Mini Review
Austin J Vaccines & Immunother. 2014;1(1): 4.
Malaria Liver Stage Parasites Strategies for Immune Evasion and Host Modulation: Implication for Vaccine Design
Dabbu Kumar Jaijyan1, Himanshu Singh1 and Agam Prasad Singh1*
1Infectious Diseases Laboratory, National Institute of Immunology, India
*Corresponding author: Singh AP, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi-110067, India
Received: July 19, 2014; Accepted: September 30, 2014; Published: October 16, 2014
Abstract
Malaria is a devastating infectious disease caused by protozoan parasite of the genus Plasmodium. When an infected mosquito bites a vertebrate host it leaves a small number of sporozoites under the skin. Sporozoites from the site of bite, end up in lymph node or liver. In the lymph node these parasite are degraded and act as source of antigen for immune system. In liver, the sporozoites invade into a hepatocyte and develop into mature schizonts. Liver stage parasites are able to evade host immune responses as well as modulate the various host pathways. Study of Plasmodium liver stage exportome may provide valuable vaccine candidates and those with strong T cell epitope may be inducted into pre erythrocytic stage vaccine pipeline. Hepatocytes process and present foreign antigens on its surface hence Plasmodium exported proteins are also likely presented on its surface similar to circumsporozoite protein. In terms of designing vaccine delivery system and the antigen load in liver, recent advances suggest that one need to deliver antigen in <20% of hepatocytes. Presence of antigen in large population of hepatocytes (>25%) leads to silencing of CTLs, which are no longer effective in killing the pathogens.
Keywords: Liver stage Malaria; Plasmodium; Exported proteins; Novel antigen; Vaccine
Abbreviations
Ag: Antigen; EEF: Exo-Erythrocytic Forms; KCs: Kupffer Cells; mTOR: Mammalian Target of Rapamycin; PEXEL: Plasmodium Export Element; PVM: Parasitophorous Vacuole Membrane; CSP: Circumsporozoite Protein
Introduction
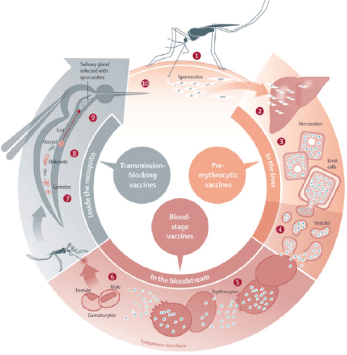
Malaria, a deadly diseases spread worldwide, is caused by protozoan parasites of the genus Plasmodium. Infection in the vertebrate host is comprised of two stages: an asymptomatic pre-erythrocytic stage, and a symptomatic erythrocytic stage. The pre-erythrocytic stage is short-lived, yet critical for the establishment of malaria infection. This phase is an amplification stage where parasite increases its number from one to 10-30 thousand. Sporozoites, inoculated by infected mosquitoes, initiate the infection in vertebrate host, which leads to development of mature liver schizont containing thousand of merozoites ready for blood stage infection (Figure 1). In this mini review we discuss on the early stages of malaria infection, how parasite deals with host immune response and host modulation. In the end, we conclude with some thoughts as to how this knowledge could be utilized for vaccine design for the generation of improved malaria vaccine.
Figure 1: Sites in the malaria life cycle that could be interrupted by vaccines (1) Malaria infection begins when an infected female anopheles mosquito bites a person, injecting Plasmodium spp parasites, in the form of sporozoites, into the bloodstream. (2) The sporozoites pass quickly into the human liver. (3) The sporozoites multiply asexually in the liver cells over the next 7–10 days, causing no symptoms. (4) In an animal model, the parasites, in the form of merozoites, burst from the liver cells in vesicles, journey through the heart, and arrive in the lungs, where they settle within lung capillaries. The vesicles eventually disintegrate, freeing the merozoites to enter the blood phase of their development. (5) In the bloodstream, the merozoites invade erythrocytes and multiply again until the cells burst. Then they invade more erythrocytes. This cycle is repeated, causing fever each time parasites break free and invade blood cells. (6) Some of the infected blood cells leave the cycle of asexual multiplication. Instead of replicating, the merozoites in these cells develop into sexual forms of the parasite, called gametocytes,that circulate in the bloodstream. (7) When a mosquito bites an infected human, it ingests the gametocytes, which develop further into mature sex cells called gametes. (8) The gametes develop into actively moving ookinetes that burrow through the mosquito’s midgut wall and form oocysts. (9) Inside the oocyst, thousands of active sporozoites develop. The oocyst eventually bursts, releasing sporozoites that travel to the mosquito salivary glands. (10) The cycle of human infection begins again when the mosquito bites another person. Figure along with legend, reproduced with permission from Elsevier Limited, Oxford, UK [The Lancet (2010) 375:1468-81].
Innate Immune Responses are the First Hurdle Crossed by the Malaria Sporozoites
The first infective forms of malaria parasite, the sporozoites, are introduced under host skin when an infected female Anopheles mosquitoes bite the host [1,2]. Sporozoites cross the cellular barrier and reach to blood and lymphatic systems [3]. Sporozoites in lymph nodes transform into early liver stages and act as a source of antigen for immune system [4,5]. Sporozoites circulating in the blood home in liver and invade hepatocytes. Before invading a hepatocyte sporozoites traverse through Kupffer cells and several hepatocytes [6]. Sporozoite traversal not only inactivate kupffer cell defenses, but it also help in the activation of sporozoite (exocytosis) for productive invasion [7]. In productive invasion, sporozoites are enclosed by a parasitophorous vacuole membrane (PVM), which physically separates it from host cytoplasm. Inside PVM sporozoite transform into round form and develop into liver schizonts containing thousands of merozoites, which are released into blood stream in the form of merosomes [8].
In the end only 10% or less sporozoite successfully develops into mature schizonts and the remaining sporozoites and liver forms are cleared by the immune system [1]. During cell traversal parasite may shed molecules that can trigger innate immune responses. Recently it was shown that parasite RNA is recognized by the host MDA5 and MAVS and activates innate immune response in the form of type I interferon secretion [9]. Both, type I and IFN-γ interferons are known to inhibit EEF growth [10]. Innate immune response mediated by NK, NKT, and γδT cells inhibits EEF growth through secretion of interferon [11-13]. Hepcidin is also implicated in inhibition of EEF growth [14]. In each bite of mosquito 100-200 sporozoites are deposited and despite innate immune hurdle some parasites still able to establish successful infection [15]. Recent findings suggest that liver stage parasites have developed host immune evasion strategies [16-17].
Kupffer Cells Form the Second Barrier for Sporozoites
In a study using intravital microscopy it was proposed that sporozoites pass through kupffer cells (KC) but not the endothelial cells (EC) [18-19]. However recently it was demonstrated that sporozoites have multiple mechanism to cross the sinusoidal barrier. Study showed that most of the sporozoites penetrate through ECs (53%) and some through KCs (24%) and remaining pass through gaps without traversal [20]. Traversal deficient parasite lines (SPECT1, SPECT2 and CelTOS) may primarily reach hepatocytes using the gaps [21-23].
For the sporozoites passing through KCs, it is amazing that parasites are not killed by KCs although they are known to kill other microorganism present in the liver. We partly know why this does not happen. Studies have shown that sporozoite abundant surface protein circumsporozoite (CSP) binds to LRP1 receptor and proteoglycans on KC surface and it leads to increased level of EPAC and reduced levels of ROS [24]. Sporozoite contact with KC, and upon stimulation with IFN-γ or LPS, leads to increase in anti-inflammatory cytokine IL-10 and decrease in inflammatory molecules like IL6, TNFα and MCP1 [25]. Sporozoite traversal through KCs also reduces their antigen presentation capacity [26] and thus affecting the adoptive immune responses.
Processing and Presentation of Liver-Stage Antigens by Hepatocytes
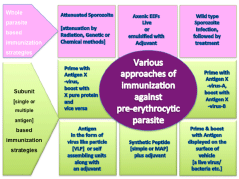
Hepatocytes are capable of processing and presentation of foreign antigens present in its cytoplasm. Bongfen et al. [27] showed that both infected and traversed primary hepatocytes process and present the CSP. The processing and presentation pathway was found to involve the proteasome, Ag transport through a post endoplasmic reticulum compartment, and aspartic proteases [27]. It has been suggested that in malaria, hepatocytes express MHC class I-peptide complexes on their surface and that the recognition of these complexes by CD8+ T cells is necessary for protection [28,29]. This notion is supported by studies that show irradiated sporozoite-immunized β2m-/- mice are not protected against sporozoite challenge, despite induction of IL-12, IFN-γ, and proliferating T cells [30]. The finding that infected hepatocytes present parasite Ags has profound consequences for vaccine development. CSP is the leading pre-erythrocytic stage vaccine candidate and is an immunodominant protective Ag in irradiated sporozoites (Figure 2) [31]. However, the developing parasite also expresses other Ags inside the infected cell. If these Ags are presented on the surface of the infected hepatocyte, they could be potential candidates for a pre-erythrocytic stage vaccine. In fact two of the liver stage exported proteins (with PEXEL motif) when used for immunization in mice and subsequently challenged with high dose of sporozoites both offered 10000 fold reduction in liver parasite burden compared to controls (Figure 2) (Jaijyan D.K., Singh, H. and Singh A.P. unpublished data). Thus it is not far fetched to say that study of liver stage exportome holds promise for pre erythrocytic vaccine candidates (Figure 3).
Approaches of immunization against liverstage parasites.
In the last four-decade various methods of immunization against liver stage parasite has been tried. Some of them are successful others failed. In the figure listed are some of them that have shown promise. Both methods (whole parasite and subunit) are currently being pursued. So far the gold standard is immunization with radiation-attenuated sporozoites. This method although very effective, but has problems in implementation at the mass scale. Use of axenic EEFs should provide means to circumvent some of the problems associated with irradiated sporozoite immunization, for example storage, viability, transportation and route of immunization. Whole parasite immunizations have shown highly effective immune responses capable of clearing the high doses of challenge. Subunit based (protein) immunization either single or multiple antigens have shown so far limited efficacy. The most advanced subunit based vaccine is CS based [RTS, S] which recently completed phase-III trials. RTS,S is partially effective (50 % protection from sever disease) but in absence of any other malaria vaccine, it may be licensed very soon for mass scale use.Figure 2:Approaches of immunization against liverstage parasites.
In the last four-decade various methods of immunization against liver stage parasite has been tried. Some of them are successful others failed. In the figure listed are some of them that have shown promise. Both methods (whole parasite and subunit) are currently being pursued. So far the gold standard is immunization with radiation-attenuated sporozoites. This method although very effective, but has problems in implementation at the mass scale. Use of axenic EEFs should provide means to circumvent some of the problems associated with irradiated sporozoite immunization, for example storage, viability, transportation and route of immunization. Whole parasite immunizations have shown highly effective immune responses capable of clearing the high doses of challenge. Subunit based (protein) immunization either single or multiple antigens have shown so far limited efficacy. The most advanced subunit based vaccine is CS based [RTS, S] which recently completed phase-III trials. RTS,S is partially effective (50 % protection from sever disease) but in absence of any other malaria vaccine, it may be licensed very soon for mass scale use.
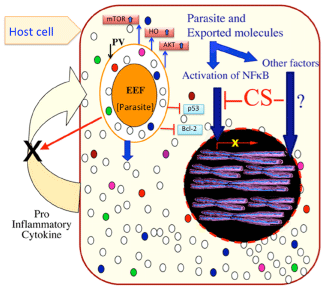
Host modulation by malaria liverstage parasites.
A host cell infected with Plasmodium liverstage parasite (EEF), undergo lots of changes in gene expression. EEFs export many proteins into the host cytoplasm (represented with white and colored dots) to manipulate the host. The upregulation of mTOR, heamoxygenase (HO), Akt and downregulation of p53, Bcl-2 has been experimentally verified. The CS protein has been shown to interfere with translocation of activated NFκB to the host nucleus, leading to down regulation of 40 NFκB dependent genes expression. CS and HO are involved in inhibiting host inflammatory response (inflammatory cytokines) and promote parasite growth and survival. Besides NFκB, inhibition of host transcription factor by parasite proteins is an area open for exploration.Figure 3:Host modulation by malaria liverstage parasites.
A host cell infected with Plasmodium liverstage parasite (EEF), undergo lots of changes in gene expression. EEFs export many proteins into the host cytoplasm (represented with white and colored dots) to manipulate the host. The upregulation of mTOR, heamoxygenase (HO), Akt and downregulation of p53, Bcl-2 has been experimentally verified. The CS protein has been shown to interfere with translocation of activated NFκB to the host nucleus, leading to down regulation of 40 NFκB dependent genes expression. CS and HO are involved in inhibiting host inflammatory response (inflammatory cytokines) and promote parasite growth and survival. Besides NFκB, inhibition of host transcription factor by parasite proteins is an area open for exploration.
Host Modulation by Liver-Stage Parasite
Liver stage parasites develop with in PVM. For its survival parasites have developed several strategies to suppress host immunity [16,17], apoptosis [32] and selective autophagy [33,34]. Parasite also depends on host metabolites like lipids, nucleotide, sugars, amino acids and metals like iron to maintain its rapid growth rate. Circumsporozoite protein, a major surface protein of sporozoites and EEFs is cleaved at it N terminus and exported across PVM into the hepatocyte cytoplasm [16]. Cleaved CSP also has a functional nuclear localization signal which drives CSP into host nucleus. To enter the host nucleus, CSP uses importin alpha 3, alpha 4, the same importins also utilized by activated NFκB [16]. Large amount of CSP present in host cytoplasm and its import into host nucleus competes with tiny amounts of activated NFκB. This competition for utilization of same importins by CSP and NFκB leads to reduction in or complete loss of NFκB mediated host gene expression (Figure 3). Microarray results from CSP over-expressing cell line showed that several NFκB regulated immunity related genes were down regulated [16]. CSP ectopic expression also lead to changes in the transcript levels of over 1000 host genes that fall in various host pathways. CSP ectopic expression causes enhanced growth of the EEFs [16]. Up regulation of host heme oxygenase (HO) is another example of host modulation. HO-1 overexpression in the liver leads to a proportional increase in parasite liver load. Findings suggest that, while stimulating inflammation, the liver stage of Plasmodium also induces HO-1 expression, which modulates the host inflammatory response, protecting the infected hepatocytes and promoting the liver stage of infection (Figure 3) [17]. Using protein microarrays it was discovered that sporozoite infection of hepatocytes causes altered levels of proteins involved in cell survival, proliferation and autophagy/anabolism/cell growth (mTOR) [35]. Autophagy was originally thought to be essential for cell survival, development, and homeostasis, but growing evidence suggests that it could also play role in inhibiting the growth of viruses or intracellular pathogens (Figure 3) [36]. Autophagy was found to play role in the transformation of sporozoites into the liver stage [37], its role in EEF development is still not known. Hepatic merozoites also evade host defenses when they exit hepatocytes. To avoid host cell defense mechanisms, merozoites bud from detached hepatocytes in the form of merosomes [8], which are covered with host cell-derived membranes [38]. Hepatic merozoites uptake Ca2+ and maintain low Ca2+ levels in the host cell to inhibit host cell apoptosis [8]. Thus, dying hepatocytes avoid recognition by phagocytes, and merosomes are safeguarded from immune attack [39].
Conclusion
The finding that infected hepatocytes present parasite Ags has profound bearing on vaccine development. Developing EEFs also expresses other Ags (than CSP) and export across PVM inside the infected cell. If these Ags are presented on the surface of the infected hepatocyte, they could be potential candidates for a pre-erythrocytic stage vaccine (Figure 2). Understanding how EEFs modulate host immune responses (Figure 3) may help in formulating strategies that nullify the parasite tricks and thus providing better vaccines against malaria liver stage parasites. Liver possesses unique tolerogenic properties, yet it is also target of immune-mediated damage in chronic viral infections. Liver tolerogenic properties likely evolved to maintain immunological unresponsiveness toward food-derived and microbial antigens that enter the circulation via the gut. Several studies [40,41] have shown that effective immune responses can be sustained in the liver despite its apparent predisposition towards tolerance.
In a recent study [42], findings reveal a threshold of antigen expression within the liver as the dominant factor determining T-cell fate, irrespective of T-cell receptor affinity or antigen cross-presentation. In mice, when a low percentage of hepatocytes expressed cognate antigen, high-affinity T cells developed and maintained effector function, whereas, at a high percentage (> 25%), they became functionally exhausted and silenced [42]. Authors also show that exhaustion was not irreversibly determined by initial activation, but was maintained by high intrahepatic antigen load during the early phase of the response [42]. These finding should be taken into consideration for future malaria vaccine design where antigens are directly expressed in hepatocyte using viral vector or other delivery methods. Keeping the low percentage of hepatocytes having antigens will maintain the effector CTLs for longer duration.
Acknowledgment
We apologize to all whose work could not be cited, due to space limitation. Work in APS lab, was supported by Department of Biotechnology, Government of India, and NII, New Delhi, Core fund.
References
- Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, et al. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe. 2008; 3: 88-96.
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nature medicine. 2006; 12: 220-224.
- Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007; 9: 1215-1222.
- Sinnis P, Zavala F. The skin: where malaria infection and the host immune response begin. Semin Immunopathol. 2012; 34: 787-792.
- Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007; 13: 1035-1041.
- Frevert U, Engelmann S, Zougbédé S, Stange J, Ng B, Matuschewski K,et al. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 2005; 3: e192.
- Mota MM, Hafalla JC, Rodriguez A. Migration through host cells activates Plasmodium sporozoites for infection. Nat Med. 2002; 8: 1318-1322.
- Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006; 313: 1287-1290.
- Liehl P, Zuzarte-Luís V, Chan J, Zillinger T, Baptista F, Carapau D, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med. 2014; 20: 47-53.
- Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SH. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep. 2014; 7: 436-447.
- Roland J, Soulard V, Sellier C, Drapier AM, Di Santo JP, Cazenave PA, et al. NK cell responses to Plasmodium infection and control of intrahepatic parasite development. J Immunol. 2006; 177: 1229-1239.
- Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D, et al. Liver CD4-CD8- NK1.1+ TCR alpha beta intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. Journal of immunology. 2000; 164: 1463-1469.
- McKenna KC, Tsuji M, Sarzotti M, Sacci JB Jr, Witney AA, Azad AF. gammadelta T cells are a component of early immunity against preerythrocytic malaria parasites. Infect Immun. 2000; 68: 2224-2230.
- Portugal S, Carret C, Recker M, Armitage AE, Gonçalves LA, Epiphanio S, et al. Host-mediated regulation of superinfection in malaria. Nat Med. 2011; 17: 732-737.
- Medica DL, Sinnis P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect Immun. 2005; 73: 4363-4369.
- Singh AP, Buscaglia CA, Wang Q, Levay A, Nussenzweig DR, et al. Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell. 2007; 131: 492-504.
- Epiphanio S, Mikolajczak SA, Gonçalves LA, Pamplona A, Portugal S, et al. Heme oxygenase-1 is an anti-inflammatory host factor that promotes murine plasmodium liver infection. Cell Host Microbe. 2008; 3: 331-338.
- Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology. 2001; 33: 1154-1165.
- Yuda M, Ishino T. Liver invasion by malarial parasites-how do malarial parasites break through the host barrier? Cell Microbiol. 2004; 6: 1119-1125.
- Tavares J, Formaglio P, Thiberge S, Mordelet E, Van Rooijen N, Medvinsky A, et al. Role of host cell traversal by the malaria sporozoite during liver infection. J Exp Med. 2013; 210: 905-915.
- Ishino T, Yano K, Chinzei Y, Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004; 2: E4.
- Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005; 58: 1264-1275.
- Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol. 2006; 59: 1369-1379.
- Usynin I, Klotz C, Frevert U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell Microbiol. 2007; 9: 2610-2628.
- Klotz C, Frevert U. Plasmodium yoelii sporozoites modulate cytokine profile and induce apoptosis in murine Kupffer cells. Int J Parasitol. 2008; 38: 1639-1650.
- Steers N, Schwenk R, Bacon DJ, Berenzon D, Williams J, Krzych U. The immune status of Kupffer cells profoundly influences their responses to infectious Plasmodium berghei sporozoites. Eur J Immunol. 2005; 35: 2335-2346.
- Bongfen SE, Torgler R, Romero JF, Renia L, Corradin G. Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J Immunol. 2007; 178: 7054-7063.
- Good MF, Doolan DL. Immune effector mechanisms in malaria. Curr Opin Immunol. 1999; 11: 412-419.
- Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006; 28: 51-60.
- White KL, Snyder HL, Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J Immunol. 1996; 156: 3374-3381.
- Kumar KA, Sano G, Boscardin S, Nussenzweig RS, Nussenzweig MC, Zavala F, Nussenzweig V. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006; 444: 937-940.
- van de Sand C, Horstmann S, Schmidt A, Sturm A, Bolte S, Krueger A, et al. The liver stage of Plasmodium berghei inhibits host cell apoptosis. Mol Microbiol. 2005; 58: 731-742.
- Lassen KG, Kuballa P, Conway KL, Patel KK, Becker CE, Peloquin JM, et al. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci U S A. 2014; 111: 7741-7746.
- Heusler V. Two sides of the same coin: Beneficial and deleterious effects of host cell autophagy in Plamsmodium infected Hepatocytes. 2014.
- Kaushansky A, Ye AS, Austin LS, Mikolajczak SA, Vaughan AM, Camargo N, et al. Suppression of host p53 is critical for Plasmodium liver-stage infection. Cell Rep. 2013; 3: 630-637.
- Kuballa P, Nolte WM, Castoreno AB, Xavier RJ. Autophagy and the immune system. Annu Rev Immunol. 2012; 30: 611-646.
- Coppens I. Metamorphoses of malaria: the role of autophagy in parasite differentiation. Essays Biochem. 2011; 51: 127-136.
- Graewe S, Rankin KE, Lehmann C, Deschermeier C, Hecht L, Froehlke U, et al. Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog. 2011; 7: e1002224.
- Baer K, Klotz C, Kappe SH, Schnieder T, Frevert U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 2007; 3: e171.
- Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005; 436: 946-952.
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005; 5: 215-229.
- Tay SS, Wong YC, McDonald DM, Wood NA, Roediger B, Sierro F, et al. Antigen expression level threshold tunes the fate of CD8 T cells during primary hepatic immune responses. Proc Natl Acad Sci U S A. 2014; 111: 2540-2549.