Review Article
Austin J Vasc Med. 2014;1(2): 9.
Angiogenic Integrity Preservation/Restoration: Hit for Vascular Endothelial Growth Factor Prone Therapeutic Coronary Revascularization
Anita A. Mehta* and Kiranj K Chaudagar
Department of Pharmacology, L. M. College of Pharmacy, India
*Corresponding author: Anita A. Mehta, Department of Pharmacology, L. M. College of Pharmacy, Opp Gujarat University, Navarangpura, Ahmedabad-380 009, India
Received: November 11, 2014; Accepted: December 04, 2014; Published: December 05, 2014
Abstract
Various mediators such as acetylcholine, bradykinin, and Vascular Endothelial Growth Factor (VEGF) are widely known for endothelial cells dependent Nitric Oxide (NO) release. However, the VEGF-A165/VEGFR-2 binding stimulated NO is associated with induction of angiogenesis. This axis is disturbed in coronary endothelial cells of heart failure patients, and VEGF prone therapeutic revascularization establishes the integrity of such axis in preclinical studies but not in clinical trials. The present review discusses possible approaches that can be useful for angiogenic integrity restoration, and preservation to succeed the clinical trials of VEGF.
Keywords: Vascular Endothelial Growth Factor; Angiogenic integrity; Therapeutic revascularization; Nitric oxide; Heart failure
Introduction
Heart Failure (HF) is a prominent cause of mortality and morbidity worldwide. It is characterized by loss of blood pumping capacity of heart to compensate the demand of body tissue. Approximately, 5.7 million people have been suffering from HF in the US. It is predicated that half of these patient to be die within 5 years of diagnosis [1]. General Practice Research Database of UK suggested that 800,000 people are suffering from HF. It is more prevalent in male than female, and elders than younger [2].The high prevalence rate of HF have been predicted in developing countries India, however, there is no specific epidemiological data due to lack of surveillance program [3,4]. It is exceptionally more prevalent in Indian young age population than other developed countries [5]. Ultimately, HF is a threatening issue for both developed and developing countries.
Angiogenesis is a process of new blood vessel formation from pre-existing endothelial cells. It involves simultaneous coordination of parent vessel vasodilation, basement membrane degradation-reformation, endothelial migration-proliferation, lumen formation, loop development, and pericytes incorporation [6]. The cardiac contractility is maintained in the acute phase HF by cardiac hypertrophy and angiogenesis [7].The co-ordination of such hypertrophy and angiogenesis require to fulfill the oxygen demand of heart [8]. The disturbance of coordination due to loss of angiogenesis transits cardiac insults into heart failure in late phase [7,9,10]. This is indicated by absence of coronary collateralization (anastomotic connections between portions of the same coronary artery and between different coronary arteries without an intervening capillary bed which is developed by angiogenesis) in HF patients [11,12]. The collateral vessels formation is a phenomenon of compensatory physiological revascularization that preserves the heart in late phase of chronic injury. However, it is not fully combat the worsening inside the heart. Therefore, there is need of therapeutic revascularization (cells or growth factor induced coronary angiogenesis).
Many angiogenesis inducers such as fibroblast growth factor (FGF), Transforming Growth Factors (TGF) [13], angiopoietin [14], Hepatocyte Growth Factor (HGF) [15], interleukin-8 (IL-8) [16], angiogenin [17] and Vascular Endothelial Growth Factor (VEGF) have been reported [18]. One of them, VEGF is only an endothelial cell selective potent mitogen (ED50=2-10pM). It stimulates the proliferation of micro-macrovascular endothelial cells (EC) of arteries, veins, and lymphatic vessels [19-21]. VEGF possess dose (10-100ng/mL) dependent anti-apoptotic effects on endothelial cells [22], and promote survival rate in serum starved ECs [23]. It require for survival of EC during neonatal life until vessels are rich in the supporting cells such as pericyte [24,25]. It is a key player for tumor induced angiogenesis. VEGF is also known as a Vascular Permeability Factor (VPF), and increase permeability at concentration less than 1nmol/L in Miles assay [26]. It involve in tumor dependent ascites [27,28]. Other than EC proliferation, survival, it also auto-regulates the response of cells for the angiogenesis. The angiogenic integrity of Endothelial Cells (EC) means proliferative, migratory, adhesive, organization response of EC to VEGF.
The hypertrophy induced coronary angiogenesis is mediated through activation of mammalian target of rapamycin (mTOR) dependent myocardial VEGF expression. The decoy of VEGF receptor or loss of angiogenic integrity decreases capillary density, induces hypoxia, contractile dysfunction, interstitial fibrosis, maladaptive cardiac hypertrophy, and heart failure [7,9]. The angiogenic integrity of EC is modulated by various phenomena such as hypercholesterolemia, diabetes, oxidative stress, shear stress, drug treatment and preconditioning [29,30]. Various preclinical experiments of VEGF induced therapeutic angiogenesis has been indicated promising data of increase in cardiac contractility, collateralization and perfusion volume. However, the clinical trials are failure to show benefit similar to preclinical studies. Because preclinical studies are well control experiments on normal or pathology-possessing animals whereas clinical trials include the patients with disturbed EC due to more than one disease, and they are also vary in VEGF responsiveness. This review focuses VEGF signaling, angiogenic integrity, outcome of VEGF prone therapeutic angiogenesis, and approaches for the preservation/restoration of angiogenic integrity to fill the gap between preclinical and clinical outcomes.
VEGF family
Molecular cloning revealed first time that multiple forms of VEGF such as 206, 186, 165 and 121 (VEGF206, VEGF186, VEGF165, and VEGF121) arose due to alternative splicing of RNA transcript in vascular smooth muscle cells [31]. Transfection experiment on human embryonic kidney 293 cells showed that VEGF189, VEGF206 was predominately cell associated and only very poorly secreted whereas VEGF121 and VEGF165 were efficiently exported from the cell. Vascular permeability activity was detected in the medium of 293 cells transfected with all four forms of VEGF but endothelial cell mitogenic activity was present only with VEGF121 and VEGF165 [32]. Immunostaining of VEGF isoform in transfected human embryonic kidney CEN4 cells revealed that VEGF189 or VEGF206 localized in the subepithelial Extracellular Matrix (ECM). ECM bound VEGF released into a soluble and bioactive form by heparin or plasmin that possessed molecular masses consistent with the intact polypeptides. This study also showed that VEGF165 possessed intermediate ECM binding properties [33]. Plasmin mediated cleavage of VEGF generated two smaller fragments, an amino terminal homodimeric protein containing receptor binding determinants and a carboxyl-terminal polypeptide which bound heparin. The various isoforms of VEGF (165, 165/110, 110 and 121) bound soluble kinase domain region receptor with similar affinity (approximately 30pM) whereas bound soluble Flt-1 receptor with different affinities (10, 30, 120, and 200pM, respectively). VEGF110 and VEGF121 showed 100 fold less endothelial cell mitogenic potencies as compared to VEGF165 [34]. VEGF164 and VEGF188 lacking mice possessed defect in myocardial angiogenesis but not due to VEGF120 [35]. Thus, VEGF165 is a most potent angiogenesis inducing isoform of VEGF family. Currently, the VEGF family has seven members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF. VEGF-A, VEGF-B, VEGF-C and VEGF-D are found in human. VEGF121, 165, 189 and 206 are sub members of VEGF-A [36].
VEGF signalling and angiogenic integrity
VEGF interact with tyrosine kinase receptor and coreceptor on the endothelial cells. VEGF receptor-1 (VEGFR-1/ Fms like tyrosine kinase-1-Flt-1), VEGFR-2 (Kinase Domain Containing Receptor (KDR)/ Fetal liver kinase-1 (Flk-1)) and VEGFR-3 (Flt-4) are receptors and Neuropilins-1 (NP-1) and NP-2 are coreceptors [37]. VEGFR-1 and VEGFR-2 deficient mice died in utero between 8.5 and 9.5 day due to vascular disorganization [38,39]. In VEGFR-1 deficient mice, vascular disorganization was related to excessive angioblast activity whereas proliferating endothelial cells were present [40]. In VEGFR-2 deficient mice, vascular disorganization was related to loss of blood islands and proliferating endothelial cells [38]. Flt-1 tyrosine kinase domain deficient mice showed normal development of vasculature [41]. VEGFR-2 selectively expressed in vascular endothelial cells and possessed lower binding affinity (Kd= 400-800pM) to VEGF-A as compared to VEGFR-1 [42,43]. Binding of VEGF to VEGFR-2 resulted in activation of phosphoinositide 3 kinase (PI3K)/Akt-dependent integrins such as αvβ3, αvβ5, α5β1 and α2β1, activation of Protein Kinase C (PKC)-dependent-Ras-independent Raf/ MEK/ Mitogen Activated Protein Kinase (MAPK) and induction of angiogenesis cascade such as endothelial cell proliferation, migration, adhesion and tube formation [44-47]. VEGFR-3 selectively expressed on lymphatic endothelial cells and bound only with VEGF-C and VEGF-D but not with other forms of VEGF [48,49]. Defect in VEGF-C dependent VEGFR-3 activation caused severe embryonic lethality due to loss of embryonic lymphatic system and embryonic tissue oedema [50]. Neuropilin (NP) coexpressed with VEGFR-2 in endothelial cells, enhanced the binding of VEGF165 to VEGFR-2 and increased effectiveness of VEGF165-VEGFR-2-mediated signal transduction [51]. Therefore, VEGF165-VEGFR-2 mediated signalling is a determinant of angiogenic integrity.
VEGF prone therapeutic revascularization
The angiogenesis appears at the border zone of the infracted area for 3 day immediately after insults. After 7 days, it gradually disappears in various experiment model of ischemia reperfusion injury. Early phase angiogenesis is regulated by VEGF-A and VEGFR level which are downregulated in the late phase. The VEGF-A/VEGFR expression is not affected in the noninfarcted area [10]. However, the VEGF liposomes showed improvement in systolic function and increase myocardial perfusion due to 21% increase in collateralization and 74% increase in number of perfused vessels in the MI region [52]. Other preclinical studies has reported the similar results due to VEGF therapy [53,54]. According to such preclinical reports, VEGF administration is itself restore and preserve the angiogenic integrity.
In angina patients, the intramyocardial administration of plasmid based VEGF165 (phVEGF165) gene increased plasma VEGF level that returned to baseline on 90th day. The 13 out of 17 patients were angina free at the end of 6 months. There was no report on adverse events related to gene delivery [55]. The 14 coronary artery disease patients (who were not candidates for mechanical revascularization) treated with low-dose (0.005 and 0.017mg/kg, intracoronary, for 20min) and high-dose (0.05 and 0.167mg/kg, intracoronary, for 20min) of recombinant human VEGF (rhVEGF) showed improvement in myocardial perfusion dose dependently under stress and rest conditions visual analysis using Single Photon Emission CT (SPECT) at 60th day after treatment of rhVEGF. This visual improvement was not significant as per statistical analysis [56]. In the first placebo-controlled phase-II trial of VEGF (Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis-VIVA), high-dose rhVEGF (50ng/kg/min, intracoronary infusion on 0day for 20min, followed by 4hr intravenous infusions on 3, 6, and 9day) significantly ameliorated angina class on 120day but did not show significant increase in ETT time and angina frequency. Whereas low-dose treatment of rhVEGF (17ng/kg/min) did not show any significant benefit as compared to placebo [57]. Direct intramyocardial injections of adenovirus mediated VEGF121 (AdVEGF121) and plasmid VEGF165 DNA in anginal patients with optimal medical therapy showed site specific delivery of gene but did not show significant improvement in treadmill testing as compared to patients treated with only medical [58,59]. Ultimately, the failure of clinical trials on VEGF induced cardiac angiogenesis compared to preclinical studies is related to loss of angiogenic integrity restoration and preservation by VEGF. Therefore, there is need of approaches that fulfills the gap of VEGF prone therapeutic revascularization in preclinical, and clinical studies by restoration and preservation of angiogenic integrity.
Angiogenic integrity preservation/restoration
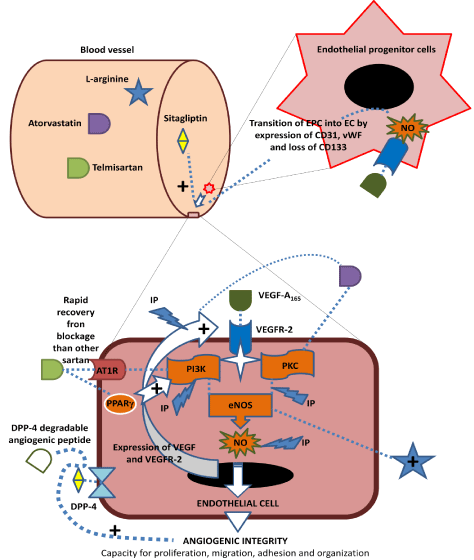
Endothelial cells, cover luminal side of vessels, have been prominently reported for regulating vascular homeostasis [60]. The angiogenic integrity of EC is autoregulated by VEGF/KDR/ PKC+PI3K/eNOS/NO pathway [61]. There are mainly three approaches, such as cell therapy, drug treatment, and food supplementation for recoupling or preservation of VEGF dependent nitric oxide releasing axis in endothelial cells (Figure 1).
Figure 1: Mechanisms for the angiogenic integrity restoration, and preservation. The dash line and arrow indicates direct or indirect connection. The plus sign suggests stimulation of the target.
Cell therapy
The circulating mononuclear cells those possess endothelial cell–like phenotype and have capacity to incorporate into capillaries [62], are known as Endothelial Progenitor Cells (EPC) [63]. There also derive from bone marrow. Transplantation of bone marrow EPC restored endothelial lining in the Dacron graft of adult dogs thoracic aorta [64]. Intracoronary administration of autologous bone-marrow-cells accelerated improvement in left-ventricular systolic function in patients after acute myocardial infarction [65]. Autologous bone marrow–derived mononuclear cell injection in patients with ischemia reduced anginal symptoms and increased left ventricular functions, via improved myocardial perfusion [66]. After primary Percutaneous Intervention (PCI), intracoronary infusion of autologous mononuclear bone marrow cells or peripheral mononuclear blood cells showed beneficial effects on cardiac functioning and prevented heart failure related morbidity and mortality in patients [67]. In long term study on acute myocardial infarct patients, EPC administration showed modest and long standing increased in left ventricular performance and myocardial revascularization [68- 70]. After transplantation of EPC, the improvement in ventricular functioning is associated with modulation in functioning of border zone of infarct area without change in infarct size and remodeled tissue [71,72]. EPC administration therapy do not produce any major adverse events such as arrhythmia and inflammation [73]. Due to reendothelialization and angiogenic integrity stabilizing nature, EPC therapy has been proved for partial restoration of the cardiac functioning in the damage area. The combination of EPC therapy and VEGF prone therapeutic revascularization might be beneficial for the total restoration of cardiomyocytes networking.
Ischemia preconditioning
Ischemia Preconditioning (IP) is an inherent property of actively working cells that activate by brief repeated period of ischemia-reperfusion. It is protective response because it decreases the rate of ATP degradation, catabolite accumulation and infarct size and protects heart against ischemia reperfusion induced damage [74]. This also reduces post-ischemic ventricular dysfunction and decreases incidence of arrhythmias [75]. IP has been widely reported for stimulation of NF-kB mediated PI3K expression and activation [76], increasing nuclear transportation of PKC, phosphorylation of Akt, synthesis of NO [77], stimulation of NO-dependent ATP dependent potassium (KATP) channel functioning, increasing superoxide production and cytosolic PKC activation [78-80]. These pathways ultimately lead to increase in DNA binding of angiogenic transcription factors such as signal transducer and activator of transcription 3 (Stat3), paired box protein-5 (Pax5), nuclear factor-kB (NF-kB), transcription factor-IID (TFIID), specificity factor 1 (SP1), [81], and stimulate the expression of survivin, B-cell lymphoma 2 (Bcl2), KDR and VEGF [82,83]. In our previous experiments, ischemia preconditioning improved the angiogenic phenotype of coronary EC in normal and diabetic conditions via VEGF sensitization [30]. Therefore, IP is an inherent mechanism of EC for preservation of angiogenic integrity, and might be promoting VEGF prone therapeutic angiogenesis. The clinical evidence of IP is not yet clear whereas few reports indicated the chemical preconditioning by resveratrol, and adenosine. Adenosine has reduced the degree of vascular injury after ischemia and reperfusion by inhibition of oxygen free radical release and preservation endothelial cell function and microvascular perfusion [84]. Thus, such agents can be useful in combination with VEGF to promote therapeutic revascularization.
RAAS inhibitors
Renin-Angiotensin-Aldosterone System (RAAS) is a crucial regulator of blood pressure and vascular activity. The over activation of RAAS is associated with development of hypertension, atherosclerosis, vascular remodeling, and cardiac failure [85,86]. Angiotensin II and aldosterone are an important mediators of this system that causes vasoconstriction, increase vascular hypertrophy, induce endothelial and vascular smooth muscle cell proliferation, migration, increase vascular oxidative stress, and increase extracellular matrix deposition [86-91]. This suggests the cardiovasculoprotective role of RAAS inhibitors.
Candesartan is an angiotensin type 1 receptor (AT1R) blocker (ARB). It inhibited VEGF mediated coronary angiogenesis via decreasing expression of VEGF165 and VEGFR-2 in normal heart [92], Contrast to this, It increased VEGF expression and capillary density in infracted rat heart due to its anti-fibrotic actions [93]. Similar to candesartan, telmisartan has increased VEGF expression in EC by PI3K activation whereas decreased its expression in diabetic mice by inhibiting AT1R [94,95]. Spironolactone is a mineralocorticoid receptor antagonist that attenuates angiotensin induced aldosterone mediated abnormalities [96]. Aldosterone increased VEGF164 and VEGF120 in cell line experiment that abolished by spironolactone [97]. Hyperaldosteronism impaired the function and numbers of EPC by downregulating VEGF and the VEGF receptor in rats. Spironolactone antagonized these effects [98]. From these data, it is clear that the Angiotensin Converting Enzyme Inhibitor (ACEI), angiotensin type-1-receptor blocker (ARB), and aldosterone antagonist exerts conditional and tissues specific effects on VEGF expression.
Enalapril, ACEI, inhibited VEGF expression, and downregulated expression of VEGF receptors in various in vivo studies [92,99,100]. Our experiments indicated the inhibitory actions of enalapril on VEGF induced angiogenesis in CAM assay by novel antiangiogenic mechanism that independent on condition and cell specificity (unpublished data). Telmisartan has ARB, peroxisome proliferation activated receptor γ (PPARγ) agonistic, anti-inflammatory and antioxidant actions [101,102]. Due to Peroxisome Proliferator- Activated Receptor Gamma (PPARγ) agonist action, telmisartan has been reported for increase in vascular endothelial growth factor receptor 2 (VEGFR2) tyrosine phosphorylation [103]. Previously, we reported the VEGF independent and VEGF induced angiogenic integrity of coronary EC stimulatory actions of telmisartan but not olmesartan [104]. Because olmesartan have no affinity for PPARγ [101], and possess prolong inhibitory actions on Angiotensin Type- 1-Receptor (AT1R). The half of telmisartan dissociates from the AT1R within 29min, and restores the maximum response within 24min, whereas these values for olmesartan are 72min and 76min, respectively. This difference is related to stabilization of the carboxyl group in imidazole core of the sartan [105]. Therefore, telmisartan is an angiogenic integrity preserving and restoring agent for VEGF due to rapid rate of AT1R recovery and PPARγ agonistic activity but not olmesartan.
Spironolactone has shown inhibitory actions on proliferation and neovascularisation in Human Umbilical Vein Endothelial Cells (HUVEC) and fibrin gel chamber assay, respectively. This effect is independent on antimineralocorticoid activity and not reversed by VEGF or Fibroblast Growth Factor (FGF) [106]. Spironolactone also inhibited VEGF expression in neonatal rat kidney [107], and in Chorioallantoic Membrane (CAM) assay. Thus, it is prominently used for the treatment of cardiac remodeling but not for angiogenic integrity restoration/preservation.
HMG-CO-A reductase inhibitors
The 3-Hydroxy Methyl Glutaryl Coenzyme A (HMG-CO-A) reductase inhibitors (statins) are widely known for their lipid lowering effects. Fluvastatin has significantly increased myocardial perfusion in ischemic segments of hypercholesterolemic patients on long term treatment [108]. Simvastatin therapy increased acetylcholine induced vasodilation in subjects with higher cholesterol levels [109]. Apart from these, atorvastatin improved endothelial function within 24 hours of administration in normocholesterolemic young men [110]. It also improved endothelium dependent vasodilatation in type 1 and type 2 diabetic patients [111,112]. Atorvastatin improved endothelial regeneration capacity in coronary artery disease patients [113]. In normocholesterolemic rabbits, it promoted angiogenesis and collateral vessels development in ischemic area [114]. Atorvastatin therapy increased inflammatory angiogenesis in hyperlipidemic apolipoprotein E–deficient mice without reducing lipid levels [115]. Statin therapy increased circulating EPC in mice [116] and stable coronary artery disease patients [117]. They also increased mobilization of EPC, and promoted corneal neovascularisation in normocholesterolemic mice [118]. Recently, atorvastatin has reported to decrease circulating CXCL12, an angiogenic chemokine, level in coronary artery disease, congestive heart failure and myocardial infarction patients without modulation in lipid profile [119]. In venous-specific zebrafish model, inhibition of HMG-CO-A reductase expression attenuated development of new blood vessels by inhibition of geranylgeranyl transferase [120]. Due to this mechanism, statins have angiogenic integrity restoring and preserving pleiotropic actions.
Other these effects, statin stimulates hypoxic angiogenesis via upregulation of hypoxia inducible factor-1α (HIF-1α), VEGF and Akt. They also inhibit tumor necrosis factor-α (TNF-α) induced angiogenesis via downregulation of HIF-1α, VEGF and Akt [121]. In human human vascular smooth muscle cells and microvascular endothelial cells, atorvastatin, simvastatin and lovastatin reduced basal and cytokine-, nitric oxide- or Lysophosphatidyl Choline (LPC)-induced VEGF synthesis but upregulated VEGF generation in HUVEC [122]. Atorvastatin possesses angiogenic activity at 10 nM whereas antiangiogenic activity at 10 μM [115]. Atorvastatin (10μM) attenuated VEGF induced ACE upregulation in HUVEC [123]. Atorvastatin increased expression of VEGF and VEGF receptor in mouse brain EC and stroke model [124]. In Coronary Artery Disease (CAD) patients, atorvastatin decreased serum VEGF level but pravastatin did not [125]. Such data suggested that the actions of statins on angiogenesis is affected by type of statin, concentration of statin and type of cells or tissue [126]. We has revealed the coronary angiogenic integrity restoring actions of atorvastatin at low dose (similar to ~10mg/day in human) and inhibitory effects at high dose (similar to ~80mg/day in human) in rats whereas pravastatin inhibited VEGF induced angiogenesis at low and high dose in CAM assay [127]. Therefore, low dose of atorvastatin is preferable in heart failure patients those are prime candidate for VEGF prone therapeutic angiogenesis.
L-arginine supplementation
L-arginine, is a substrate of eNOS for synthesis of NO, and biotransformed into citrulline. The supplementation of L-arginine has improved endothelial cell functioning, flow mediated vasodilation, acetylcholine mediated vasodilaton and microvascular reactivity in preclinical and clinical studies. This effect of L-arginine is dependent on restoration of nitric oxide releasing axis in endothelial cells [128- 132]. Also, a meta-analysis indicated that L-arginine improved Flow Mediated Vasodilation (FMD) in individuals who possessed lower baseline value of FMD [133]. Hypercholesterolemic pigs did not respond to VEGF based angiogenic therapy because presence of defective endothelial cells. L-arginine supplementation showed improvement in myocardial perfusion due to presence of VEGF responding functional endothelial cells in these hypercholesterolemic pigs. This effect of L-arginine associated with increased vascular endothelial growth factor, endothelial nitric oxide synthase, and Akt protein levels in the ischemic area [134]. Supplementation of L-arginine (6g/day, orally) in VEGF165 plasmid DNA (200μg) treated coronary artery disease patients improved myocardial perfusion and contractility at 12wk as compared to placebo group [135]. Therefore, L-arginine supplementation is a key therapy for maintaining angiogenic integrity, and outcome of VEGF prone therapeutic revascularization.
DPP-4 inhibitors
DPP-4 (dipeptidylpeptidase-4) inhibitors are widely reported for treatment of diabetes via increasing incretin dependent insulin release [136]. They also have cardioprotective actions that are independent on antidiabetic effects [137]. In the Acute Coronary Syndrome Israeli Survey (ACSIS), sitagliptin (DPP4i) pretreatment significantly decreased in-hospital complication rate (post MI angina, re-infarction, pulmonary edema, infections, acute renal failure and better KILLIP class), rates of 30day Major Adverse Cardiovascular Events (MACE) and hospital stay in diabetic patients [138]. Similar cardioprotection has been reported for other DPP-4 inhibitors [139]. In vivo treatment of sitagliptin increased eNOS expression in spontaneously hypertensive rat renal arteries [140]. Sitagliptin improved flow mediated dilation in type 2 diabetic patients without cardiovascular disease [141]. Sitagliptin increased EPC in type 2 diabetic patients by upregulation of stromal cell derived factor-1α (SDF-1α) [142]. In our experiments, it did not stimulate or inhibit the VEGF mediated angiogenesis in CAM assay (unpublished data). Thus, sitagliptin may facilitate the VEGF prone therapeutic angiogenesis by activating EPC dependent reendothelialisation, restoring endothelial NO release, and angiogenic integrity in diabetic patients.
Conclusion
The angiogenic integrity of coronary EC is a valuable property for facilitating VEGF prone therapeutic revascularization in heart failure patients. This signalling includes the downstream mediators of VEGF such as KDR, PI3K, PKC, eNOS and NO for proliferation, migration, adhesion and reorganization of EC. Such mediators are modulated by various factors such as drugs, pathological conditions, exercise, and food. In the heart failure patients who are candidates for therapeutic revascularization, the pretreatment or co-administration of angiogenic integrity preserving or restoring therapy such as EPC, DPP4i, telmisartan, atorvastatin, preconditioning and L-arginine with VEGF may leads to increase in collateralization, microvessels density, and cardiac output whereas others therapy such as pravastatin, olmesartan, enalapril, and spironolactone those inhibit remodeling, attenuate the VEGF prone coronary angiogenesis, and damaged the heart on long term.
References
- Roger V, Go A, Lloyd-Jones D, Benjamin E, Berry J, Borden W, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012; 125: 2-220.
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary heart disease statistics A compendium of health statistics 2012 edition. British Heart Foundation: London. 2012: 1-211.
- Vakil RJ. A statistical study of 1281 cases of congestive cardiac failure or myocardial insufficiency in India. Indian Physician. 1949; 8: 281-289.
- Huffman MD, Prabhakaran D. Heart failure: epidemiology and prevention in India. Natl Med J India. 2010; 23: 283-288.
- Reddy S, Bahl A, Talwar KK. Congestive heart failure in Indians: how do we improve diagnosis & management? Indian J Med Res. 2010; 132: 549-560.
- Moulton K, Folkman J. Chien K. Molecular Basis of Cardiovascular Disease. Saunders WB. 1998: 93–409.
- Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005; 115: 2108-2118.
- De Boer R, Pinto Y, Van Veldhuisen D. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Microcirculation. 2003; 10: 113-126.
- Gavin JB, Maxwell L, Edgar SG. Microvascular involvement in cardiac pathology. J Mol Cell Cardiol. 1998; 30: 2531-2540.
- Zhao T, Zhao W, Chen Y, Ahokas RA, Sun Y. Vascular endothelial growth factor (VEGF)-A: role on cardiac angiogenesis following myocardial infarction. Microvasc Res. 2010; 80: 188-194.
- Sasayama S, Fujita M. Recent insights into coronary collateral circulation. Circulation. 1992; 85: 1197-1204.
- Baroldi G, Mantero O, Scomazzoni G. The collaterals of the coronary arteries in normal and pathologic hearts. Circ Res. 1956; 4: 223-229.
- Grundmann S, van Royen N, Pasterkamp G, Gonzalez N, Tijsma EJ, Piek JJ, et al. A new intra-arterial delivery platform for pro-arteriogenic compounds to stimulate collateral artery growth via transforming growth factor-beta1 release. J Am Coll Cardiol. 2007; 50: 351-358.
- Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003; 12: 933-941.
- Morishita R, Aoki M, Hashiya N, Yamasaki K, Kurinami H, Shimizu S, et al. Therapeutic angiogenesis using hepatocyte growth factor (HGF). Curr Gene Ther. 2004; 4: 199-206.
- Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011; 128: 2038-2049.
- Shestenko O, Nikonov S, Mertvetsov N. Angiogenin and Its Functions in Angiogenesis. Molecular Biology. 2001; 35: 294-314.
- Renault MA, Losordo DW. Therapeutic myocardial angiogenesis. Microvasc Res. 2007; 74: 159-171.
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18: 4-25.
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989; 246: 1306-1309.
- Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002; 196: 1497-1506.
- Gerber H, Dixit V, Ferrara N. Vascular Endothelial Growth Factor Induces Expression of the Antiapoptotic Proteins Bcl-2 and A1 in Vascular Endothelial Cells. The Journal of Biological Chemistry. 1998; 273: 13313–13316.
- Gerber H, McMurtrey A, Kowalski J, Yan M, Key B, Dixit V, et al. Vascular Endothelial Growth Factor Regulates Endothelial Cell Survival through the Phosphatidylinositol 3*-Kinase/Akt Signal Transduction Pathway. The Journal of Biological Chemistry. 1998; 273: 30336–30343.
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD. VEGF is required for growth and survival in neonatal mice. Development. 1999; 126: 1149-1159.
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999; 103: 159-165.
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989; 246: 1309-1312.
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983; 219: 983-985.
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995; 146: 1029-1039.
- Goligorsky MS. Endothelial cell dysfunction: can't live with it, how to live without it. Am J Physiol Renal Physiol. 2005; 288: F871-880.
- Chaudagar K, Mehta A. Effect of ischemia reperfusion injury and ischemia preconditioning on angiogenic responsiveness of coronary endothelial cells in normal and streptozotocin (STZ) induced diabetic rats. World Journal of Pharmacy and Pharmaceutical Sciences. 2013; 2: 5329-5347.
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991; 266: 11947-11954.
- Houck K, Ferrara N, Winer J, Cachianes G, Li B, Leung D. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Molecular Endocrinology. 1991; 5: 1806-1814.
- Park J, Keller H, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993; 4: 1317-1326.
- Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, et al. The carboxyl-terminal domain (111-165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996; 271: 7788-7795.
- Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999; 5: 495-502.
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004; 56: 549-580.
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25: 581-611.
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995; 376: 62-66.
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995; 376: 66-70.
- Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999; 126: 3015-3025.
- Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998; 95: 9349-9354.
- Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993; 90: 7533-7537.
- Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Møller NP, Risau W, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993; 72: 835-846.
- Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000; 6: 851-860.
- Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999; 18: 2221–2230.
- Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001; 20: 2768-2778.
- Wu L, Mayo L, Dunbar J, Kessler K, Baerwald M, Jaffe E, et al. Utilization of distinct signalling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. The Journal of Biological Chemistry. 2000; 275: 5096–6103.
- Partanen T, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, et al. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB Journal. 2000; 14: 2087–2096.
- Pajusola K, Aprelikova O, Korhonen J, Kaipainen A, Pertovaara L, Alitalo R, et al. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992; 52: 5738-5743.
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004; 5: 74-80.
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998; 92: 735-745.
- Scott RC, Rosano JM, Ivanov Z, Wang B, Chong PL, Issekutz AC, et al. Targeting VEGF-encapsulated immunoliposomes to MI heart improves vascularity and cardiac function. FASEB J. 2009; 23: 3361-3367.
- Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, et al. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994; 89: 2183-2189.
- Lopez JJ, Laham RJ, Stamler A, Pearlman JD, Bunting S, Kaplan A, et al. VEGF administration in chronic myocardial ischemia in pigs. Cardiovasc Res. 1998; 40: 272-281.
- Symes J, Losordo D, Vale P, Lathi K, Esakof D, Mayskiy M, et al. Gene Therapy With Vascular Endothelial Growth Factor for Inoperable Coronary Artery Disease. The Annal of Thoracic Surgery. 1999; 68: 830 –837.
- Hendel RC, Henry T, Singh K, Isner J, Kereiakes D, Giordano F, et al. Effect of Intracoronary Recombinant Human Vascular Endothelial Growth Factor on Myocardial Perfusion: Evidence for a Dose-Dependent Effect. Circulation. 2000; 101: 118-121.
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003; 107: 1359-1365.
- Stewart D, Hilton J, Arnold J, Gregoire J, Rivard A, Archer S, et al. Angiogenic gene therapy in patients with non revascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF121 (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006; 13: 1503–1511.
- Stewart D, Kutryk M, Fitchett D, Freeman M, Camack N, Su Y, et al. VEGF Gene Therapy Fails to Improve Perfusion of Ischemic Myocardium in Patients With Advanced Coronary Disease: Results of the NORTHERN Trial. Molecular Therapy. 2009; 17: 1109–1115.
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003; 314: 15-23.
- Nakagawa T. Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. American Journal of Physiololgy - Renal Physiology. 2007; 292: F1665–F1672.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275: 964-967.
- Luttun A, Carmeliet G, Carmeliet P. Vascular progenitors: from biology to treatment. Trends Cardiovasc Med. 2002; 12: 88-96.
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998; 92: 362-367.
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004; 364: 141-148.
- Beeres SL, Bax JJ, Kaandorp TA, Zeppenfeld K, Lamb HJ, Dibbets-Schneider P, et al. Usefulness of intramyocardial injection of autologous bone marrow-derived mononuclear cells in patients with severe angina pectoris and stress-induced myocardial ischemia. Am J Cardiol. 2006; 97: 1326-1331.
- Hirsch A, Nijveldt R, Vander Vleuten P, Biemond B, Doevendans P, Van Rossum A, et al. Intracoronary infusion of autologous mononuclear bone marrow cells or peripheral mononuclear blood cells after primary percutaneous coronary intervention: Rationale and design of the HEBE trial — A prospective, multicenter, randomized trial. Am Heart J. 2006; 152: 434-441.
- Suárez de Lezo J, Herrera C, Romero M, Pan M, Jiménez R, Carmona D, et al. Functional Recovery Following Intracoronary Infusion of Autologous Mononuclear Bone Marrow Cells in Patients With Chronic Anterior Myocardial Infarction and Severely Depressed Ventricular Function. Rev Esp Cardiol. 2010; 63: 1127-1135.
- Yousef M, Schannwell C, Köstering M, Zeus T, Brehm M, Strauer B. The BALANCE Study Clinical Benefit and Long-Term Outcome After Intracoronary Autologous Bone Marrow Cell Transplantation in Patients With Acute Myocardial Infarction. J Am Coll Cardiol. 2009; 53: 2262–2269.
- Ye Z, Zhang B, Zhao X, Qin Y, Wu H, Cao J, et al. Intracoronary infusion of bone marrow-derived mononuclear cells contributes to longstanding improvements of left ventricular performance and remodelling after acute myocardial infarction: A meta-analysis. Heart, Lung and Circulation. 2012; 21: 725–33.
- Müller-Ehmsen J, Tossios P, Schmidt M, Scheid C, Unal N, Bovenschulte H, et al. Transmurality of scar influences the effect of a hybrid-intervention with autologous bone marrow cell injection and aortocoronary bypass surgery (MNC/CABG) in patients after myocardial infarction. Int J Cardiol. 2012; 156: 303-308.
- Zhang SN, Sun AJ, Ge JB, Yao K, Huang ZY, Wang KQ, et al. Intracoronary autologous bone marrow stem cells transfer for patients with acute myocardial infarction: a meta-analysis of randomised controlled trials. Int J Cardiol. 2009; 136: 178-185.
- Strauer BE, Steinhoff G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol. 2011; 58: 1095-1104.
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986; 74: 1124-1136.
- Das DK, Moraru II, Maulik N, Engelman RM. Gene expression during myocardial adaptation to ischemia and reperfusion. Ann N Y Acad Sci. 1994; 723: 292-307.
- Sasaki H, Ray P, Zhu L, Otani H, Asahara T, Maulik N. Hypoxia/Reoxygenation Promotes Myocardial Angiogenesis via an NFkB-dependent Mechanism in a Rat Model of Chronic Myocardial Infarction. J Mol Cell Cardiol. 2001; 33: 283–294.
- Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000; 87: 309-315.
- Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005; 97: 329-336.
- Krieg T, Landsberger M, Alexeyev MF, Felix SB, Cohen MV, Downey JM. Activation of Akt is essential for acetylcholine to trigger generation of oxygen free radicals. Cardiovasc Res. 2003; 58: 196-202.
- Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, et al. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res. 2000; 87: 460-466.
- Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, et al. PKCepsilon modulates NF-kappaB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000; 279: H1679-1689.
- Kawata H, Yoshida K, Kawamoto A, Kurioka H, Takase E, Sasaki Y, et al. Ischemic preconditioning upregulates vascular endothelial growth factor mRNA expression and neovascularization via nuclear translocation of protein kinase C epsilon in the rat ischemic myocardium. Circ Res. 2001; 88: 696-704.
- Fukuda S, Kaga S, Sasaki H, Zhan L, Zhu L, Otani H, et al. Angiogenic signal triggered by ischemic stress induces myocardial repair in rat during chronic infarction. J Mol Cell Cardiol. 2004; 36: 547-559.
- Ely SW, Berne RM. Protective effects of adenosine in myocardial ischemia. Circulation. 1992; 85: 893-904.
- Sakurabayashi-Kitade S, Aoka Y, Nagashima H, Kasanuki H, Hagiwar N, Kawana M. Aldosterone blockade by Spironolactone improves the hypertensive vascular hypertrophy and remodeling in angiotensin II overproducing transgenic mice. Atherosclerosis. 2009; 206: 54–60.
- Hilfiker-Kleiner D, Landmesser U, Drexler H. Molecular Mechanisms in Heart Failure: Focus on Cardiac Hypertrophy, Inflammation, Angiogenesis, and Apoptosis. Journal of the American College of Cardiology. 2006; 48: A56–A66.
- Huckle WR, Earp HS. Regulation of cell proliferation and growth by angiotensin II. Prog Growth Factor Res. 1994; 5: 177-194.
- Itoh H, Mukoyama M, Pratt RE, Gibbons GH, Dzau VJ. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J Clin Invest. 1993; 91: 2268-2274.
- Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991; 68: 450-456.
- Bell L, Madri JA. Influence of the angiotensin system on endothelial and smooth muscle cell migration. Am J Pathol. 1990; 137: 7-12.
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994; 74: 1141-1148.
- Siddiqui A, Månsson-Broberg A, Gustafsson T, Grinnemo K, Dellgren G, Hao X, et al. Antagonism of the Renin-Angiotensin System Can Counteract Cardiac Angiogenic Vascular Endothelial Growth Factor Gene Therapy and Myocardial Angiogenesis in the Normal Heart. American Journal of Hypertension. 2005; 18: 1347–1352.
- DeBoer R, Pinto Y, Suurmeijer A, Pokharel S, Scholtens E, Humler M, et al. Increased expression of cardiac angiotensin II type 1 (AT (1)) receptors decreases myocardial microvessel density after experimental myocardial infarction. Cardiovascular Research. 2003; 57: 434–442.
- Bian C, Lin J, Li X, Wang Y, Hu H, Chen P. Telmisartan-enhanced hypercholesterolaemic serum-induced vascular endothelial growth factor expression in immortalized human umbilical vascular endothelial cells. Scandinavian Journal of Clinical & Laboratory Investigation. 2007; 67: 619-631.
- Yao LJ, Wang JQ, Zhao H, Liu JS, Deng AG. Effect of telmisartan on expression of protein kinase C-alpha in kidneys of diabetic mice. Acta Pharmacol Sin. 2007; 28: 829-838.
- Abiose AK, Mansoor GA, Barry M, Soucier R, Nair CK, Hager D. Effect of spironolactone on endothelial function in patients with congestive heart failure on conventional medical therapy. Am J Cardiol. 2004; 93: 1564-1566.
- Lai L, Pen A, Hu Y, Ma J, Chen J, Hao CM, et al. Aldosterone upregulates vascular endothelial growth factor expression in mouse cortical collecting duct epithelial cells through classic mineralocorticoid receptor. Life Sci. 2007; 81: 570-576.
- Ladage D, Schützeberg N, Dartsch T, Krausgrill B, Halbach M, Zobel C, et al. Hyperaldosteronism is associated with a decrease in number and altered growth factor expression of endothelial progenitor cells in rats. Int J Cardiol. 2011; 149: 152-156.
- Kakizawa H, Itoh Y, Imamura S, Matsumoto T, Ishiwata Y, Ono Y, et al. Possible role of VEGF in the progression of kidney disease in streptozotocin (STZ)-induced diabetic rats: effects of an ACE inhibitor and an angiotensin II receptor antagonist. Horm Metab Res. 2004; 36: 458-464.
- Kim HW, Kim JL, Lee HK, Hur DY, Yun IH, Kim SD. Enalapril alters expression of key growth factors in experimental diabetic retinopathy. Curr Eye Res. 2009; 34: 976-987.
- Yamagishi S, Takeuchi M. Telmisartan is a promising cardiometabolic sartan due to its unique PPAR-gamma-inducing property. Med Hypotheses. 2005; 64: 476-478.
- Cianchetti S, Del Fiorentino A, Colognato R, Di Stefano R, Franzoni F, Pedrinelli R. Anti-inflammatory and anti-oxidant properties of telmisartan in cultured human umbilical vein endothelial cells. Atherosclerosis. 2008; 198: 22-28.
- Watanabe T, Suzuki J, Yamawaki H, Sharma VK, Sheu SS, Berk BC. Losartan metabolite EXP3179 activates Akt and endothelial nitric oxide synthase via vascular endothelial growth factor receptor-2 in endothelial cells: angiotensin II type 1 receptor-independent effects of EXP3179. Circulation. 2005; 112: 1798-1805.
- Chaudagar KK, Mehta AA. Effect of telmisartan on VEGF-induced and VEGF-independent angiogenic responsiveness of coronary endothelial cells in normal and streptozotocin (STZ)-induced diabetic rats. Clin Exp Hypertens. 2014; 36: 557-566.
- Le MT, Pugsley MK, Vauquelin G, Van Liefde I. Molecular characterisation of the interactions between olmesartan and telmisartan and the human angiotensin II AT1 receptor. Br J Pharmacol. 2007; 151: 952-962.
- Miternique-Grosse A, G-riffon C, Siegel L, Neuville A, Weltin D, Stephan D. Antiangiogenic effects of spironolactone and other potassium-sparing diuretics in human umbilical vein endothelial cells and in fibrin gel chambers implanted in rats. Journal of Hypertension. 2006; 24: 2207-2213.
- Yim HE, Kim JH, Yoo KH, Bae IS, Hong YS, Lee JW. Spironolactone and enalapril differentially up-regulate the expression of VEGF and heme oxygenase-1 in the neonatal rat kidney. Pediatr Res. 2011; 69: 378-383.
- Eichstädt HW, Eskötter H, Hoffman I, Amthauer HW, Weidinger G. Improvement of myocardial perfusion by short-term fluvastatin therapy in coronary artery disease. Am J Cardiol. 1995; 76: 122A-125A.
- O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997; 95: 1126-1131.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Böhm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol. 2001; 88: 1306-1307.
- Tan KC, Chow WS, Tam SC, Ai VH, Lam CH, Lam KS. Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2002; 87: 563-568.
- Mullen MJ, Wright D, Donald AE, Thorne S, Thomson H, Deanfield JE. Atorvastatin but not L-arginine improves endothelial function in type I diabetes mellitus: a double-blind study. J Am Coll Cardiol. 2000; 36: 410-416.
- Schmidt-Lucke C, Fichtlscherer S, Rossig L, Kamper U, Dimmeler S. Improvement of endothelial damage and regeneration indexes in patients with coronary artery disease after 4 weeks of statin therapy. Atherosclerosis. 2010; 211: 249–254.
- Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer D, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nature Medicine. 2000; 6: 1004-1010.
- Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002; 105: 739-745.
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001; 108: 391-397.
- Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001; 103: 2885-2890.
- Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, et al. HMG-CoA reductase inhibitor mobilizes bone marrow--derived endothelial progenitor cells. J Clin Invest. 2001; 108: 399-405.
- Camnitz W, Burdick MD, Strieter RM, Mehrad B, Keeley EC. Dose-dependent Effect of Statin Therapy on Circulating CXCL12 Levels in Patients with Hyperlipidemia. Clin Transl Med. 2012; 1: 23.
- Choi J, Mouillesseaux K, Wang Z, Fiji HD, Kinderman SS, Otto GW, et al. Aplexone targets the HMG-CoA reductase pathway and differentially regulates arteriovenous angiogenesis. Development. 2011; 138: 1173-1181.
- Zhu XY, Daghini E, Chade AR, Lavi R, Napoli C, Lerman A, et al. Disparate effects of simvastatin on angiogenesis during hypoxia and inflammation. Life Sci. 2008; 83: 801-809.
- Frick M, Dulakemail J, Cisowski J, Józkowicz A, Zwick R, Alber H, et al. Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis. 2003; 170: 229-236.
- Saijonmaa O, Nyman T, Stewen P, Fyhrquist F. Atorvastatin completely inhibits VEGF-induced ACE upregulation in human endothelial cells. Am J Physiol Heart Circ Physiol. 2004; 286: H2096-2102.
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005; 25: 281-290.
- Takahashi Y, Satoh M, Tabuchi T, Nakamura M. Prospective, randomized, single-blind comparison of effects of 6 months' treatment with atorvastatin versus pravastatin on leptin and angiogenic factors in patients with coronary artery disease. Heart and Vessels. 2012; 27: 337-343.
- Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005; 19: 117-125.
- Chaudagar KK, Mehta AA. Effect of atorvastatin on the angiogenic responsiveness of coronary endothelial cells in normal and streptozotocin (STZ) induced diabetic rats. Can J Physiol Pharmacol. 2014; 92: 338-349.
- Bode-Böger SM, Muke J, Surdacki A, Brabant G, Böger RH, Frölich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003; 8: 77-81.
- Lerman A, Burnett JC Jr, Higano ST, McKinley LJ, Holmes DR Jr. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998; 97: 2123-2128.
- Zhan Z, Ou D, Piao X, Kim SW, Liu Y, Wang J. Dietary arginine supplementation affects microvascular development in the small intestine of early-weaned pigs. J Nutr. 2008; 138: 1304-1309.
- Tousoulis D, Davies G, Tentolouris C, Crake T, Katsimaglis G, Stefanadis C, et al. Effects of changing the availability of the substrate for nitric oxide synthase by L-arginine administration on coronary vasomotor tone in angina patients with angiographically narrowed and in patients with normal coronary arteries. American Journal of Cardiology. 1998; 82: 1110-1113.
- Lekakis JP, Papathanassiou S, Papaioannou TG, Papamichael CM, Zakopoulos N, Kotsis V, et al. Oral L-arginine improves endothelial dysfunction in patients with essential hypertension. Int J Cardiol. 2002; 86: 317-323.
- Bai Y, Sun L, Yang T, Sun K, Chen J, Hui R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2009; 89: 77-84.
- Voisine P, Bianchi C, Khan T, Ruel M, Xu S, Feng J, et al. Normalization of coronary microvascular reactivity and improvement in myocardial perfusion by surgical vascular endothelial growth factor therapy combined with oral supplementation of L-arginine in a porcine model of endothelial dysfunction. The Journal of Thoracic and Cardiovascular Surgery. 2005; 129: 1414-1420.
- Ruel M, Beanlands R, Lortie M, Chan V, Camack N, DeKemp R, et al. Concomitant treatment with oral L-arginine improves the efficacy of surgical angiogenesis in patients with severe diffuse coronary artery disease: The Endothelial Modulation in Angiogenic Therapy randomized controlled trial. The Journal of Thoracic and Cardiovascular Surgery. 2008; 135: 762-770.
- Gallwitz B. Review of sitagliptin phosphate: a novel treatment for type 2 diabetes. Vasc Health Risk Manag. 2007; 3: 203-210.
- Yousefzadeh P, Wang X. The effects of dipeptidyl peptidase-4 inhibitors on cardiovascular disease risks in type 2 diabetes mellitus. J Diabetes Res. 2013; 2013: 459821.
- Leibovitz E, Gottlieb S, Goldenberg I, Gevrielov-Yusim N, Matetzky S, Gavish D. Sitagliptin pretreatment in diabetes patients presenting with acute coronary syndrome: results from the Acute Coronary Syndrome Israeli Survey (ACSIS). Cardiovascular Diabetology. 2013; 12: 1-7.
- Engel SS, Golm GT, Shapiro D, Davies MJ, Kaufman KD, Goldstein BJ. Cardiovascular safety of sitagliptin in patients with type 2 diabetes mellitus: a pooled analysis. Cardiovasc Diabetol. 2013; 12: 3.
- Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012; 60: 833-841.
- Suzuki K, Watanabe K, Suzuki T, Ouchi M, Futami-Suda S, Igari Y, et al. Sitagliptin improves vascular endothelial function in Japanese type 2 diabetes patients without cardiovascular disease. Journal of Diabetes Mellitus. 2012; 2: 338-345.
- Fadini G, Boscaro E, Albiero M, Menegazzo L, Frison V, De Kreutzenberg S, et al. The Oral Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Increases Circulating Endothelial Progenitor Cells in Patients With Type 2 Diabetes. Diabetes Care. 2010; 33: 1607–1609.