Research Article
Austin J Vasc Med. 2014;1(1): 5.
Anaplerosis of Estrogen Confers Protection of Cardiac Injury and Cardiomyocyte Apoptosis in Isoproterenol Challenged Rat
Zhang Y1*, Wu Y2,3, Wang H1, Meng J1, Tang HY4
1Department of Anesthesiology, Xuzhou Medical College, China
2Department of Nephrology, The First People’s Hospital of Xuzhou, China
3Departmentof Anesthesiology, University of Illinois, USA
4Department of Medicine, University of Illinois, USA
*Corresponding author: Zhang Y, Department of Anesthesiology, Xuzhou Medical College, 209 Tongshan Road, Xuzhou, Jiangsu, 221000, China
Received: September 30, 2014; Accepted: October 30, 2014; Published: November 03, 2014
Abstract
Previous investigations have indicated that premenopausal women show lower cardiovascular risk than men and postmenopausal women. It is suggested that estrogen has protective effects on myocardial function. However, the cardioprotective mechanisms of estrogen in humans and mammals are still elusive. We studied the effect of ovarian hormone deficiency and estrogen anaplerosison cardiac injury and cardiomyocyte apoptosis in Isoproterenol (ISO) challenged rats. Female Sprague–Dawley rats with bilateral Ovariectomy (OVX) or sham operation (Sham) were divided into 5 groups: sham surgery (Sham), OVX+vehicle (vehi), OVX+Isoproterenol (ISO), OVX+E2a (estrogen, 4 μg/kg/d), OVX+ISO+E2b (estrogen, 40 μg/kg/d). ISO (100 mg/kg/d, sc) was administrated for 5 days to induce myocardial injury, followed by estrogen anaplerosis (0-40 μg/ kg/d) for 4 weeks. Our results indicated that ISO significantly reduced myocardial pump function, increased hypertrophy and apoptosis of cardiomyocytes, and reduced single cardiomyocyte contractility. Anaplerosis of high-dose estrogen (40 μg/kg/d) dramatically restored ISO induced cardio injury and dysfunction, and inhibited ISO induced decrease of cardiomyocyte contractility, myocardial hypertrophy and cardiomyocyte apoptosis through increasing the expression of Bcl-2, inhibition of caspas-3 activation and expression of Bax. Interestingly, low-dose treatment with estrogen (4 μg/kg/d) has also shown marginally protective effects on ISO induced heart injury and cardiomyocyte dysfunction. These data suggest that estrogen anaplerosis has cardioprotective effects on ISO treated rats through the protection of cardiomyocyte apoptosis and hypertrophy and decrease of contractility.
Keywords: Estrogen; Cardiomyocyte; Apoptosis; Isoproterenol
Introduction
Studies in cardiovascular diseases have indicated that males and females show different prevalence and severity, and premenopausal women display lower heart disease risk than men of the same age [1,2]. This advantage of women over men disappears after menopause, suggesting that estrogen (E2) plays an important role in maintaining healthy cardiovascular conditions [3,4]. However, the mechanism of this myocardial protection effect is unclear.
The sympathetic adrenal medullary system is one of the most important extrinsic mechanisms regulating cardiac function. An enhancement in activity of this system leads to excessive increase in catecholamine secretion, which will cause acute or chronic cardiac injury in post-menopausal women [5]. Apoptosis also plays a key role in myocardial injury [6]. The relative ratio of pro-apoptotic proteins to anti-apoptotic proteins plays an important role in determining cell survival or cell death. It has been found that high expression of Bax and low expression of Bcl-2 are associated with vulnerability to apoptotic activation [7,8]. In principle, protection of cardiomyocytes from ISO-induced apoptosis increases the percentage of viable myocardium. Therefore, apoptosis of cardiomyocytes may be an excellent target for therapeutic modulation in the context of myocardial injury subject to ISO [9,10].
The goals of this work were to determine the role of estrogen replacement in reducing cardiac injury in response to ISO treatment and to demonstrate the mechanism of estrogen in inhibiting myocardial apoptosis induced by ISO.
Materials and Methods
Animals and reagents
Adult female Sprague-Dawley (SD) rats (200±20 g) were obtained from the Experimental Animal Centre of Xuzhou Medical College and all studies were approved by the Animal Ethics Committee of the Medical College of Xuzhou (permit number: xz11-12540). The main reagents used in the experiments are as follows: isoproterenol hydrochloride (Sigma-Aldrich, St. Louis, MO, USA), 17β-estradiol (ABCR, Germany), primary antibodiesCaspase3, Bcl-2, Bax, GAPDH and HRP-conjugated secondary antibodies (Santa Cruz, CA, USA). The animals were anesthetized with sodium pentobarbitone (60 mg/ kg, I.P.). Incision was chosen at the side of the spine, the muscle layer was separated and the peritoneum was opened, followed by removing ovary in OVX group.ISO (100 mg/kg, sc) treatment was performed for 5 days to get the myocardial injury model [5]. Vehicle and 17β-estradiol (0-40 μg/kg/d) were administrated for 4 weeks [11]. In general, the rats were divided into 5 groups: Sham, OVX, OVX+ISO+Vehi, OVX+ISO+ E2a, OVX+ISO+ E2b.
Determination of cardiac function
The French high-fidelity pressure transducer (Millar Instruments, Houston, TX) was introduced into the left common carotid artery in the different treatment groups. Mean arterial blood pressure (MABP), LV end-diastolic pressure (LVEDP), the maximalrates of pressure rise (+dP/dt) and of pressure fall (-dP/dt) were measured [12].
Isolation and culture of myocardial cells
Left ventricular myocytes were isolated as described previously [13]. In brief, isolated hearts were perfused for 5minwithCa2+-free buffer. Then the hearts were switched to the same perfusion buffer containing 0.04% collagenase, 0.1% bovine serum albumin and 50 μM of CaCl2. The perfusate was recirculated at a flow rate of 6–10 ml min-1. After about 25 min recirculation period, hearts were removed from the cannula and the left ventricles were cut into small pieces in Krebs–Bicarbonate (KB) solution (pH7.2) and gassed with 100% O2 at 37°C. Myocytes were harvested and filtered through 200 nylon meshes. Then they were resuspended in preoxygenated KB solution and was ahed three times to discard the dead myocytes.
Measurement of contraction in isolated cardiomyocytes
Contractile function was monitored by a video edge detector system as described previously [14]. Ventricular myocytes were added to an open chamber on the stage of an inverted microscope (Olympus, Tokyo, Japan). After 5 minutes, attached myocytes were filled with Krebs-Henseleit Buffer (KHB) and 100 n Misoprenaline to mimic the in vivo situation when there is sympathetic activity. KHB (2 ml/min, containing 2.0 mM Ca2+) was adjusted to pH 7.4 by equilibration with 95% O2- 5% CO2. The ventricular myocytes were paced with electrical field stimulation (0.5 Hz). The myocytes used were rod-shaped with clear sarcomeres. At least 10myocytes per experimental group per heart were studied. The outputs of the video edge detector were sent to a computer. Contractile function was assessed using the following indices: shortening amplitude, Time-To- Peak (TTP) contraction and time-to-90% relaxation (R90).
Evaluation of cell apoptosis
Annexin–PI staining was performed for apoptosis detection [15]. The ratcardiomyocytes were plated in 60-mm dishes with a cell density of 5 × 105 cells/dish. The cells were washed with ice-cold PBS, resuspended in 100 μlannexin V incubation reagent (10 μl10× binding buffer, 10 μl PI, 1 μlannexin V and 79 μl dH2O) at a concentration of 3-5 × 105 cells/100 μl, and incubated for 15 minutes at room temperature in the dark. Samples were washed with binding buffer and analyzed by Flow Cytometry (Bio-Rad S3). Apoptotic cells were identified as an annexin V-FITC-positive/PI-negative population.
Western blot
We performed Western-Blotting assays to determine the possible mechanisms responsible for estrogen-induced cardioprotection, expressions of Caspase3, Bcl-2 and Bax (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in cardiomyocytes. The expression of GAPDH was measured as an internal standard. It was performed as described previously [16]. After reperfusion, the cells were harvested and homogenized in lysisbuffer containing proteinase inhibitor. The protein concentration quantitation was done with modified Bradford assay (Bio-Rad, CA,USA). Equivalent amounts (20μg) of protein samples were loaded and separated on SDS-polyacrylamide gel electrophoresis and then transferred to Polywinylidene Fluoride (PVDF) membranes. The membranes were incubated overnight at 4°C with primary antibodies at a dilution of 1:1000. The membranes were then incubated with secondary antibody for 2 hours. Finally, the films were scanned into the computer, and relative intensity of bands was analyzed by Image J 3.0 system.
Statistical analysis
For each experimental series, data were presented as mean±SEM. Statistical analysis was performed with GraphPad Prism4.0. Statistical significance (p<0.05) for each variable was estimated by 1-way or 2-way analysis of variance followed by Bonferronipost hoc tests.
Result
General features of experimental animals
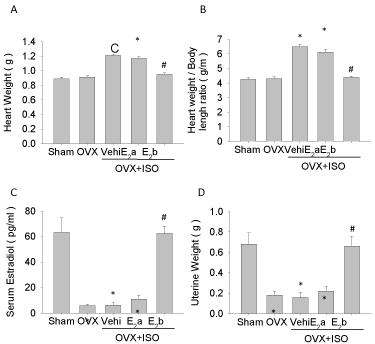
To check the role of estrogen in cardioprotection, bilateral Ovariectomy (OVX) was used to attenuate the serum estrogen level, and ISO administration was used to induce heart injury and cardiomyocyte apoptosis. As shown in Figure 1, the serum estrogen levels (Figure 1C) and uterine weight (Figure 1D) dramatically decreased after OVX, but body length was not changed (data not shown). Compared with the Sham or OVX group, ISO increased heart weight and heart weight/body length ratio (Figure 1A, B) in OVX rats. OVX+ISO+E2b (40 μg/kg/d) dramatically increased the serum estradiol level (Figure 1C), protected the loss of uterine weight due to the OVX (Figure 1D) and eliminated the increasing of the heart weight (Figure 1A), heart weight/body length ratio (Figure 1B) due to ISO challenge. Lower dose of estrogen (4 μg/kg/d) marginally increased serum estradiol level (Figure 1C), but showed limited effect on the protection of the loss of uterine weight after OVX. Interestingly, lower dose of estrogen (4 μg/kg/d) slightly attenuated ISO induced increase of heart weight (Figure 1A) and heart weight/ body length ratio (Figure 1B).
Figure 1: General features of experimental animals. The general features were measured before rat heart isolation. A, heart weights of rats; B, ratios of heart weight to body length of rats; C, serum estrogen levels in rats; D, uterine weight of rats. Each value represents mean ± S.E.M. n = 10 hearts in each group, *P < 0.05 versus Sham, #P < 0.05 versus OVX+ISO+Vehi.
Anaplerosis of estrogen improves myocardial contractile dysfunction following iso challenge
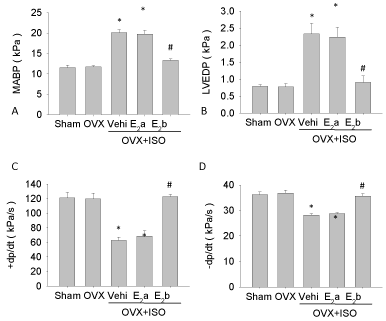
Left carotid artery of rat was cannulated. We then recorded the data of cardiac function of each group and found that there were no significant differences between the Sham group and the OVX group before ISO treatment. But after ISO treatment, the ±dP/dt was decreased, and the MABP and LVEDP were increased significantly. Administration of E2 (40 ug/kg/d) led to an increase in ±dP/dt, and decreases in MABP and LVEDP in the OVX+ISO+E2b group. However, E2 (4 ug/kg/d) treatment did not cause similar changes (Figure 2).
Figure 2: Detection of myocardial contractile function indicators. Myocardial contractile function was detected with left common carotid artery catheter. A, mean arterial blood pressure, MABP; B, left ventricular end-diastolic pressure, LVEDP; C, the maximal rates of left ventricular pressure rise, +dp/dt; D, the maximal rates of left ventricular pressure fall, –dp/dt. Each value represents mean ± S.E.M. n = 10 hearts in each group, *P < 0.05 versus Sham, #P < 0.05 versus OVX+ISO+Vehi.
Effect of estrogen anaplerosis on single ventricular myocyte contractile following iso challenge
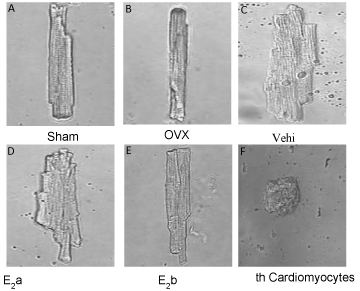
We observed contractility of myocytes isolated from the Sham, OVX, OVX+ISO, OVX+ISO+E2a, and OVX+ISO+E2b groups, which were incubated with vehicle medium. Photos were taken from videos of single cardiac myocyte contraction in each group. We found that estrogen (40 μg/kg/d) effectively suppressed the ISO-induced hypertrophy of cardiomyocytes. The average length and diameter of cardiomyocytes in the OVX+ISO+ E2b group, but not those in the OVX+ISO+E2a group, were significantly decreased (Figure 3).
Figure 3: Morphology of single left ventricular cardiomyocyte. The ventricular myocytes were paced with electrical field stimulation (0.5 Hz), and pictures of single left ventricular cardiomyocyte were captured from videos of cardiomyocyte contraction recorded for each group.
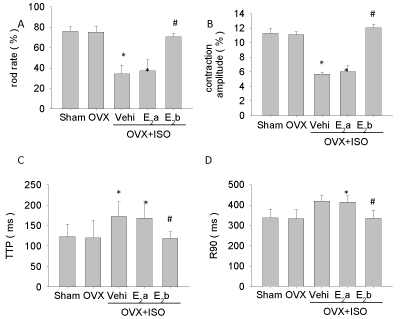
We also found that single cardiac myocyte contraction decreased in the OVX+ISO group compared with that in the OVX group, which reflected a decrease of contraction amplitude, and extension of systolic (TTP) and diastolic (R90). The myocyte contraction of cardiomyocyte in the OVX+ISO+ E2b group was improved, but not in the OVX+ISO+E2a group (Figure 4).
Figure 4: Single left ventricular cardiomyocyte contraction. Cardiomyocyte contractility was monitored after isolation and culture; A, cardiomyocyte rod rate; B, myocardial contraction amplitude; C, time of myocardial contraction to the maximum, time-to-peak (TTP); D, time of myocardial relax to 90%, timeto- 90% relaxation (R90). Each value represents mean ± S.E.M. n = 10 hearts in each group, *P < 0.05 versus Sham, #P < 0.05 versus OVX+ISO+Vehi.
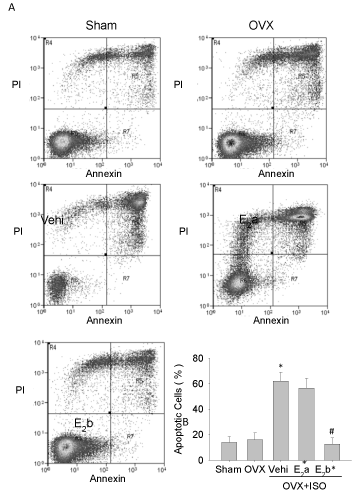
Estrogen replacement restores cardiomyocyte apoptosis following iso challenge
Based on the results that the cardiomyocytes in the OVX+ISO+E2b group were significantly decreased in injury and enhanced in myocyte shortening amplitude compared with those in the OVX+ISO groups, we next examined the apoptosis of cardiomyocytes using annexin–PI staining. The results of flow cytometry indicated that the percentage of apoptotic cells was increased in the OVX+ISO group compared with those in the sham group and the OVX group. The percentage of apoptotic cardiomyocytes was significantly reduced in the OVX+ISO+E2b group, but not in the OVX+ISO+E2a group (Figure 5).
Figure 5: Detection of myocardial apoptosis by flow cytometry. Apoptosis was examined by staining with annexin-PI and detected by flow cytometry. A, apoptosis of cardiomyocyte in each group; B, apoptosis rate of cardiomyocyte. Each value represents mean ± S.E.M. n = 10 hearts in each group, *P < 0.05 versus Sham, #P < 0.05 versus OVX+ISO+Vehi.
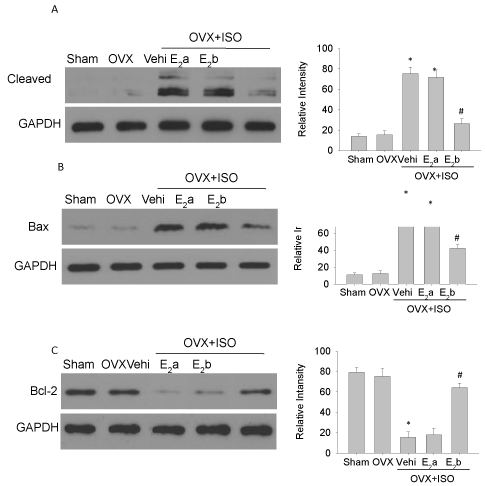
Estrogen replacement reduced the expression of caspase3, bcl-2, and bax following iso challenge
ISO challenge increased the expressions of cleaved caspase 3 and Bax proteins in the OVX group (Figure 6A and 6B). However, upon ISO challenge, the expression of Bcl-2 protein was significantly decreased in the OVX group (Figure 6C). Estrogen (40 μg/kg/d) treatment up-regulated the expression of Bcl-2 protein and down-regulated the expressions of Bax and cleaved caspase3 proteins (Figure 6).
Figure 6: Expression of apoptosis proteins in left ventricular myocytes. Western blot analyses were performed to examine the expressions of caspase 3 (A), Bax (B) and Bcl-2 (C). GAPDH was used as the internal control. Each experiment was repeated three times. Each value represents mean ± S.E.M. n = 10 hearts in each group, *P < 0.05 versus Sham, #P < 0.05 versus OVX+ISO+Vehi.
Discussion
Women have a lower incidence of cardiovascular diseases before menopause, but they lose this advantage at the onset of menopause. It is indicated that the female sex hormones, particularly estrogen, play a pivotal role in reducing the risk for cardiovascular diseases [17,18]. Also, the risk of cardiovascular disease increases with age in post-menopausal women partly due to decreased plasma estrogen levels. Administration of estrogen in female or male patients improves vasodilator responses and ameliorates myocardial ischemia [19,20]. It is suggested that estrogen may have direct protective effect on heart.
Not much information is available regarding changes in cardiomyocyte contraction and the role of apoptosis in isolated hearts in rats with heart injury. Our present study indicated that the estrogenic environment is important for cardioprotection. The replacement of estrogen increased single ventricular myocyte contraction and decreased the expressions of apoptosis proteins.
Results of our experiments indicated that estrogen replacement improved the dysfunction of cardiac contractility subjected to ISO. Simultaneously, we verified the experimental model of estrogen deficiency by reducing serum estrogen level and uterine weight. But estrogen replacement restored both serum estrogen level and uterine weight.
We also found that estrogen (40 μg/kg/d) effectively decreased the ISO-induced hypertrophy of cardiomyocytes. We demonstrated that estrogen ameliorated ISO-induced cardiomyocyte contractile function, as indicated by the significant increase in single myocyte shortening amplitude with a maximal effect at 40 μg/kg/d. These results suggested that estrogen at this concentration might improve contraction in injured myocytes subject to ISO.
Furthermore, we demonstrated with annexin V/PI staining that stimulation of ISO led to a significant increase in myocyte apoptosis, while estrogen replacement 4 weeks after ISO stimulation significantly decreased ISO-induced myocyte apoptosis. Cardiomyocyte apoptosis is one of the major contributors to the development of myocardial injury [21,22], which is related to the pathogenesis of myocardial apoptosis. Most studies have indicated that the Bcl-2 family proteins play a key role in regulating physiological and pathological apoptosis. These proteins consist of both cell death activators (eg. Bax and Bad) and cell death inhibitors (eg. Bcl-2, Bcl-X) [23,24]. The activators and inhibitors interact with each other to form a complex regulatory network. Cell survival or death is based on the relative ratio of pro-apoptotic proteins to anti-apoptotic proteins [25]. It has been identified that high ratio of Bax/Bcl-2 is associated with vulnerability to apoptotic activation [26]. In the present study, we confirmed that estrogen replacement up-regulated Bcl-2 expression and down-regulated Bax expression. These results also implied that estrogen replacement could protect cardiomyocytes from apoptosis induced by ISO injury. Therefore, we think estrogen replacement may exert its cardiomyocyte protective effect through up-regulating Bcl-2 protein expression while down-regulating Bax protein expression. What is the mechanism modulating Bcl-2 or Bax during ISO stimulation? At the present time, the exact mechanism by which estrogen modulates genes associated with apoptosis is not clear. However, the antioxidant against lipid peroxidation of estrogen may be responsible for this modulation [27], because many antioxidants affect genes related to apoptosis in cells under ISO stimulation.
Conclusion
Our experimental findings suggest that estrogen replacement plays a significant role in the regulation of cardiomyocyte contractile function and the promotion of anti-apoptotic effects following ISO injury. Estrogen replacement may have potential protective effects against ovarian hormone deficiency-induced alteration of cardiac contractile function in single ventricular myocyte. Our data also show that increased expression of Bcl-2 and decreased expressions of Bax and cleaved caspase3 play essential roles in the cardioprotective effect of estrogen. We demonstrate that estrogen replacement confers cardioprotection during ISO challenge, which is associated with improvement of cardiomyocyte contractile function and inhibition of apoptosis.
Acknowledgement
This work was supported in parts by grants from the National Natural Science Foundation for Young Scientists of China (No. 30901402 to YZ) and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China(No. 09KJD320008).
References
- Dubey RK, Imthurn B, Zacharia LC, Jackson EK. Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here. Hypertension. 2004; 44: 789–795.
- Regitz-Zagrosek V. Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov. 2006; 5: 425-438.
- Khalil RA. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmacol. 2013; 86: 1627-1642.
- Le TY, Ashton AW, Mardini M, Stanton PG, Funder JW, Handelsman DJ, et al. Role of androgens in sex differences in cardiac damage during myocardial infarction. Endocrinology. 2014; 155: 568-575.
- Brooks WW, Conrad CH. Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: structural and functional correlates. Comp Med. 2009; 59: 339-343.
- Zhuo XZ, Wu Y, Ni YJ, Liu JH, Gong M, Wang XH, Wei F, et al. Isoproterenol instigates cardiomyocyte apoptosis and heart failure via AMPK inactivation-mediated endoplasmic reticulum stress. Apoptosis. 2013;18: 800-810.
- Sun H, Zhou F, Wang Y, Zhang Y, Chang A, Chen Q. Effects of beta-adrenoceptors overexpression on cell survival are mediated by Bax/Bcl-2 pathway in rat cardiac myocytes. Pharmacology. 2006; 78: 98-104.
- Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res. 2003; 13: 385-391.
- Sahu BD, Anubolu H, Koneru M, Kumar JM, Kuncha M, Rachamalla SS, et al. Cardioprotective effect of embelin on isoproterenol-induced myocardial injury in rats: possible involvement of mitochondrial dysfunction and apoptosis. Life Sci. 2014; 107: 59-67.
- Fan Y, Wang C, Zhang Y, Hang P, Liu Y, Pan Z, et al. Genistein ameliorates adverse cardiac effects induced by arsenic trioxide through preventing cardiomyocytes apoptosis. Cellular Physiology and Biochemistry. 2013; 31: 80-91.
- Wu Q, Zhao Z, Sun H, Hao YL, Yan CD, Gu SL. Oestrogen changed cardiomyocyte contraction and beta-adrenoceptor expression in rat hearts subjected to ischaemia-reperfusion. Exp Physiol. 2008; 93: 1034-1043.
- Jiang X, Gao L, Zhang Y, Wang G, Liu Y, Yan C, et al. A comparison of the effects of ketamine, chloral hydrate and pentobarbital sodium anesthesia on isolated rat hearts and cardiomyocytes. Journal of Cardiovascular Medicine. 2011; 12: 732-735.
- Sun H, Chang AM, Zhang Y, Jiang XW. [Effects of overexpression of beta2-adrenoceptor on contraction in cardiac myocytes isolated from failure hearts of rats]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2007; 23: 410-414.
- Wu Q, Zhao Z, Sun H, Zhang Y, Hao YL, Sun YW. [The cardioprotection of estrogen on solated global myocardial ischemia/reperfusion injury in ovariectomized rats]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2009; 25: 355-360.
- Li JK, Liang HL, Li Z, Gu CH, Yi DH, Pei JM. Pigment epithelium-derived factor promotes Fas-induced cardiomyocyte apoptosis via its receptor phospholipase A2. Life Sci. 2014; 99: 18-23.
- Zhang Y, Liu G, Dull RO, Schwartz DE, Hu G. Autophagy in pulmonary macrophages mediates lung inflammatory injury viaNLRP3 inflammasome activation during mechanical ventilation. American Journal of Physiology-lung cellular and molecular physiology. 2014; 307: L173–L185.
- Renoux C, Dell'Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost. 2010; 8: 979-986.
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006; 113: 2425-2434.
- Emre A, Sahin S, Erzik C, Nurkalem Z, Oz D, Cirakoglu B, et al. Effect of hormone replacement therapy on plasma lipoproteins and apolipoproteins, endothelial function and myocardial perfusion in postmenopausal women with estrogen receptor-alpha IVS1-397 C/C genotype and established coronary artery disease. Cardiology. 2006; 106: 44-50.
- Lewandowski KC, Komorowski J, Mikhalidis DP, Bienkiewicz M, Tan BK, O’Callaghan CJ. Effects of hormone replacement therapy type and route ofadministration on plasma matrix metalloproteinases and their tissue inhibitors in postmenopausal women. Journal of Clinical Endocrinology & Metabolism. 2006; 91: 3123–3130.
- Chen HM, Hsu JH, Liou SF, Chen TJ, Chen LY, Chiu CC, et al. Baicalein, an active component of Scutellaria baicalensis Georgi, prevents lysophosphatidylcholine-induced cardiac injury by reducing reactive oxygen species production, calcium overload and apoptosis via MAPK pathways. BMC Complementary and Alternative Medicine. 2014; 14: 233.
- Zhang B, Zhou M, Li C, Zhou J, Li H, Zhu D, et al. MicroRNA-92a inhibition attenuates hypoxia/reoxygenation-induced myocardiocyte apoptosis by targeting Smad7. PLoS One. 2014; 9: e100298.
- Huang C, Gu H, Zhang W, Herrmann JL, Wang M. Testosterone-down-regulated Akt pathway during cardiac ischemia/reperfusion: a mechanism involving BAD, Bcl-2 and FOXO3a. J Surg Res. 2010; 164: e1-11.
- Deng YJ, Tan N, Zeng HK, Fu YH, Dong XL. [Effects of BNP preconditioning on myocardial cell apoptosis and expressions of bcl-2 and Bax during myocardial ischemia-reperfusion injury in rats]. Zhonghua Yi Xue Za Zhi. 2010; 90: 3431-3434.
- Penna C, Pasqua T, Amelio D, Perrelli MG, Angotti C, Tullio F, et al. Catestatin increases the expression of anti-apoptotic and pro-angiogenetic factors in the post-ischemic hypertrophied heart of SHR. PLoS One. 2014; 9: e102536.
- Zhang Y, Li H, Zhao G, Sun A, Zong NC, Li Z, et al. Hydrogen sulfide attenuates the recruitment of CD11b?Gr-1?myeloid cells and regulates Bax/Bcl-2 signaling in myocardial ischemia injury. Sci Rep. 2014; 4: 4774.
- Wang F, Xiao J, Shen Y, Yao F, Chen Y. Estrogen protects cardiomyocytes against lipopolysaccharide by inhibiting autophagy. Mol Med Rep. 2014; 10: 1509-1512.