed against few antigens
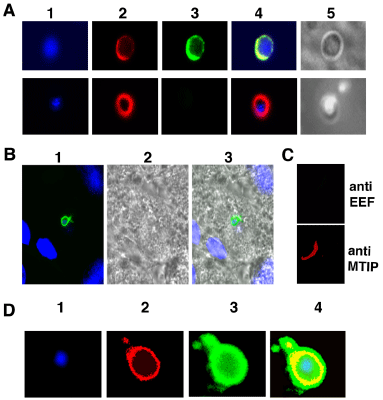
Using the sera obtained from EEF immunized animals we asked two questions: 1.Whether the antibodies in the sera recognize native molecules on axenically developed EEFs? 2.Whether the immune response is strain specific or species specific? Anti-EEF sera recognized in-vivo or axenically grown EEFs equally well. The representative images are shown in Figure 2A, 2B. A control sera generated against uninfected salivary gland tissue did not recognize any EEFs showing the specificity. Using the IFA we also determined that EEF sera recognize sporozoite poorly (IFA titer <40). This indicates that untransformed sporozoite did not contribute significantly towards antibody response. We then tested anti-P. yoelii yoelii _17XNL axenic EEF sera against P. berghei_ANKA axenic EEFs. It is evident from Figure 2D that anti- P. yoelii yoelii 17XNL EEF sera recognized P. berghei_ANKA EEFs equally well, proving that antibodies present in EEF sera are not strain specific. EFF sera recognized molecules that are conserved across species, an important feature, which could be useful in field conditions were primarily mixed infections of Plasmodium observed.
Anti-EEF sera recognized axenically or in-vivo grown EEFs.
A) P.yoelii 17XNL axenic EEFs probed with either anti-EEF sera (top panel) or control sera (bottom panel). Column 1 shows staining with DAPI, 2 MTIP, 3 anti EEF (top) or control sera (bottom), 4 merge of 1, 2, and 3. Column 5 is DIC image.
B) Liver section with P.yoelii EEFs probed with anti EFF sera generated against axenic P.yoelii 17XNL EEFs. 1) Merged image of anti-EEF sera (green) and DAPI (blue), 2) DIC image and 3) merged image of 1 and 2.
C) P.yoelii 17XNL anti- axenic EEF sera (dilution @1:100) do not crossreact with P.yoelii 17XNL sporozoites. Top panel: sporozoite probed with anti- axenic EEF sera, bottom panel: same sporozoite probed with anti-MTIP antibody.
D) P.yoelii 17XNL anti- axenic EEF sera cross react with P. berghei ANKA axenic EEF.
1) DAPI, 2) MTIP, 3) Anti Py EEF sera, 4) Overlapped image of 1, 2, and 3
Figure 2:Anti-EEF sera recognized axenically or in-vivo grown EEFs.
A) P.yoelii 17XNL axenic EEFs probed with either anti-EEF sera (top panel) or control sera (bottom panel). Column 1 shows staining with DAPI, 2 MTIP, 3 anti EEF (top) or control sera (bottom), 4 merge of 1, 2, and 3. Column 5 is DIC image.
B) Liver section with P.yoelii EEFs probed with anti EFF sera generated against axenic P.yoelii 17XNL EEFs. 1) Merged image of anti-EEF sera (green) and DAPI (blue), 2) DIC image and 3) merged image of 1 and 2.
C) P.yoelii 17XNL anti- axenic EEF sera (dilution @1:100) do not crossreact with P.yoelii 17XNL sporozoites. Top panel: sporozoite probed with anti- axenic EEF sera, bottom panel: same sporozoite probed with anti-MTIP antibody.
D) P.yoelii 17XNL anti- axenic EEF sera cross react with P. berghei ANKA axenic EEF.
1) DAPI, 2) MTIP, 3) Anti Py EEF sera, 4) Overlapped image of 1, 2, and 3
Antibody response is species specific
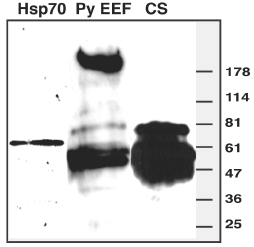
To find out the range of antigens recognized during the EEF immunization, we used anti EEF sera in a western analysis on EEF total lysate. Monoclonal antibodies against HSP70 and CS protein were used as control to compare the levels of antibody response. It is evident from Figure 3 lane 2 that antibody response is directed mainly towards two antigens. One of them appears to be CS protein and other unknown protein of ~ 220 kDa. Besides above two antigens, other seems to contribute little towards humoral response or not detectable in current immunization regimen. Figure 3 lane 3 shows the total amount of CS present in the EEF lysate, anti-EEF sera show less reactivity as compared to anti-CS monoclonal antibody.
Western blot analysis of axenic P.yoelii EEF lysate.
Equal amount of P.yoelii 17XNL EEF lyaste was probed with monoclonal antibodies against anti- HSP70, anti-CS or polyclonal anti-P.yoelii 17XNL EEF sera. On the right hand side horizontal bars indicate molecular weight size marker. Lane 1 shows a single band of 70- kDa heat shock protein expressed in liver stages. Lane 2 shows two major antigens that were recognized by EEF immune sera. Identity of top ~220 kDa molecules is not known. Lower band appears to be CS protein as a similar pattern and size is observed in lane 3 probed with 2F6 (P.yoelii-CS monoclonal antibody).
Figure 3:Western blot analysis of axenic P.yoelii EEF lysate.
Equal amount of P.yoelii 17XNL EEF lyaste was probed with monoclonal antibodies against anti- HSP70, anti-CS or polyclonal anti-P.yoelii 17XNL EEF sera. On the right hand side horizontal bars indicate molecular weight size marker. Lane 1 shows a single band of 70- kDa heat shock protein expressed in liver stages. Lane 2 shows two major antigens that were recognized by EEF immune sera. Identity of top ~220 kDa molecules is not known. Lower band appears to be CS protein as a similar pattern and size is observed in lane 3 probed with 2F6 (P.yoelii-CS monoclonal antibody).
Protection against infective sporozoite challenge was mainly due to T-cells
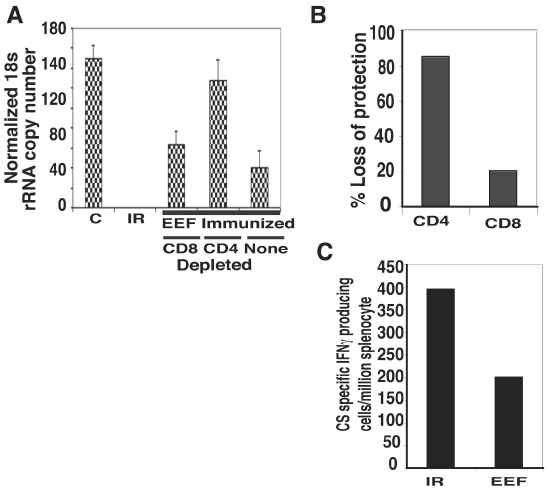
Highly protective response was obtained from EEF immunization in the absence of significant antibody response. It is now an established fact that T-cells, both CD4+ as well as CD8+, are required for protection against liver stages. To know the relative importance of CD4+ and CD8+ cells, we depleted the EEF immunized animals with antiCD4 or antiCD8 antibodies. Depletion of CD4+ T-cells lead to ~80% decrease while CD8+ T-cells depletion caused ~20% decrease in protection (Figure 4A, 4B). In an ELISPOT assay for CS specific IFN-γ producing CD8 T-cells (Figure 4C), when 30,000 RAS immunization compared with 30,000 axenic EEF immunizations, RAS gave 389 spots/106 splenocyte which was almost twice of EEF immunization (201 spots/106 splenocyte) .
cell lymphocytes play major role in axenic EEF derived immunity.
(A) Graph showing normalized 18SrRNA copy number from mice immunized or not and challenged with 10,000 sporozoite. Immunized mice received three doses of 30,000 irradiated [IR] sporozoite or 30,000 axenic EEFs. Two groups of EEF immunized mice were treated for 4 consecutive days with anti- CD4 or anti-CD8 antibody to deplete the respective cells while remaining not treated. Data represents mean of five mice per group.
(B) Graph shows percent loss of protection in EEF immunized group, when CD4 or CD8 cells were depleted, considering un-depleted as 100%. Data were from results as in Figure 4A.
(C) ELISPOT assay with splenocytes. Irradiated (IR) or axenic EEF (EEF) 30,000 each, immunized mice were analyzed for number of CS specific IFN-γ producing T-cells. Compared to EEF immunized, IR immunized mice produced twice more the number of CS specific interferon gamma producing T-cells.
Figure 4:cell lymphocytes play major role in axenic EEF derived immunity.
(A) Graph showing normalized 18SrRNA copy number from mice immunized or not and challenged with 10,000 sporozoite. Immunized mice received three doses of 30,000 irradiated [IR] sporozoite or 30,000 axenic EEFs. Two groups of EEF immunized mice were treated for 4 consecutive days with anti- CD4 or anti-CD8 antibody to deplete the respective cells while remaining not treated. Data represents mean of five mice per group.
(B) Graph shows percent loss of protection in EEF immunized group, when CD4 or CD8 cells were depleted, considering un-depleted as 100%. Data were from results as in Figure 4A.
(C) ELISPOT assay with splenocytes. Irradiated (IR) or axenic EEF (EEF) 30,000 each, immunized mice were analyzed for number of CS specific IFN-γ producing T-cells. Compared to EEF immunized, IR immunized mice produced twice more the number of CS specific interferon gamma producing T-cells.
Discussion
Based on past experiences, it appears unlikely that an effective subunit malaria vaccine that provides sterile immunity will be available soon [41]. Though a partially effective subunit pre-erythrocytic vaccine is close to market [21], search for better vaccine is ongoing. Under these circumstances efforts are being made to develop whole organism pre-erythrocytic malaria vaccine (RAS/GAS) [16,24], ITV [35] and chemically attenuated sporozoite (CAS) [42]. These efforts are based on the time tested sterile protective response obtained through irradiated sporozoite. Development of such a vaccine is not without hurdles. Several problems namely large-scale production of sporozoite, optimal irradiation, proper formulation, storage and an acceptable route of administration have to be tackled before. This work provides answers/alternates to the problems like irradiation, route of administration and formulation and storage.
In RAS immunization, the first problem is the optimal dose of sporozoite irradiation. A sub optimal dose will lead to escape of parasite from growth arrest and hence progression to blood stage and finally disease. An overdose of irradiation will lead to loss of viability of parasite hence no EEF development and abrogation of protective immune response [26-28]. Our data proves that axenically developed EEFs confer strong protective response and they are not infective (100000 axenic live EEF subcutaneous injections did not have any infection). Axenic EEFs thus could be used in place of irradiated sporozoite. Transformation conditions being simple media and temperature shift, there is little or no chance of variability. Twenty-four hour post incubation period, untransformed sporozoite are neither infective nor interfere with immune responses, thus avoiding need to separate untransformed parasites. Currently other alternatives available to RAS are GAS, CAS and ITV. Administration route is the second problem with RAS /GAS immunization. When applied subcutaneously, RAS /GAS do not give the same level of response as to intravenous application [43]. Currently any vaccine intended for human use is not allowed to administer intravenously except exemption under extraordinary condition [16,24]. Our data show that EEFs could be effectively used subcutaneously which is a well-accepted immunization route. Long-term storage is the third problem with RAS or GAS. Our results show that EEFs whether alive or dead, give strong protection hence EEFs could be formulated like any other subunit vaccine.
One important feature of EEF immunization is species-specific immune response. Since the response is not strain specific (EEF immunization confers protection against heterologous challenge) immunity will not diminish due to subtle changes in parasite antigen repertoire or it will not lead to immune selection of more virulent strains, which might be the case with single subunit vaccine. This study also shows that in EEF immunization, there are not many immuno-dominant antigens for humoral response. EEF immunization generated very low levels of antibody and the antibody response was limited to few antigens. More precisely only two dominant antigens were recognized and one of them appears to be CS, the identity of other antigen is not known. The molecular weight of unidentified antigen is ~ 220,000 daltons.
It is known that pre-erythrocytic immunity obtained from RAS is mediated by cytotoxic T cell lymphocytes (CTLs). CD8+, CD4+ and NKT cells have been implicated in liver stage immunity. By depleting CD8+and CD4+ T-cells we looked for relative contribution of T-cells in EEF immunization. Results (this study), show that contrary to RAS immunization where major contribution to protection comes from CD8+ T cells, in EEF immunization it is CD4+ T cells that are more important for protection. In EEF immunization CD8+ T-cells depletion lead to only 20% decrease in protection. This indicates that there is a qualitative difference between RAS immunization and EEF immunization. This difference may be due to context of presentation. In RAS immunization EEFs grow in liver that may have different antigen presentation and localized immune response than EEF immunization, which was administered subcutaneously. In RAS immunization CS protein is a known immuno-dominant antigen [44]. CS specific CD8+ T cells alone give high levels of protection [44]. We compared CS specific CD8+ T-cells response of RAS and EEF immunization. We found that under identical doses, EEF immunization generated 50% less CS specific CD8+ T-cells than RAS. This is most probably due to reduced levels of CS protein in in-vitro transformed EEFs as compared to sporozoite used in RAS, or due to hepatocyte specific antigen presentation [45].
In conclusion EEF immunization gives qualitatively different but highly significant protection comparable to RAS immunization. The advantages described above makes EEF immunization a realistic alternative to RAS immunization with fewer problems associated.
Acknowledgement
This work was supported in part by NIH R01 Grant Number AI053709-001 to SHIK. APS would like to thank Prof. Victor Nussenzweig, NYU, for providing lab space and permission to work in his laboratory.
Conflict of Interest
A US patent no. 20100210004 has been issued to New York University on August 19, 2010 based on the work described in this manuscript. The listed inventors are Stefan H. I. Kappe, Victor Nussenzweig, Karine Kaiser, Nelly Camargo and Agam P. Singh.
References
- Organization WH. World Malaria report 2013. WHO Library cataloguing in publication data: 2013.
- Hyde JE. Drug-resistant malaria. Trends Parasitol. 2005; 21: 494-498.
- Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Hall T, et al. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J Infect Dis. 2001; 183: 640-647.
- Moore SA, Surgey EG, Cadwgan AM. Malaria vaccines: where are we and where are we going? Lancet Infect Dis. 2002; 2: 737-743.
- Mahanty S, Saul A, Miller LH. Progress in the development of recombinant and synthetic blood-stage malaria vaccines. J Exp Biol. 2003; 206: 3781-3788.
- Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001; 358: 1927-1934.
- D'Alessandro U, Leach A, Drakeley CJ, Bennett S, Olaleye BO, Fegan GW, et al. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet. 1995; 346: 462-467.
- Genton B, Al-Yaman F, Betuela I, Anders RF, Saul A, Baea K, et al. Safety and immunogenicity of a three-component blood-stage malaria vaccine (MSP, MSP2, RESA) against Plasmodium falciparum in Papua New Guinean children. Vaccine. 2003; 22: 30-41.
- Tine JA, Lanar DE, Smith DM, Wellde BT, Schultheiss P, Ware LA, et al. NYVAC-Pf7: a poxvirus-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Infect Immun. 1996; 64: 3833-3844.
- Clyde DF. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: a review of the University of Maryland studies, 1971-75. Bull World Health Organ. 1990; 68: 9-12.
- Graves P, Gelband H. Vaccines for preventing malaria. Cochrane Database Syst Rev. 2003; CD000129.
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967; 216: 160-162.
- Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci U S A. 2005; 102: 3022-3027.
- Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005; 433: 164-167.
- van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, van Schaijk B, et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci U S A. 2005; 102: 12194-12199.
- Spring M, Murphy J, Nielsen R, Dowler M, Bennett JW, Zarling S, et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine. 2013; 31: 4975-4983.
- Collier LH. The development of a stable smallpox vaccine. J Hyg (Lond). 1955; 53: 76-101.
- Macleod DR. Current Status Of Measles And Oral Poliovirus Vaccines. Can Med Assoc J. 1964; 91: 1118-1122.
- Eyigun CP, Yilmaz S, Gul C, Sengul A, Hacibektasoglu A, Van Thiel DH. A comparative trial of two surface subunit recombinant hepatitis B vaccines vs a surface and PreS subunit vaccine for immunization of healthy adults. Journal of viral hepatitis. 1998; 5: 265-269.
- Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010; 120: 4168-4178.
- Riedmann EM. Phase 3: RTS, S almost halves malaria cases in young children. Hum Vaccin Immunother. 2013; 9: 2501.
- Luke TC, Hoffman SL. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J Exp Biol. 2003; 206: 3803-3808.
- Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973; 266: 169-177.
- Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013; 341: 1359-1365.
- Zechini B, Cordier L, Ngonseu E, D'Alessandro U, Wery M, Chatterjee S. Plasmodium berghei development in irradiated sporozoite-immunized C57BL6 mice. Parasitology. 1999; 118: 335-338.
- Nussenzweig R, Vanderberg J, Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. IV. Dose response, specificity and humoral immunity. Mil Med. 1969; 134: 1176-1182.
- Chatterjee S, Druilhe P, Wery M. Irradiated sporozoites prime mice to produce high antibody titres upon viable Plasmodium berghei sporozoite challenge, which act upon liver-stage development. Parasitology. 1999; 118: 219-225.
- Silvie O, Semblat JP, Franetich JF, Hannoun L, Eling W, Mazier D. Effects of irradiation on Plasmodium falciparum sporozoite hepatic development: implications for the design of pre-erythrocytic malaria vaccines. Parasite Immunol. 2002; 24: 221-223.
- Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987; 330: 664-666.
- Ferreira A, Schofield L, Enea V, Schellekens H, van der Meide P, Collins WE, Nussenzweig RS. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986; 232: 881-884.
- Riley EM, Stewart VA. Immune mechanisms in malaria: new insights in vaccine development. Nat Med. 2013; 19: 168-178.
- Schmieg J, Gonzalez-Aseguinolaza G, Tsuji M. The role of natural killer T cells and other T cell subsets against infection by the pre-erythrocytic stages of malaria parasites. Microbes Infect. 2003; 5: 499-506.
- Tsuji M, Zavala F. T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol. 2003; 19: 88-93.
- Seguin MC, Klotz FW, Schneider I, Weir JP, Goodbary M, Slayter M, et al. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J Exp Med. 1994; 180: 353-358.
- Belnoue E, Costa FT, Frankenberg T, Vigario AM, Voza T, Leroy N, et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004; 172: 2487-2495.
- Kaiser K, Camargo N, Kappe SH. Transformation of sporozoites into early exoerythrocytic malaria parasites does not require host cells. J Exp Med. 2003; 197: 1045-1050.
- Bruna-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol. 2001; 31: 1499-1502.
- Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol Res. 1994; 80: 16-21.
- Bergman LW, Kaiser K, Fujioka H, Coppens I, Daly TM, Fox S, et al. Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites. J Cell Sci. 2003; 116: 39-49.
- Carvalho LH, Hafalla JC, Zavala F. ELISPOT assay to measure antigen-specific murine CD8 (+) T cell responses. J Immunol Methods. 2001; 252: 207-218.
- Heppner DG. The malaria vaccine--status quo 2013. Travel Med Infect Dis. 2013; 11: 2-7.
- Purcell LA, Wong KA, Yanow SK, Lee M, Spithill TW, Rodriguez A. Chemically attenuated Plasmodium sporozoites induce specific immune responses, sterile immunity and cross-protection against heterologous challenge. Vaccine. 2008; 26: 4880-4884.
- Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8⺠T cell immunity. Science. 2011; 334: 475-480.
- Kumar KA, Sano G, Boscardin S, Nussenzweig RS, Nussenzweig MC, Zavala F, et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006; 444: 937-940.
- Bongfen SE, Torgler R, Romero JF, Renia L, Corradin G. Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J Immunol. 2007; 178: 7054-7063.