
Case Report
Austin J Vasc Med. 2015; 2(1): 1012.
Dobesilate Injection in Mid-Portion of Achilles Tendinopathy with Chronic Pain: A Case Report
Cuevas P1*, Fernandez-Jaen T2, Guillen P2 AnguloJ3 and Gimenez-Gallego G4
1Facultad de Medicina, Universidad Alfonso X, Spain
2Clinica CEMTRO, Spain
3Departamento de Investigacion, Hospital Universitario Ramon y Cajal, Spain
4Departamento de Estructura y Funcion de Proteinas, Centro de Investigaciones Biologicas, Spain
*Corresponding author: Pedro Cuevas, Facultad de Medicina, Universidad Alfonso X, Madrid, Spain
Received: November 16, 2015; Accepted: December18, 2015; Published: December 28, 2015
Abstract
Dobesilate injection performed in the peritendinous tissue of damaged midportion of Achilles tendon resulted in improved leg function, reduced pain and disappearance of pathologic neovascularization, after two weeks of treatment, and permitted patient to be back to his previous activity level.
Keywords: Achilles tendinopathy; Dobesilate injection; Fibroblast growth factor; vascular endothelial growth factor
Abbreviations
FGF: Fibroblast Growth Factor; NO: Nitric Oxide; VAS: Visual Analogue Scale; VEGF: Endothelial Vascular Growth Factor
Case Presentation
A 30-years old healthy Caucasian runner man presented with a 2 months history of chronic pain and swelling over the right midportion of his Achilles tendon, although he didn&’t reported any direct trauma. He referred a constant dull aching pain with walking, forcing to discontinue sport because of severity of his pain. Analgesic and anti-inflammatory drugs were initiated by the patient one week before presentation; however, his pain did not improve.
At presentation pain was rated as 5 out of 10 on Visual Analogue Scale (VAS). Colour Doppler ultrasound examination at the midportion of right Achilles tendon revealed significant neovascularity, formed by immature tortuous and dilated vessels, mainly at intratendinous mass (Figure 1). After discussing the various treatment options, the patient opted to try a Dobesilate injection to the Achilles tendon and signed an informed consent. Lidocaine was infiltrated into the skin overlying the mid-portion of Achilles tendon. Dobesilate (2ml of ammonium salt formulation; Etamsylate. Dicynone®. Sanofi France) was injected under ultrasound guidance into peritendinous Achilles tendon. The procedure was uneventful. After 15 minutes of Dobesilate injection colour doppler ultrasound scans revealed a significant reduction of tendon hypervascularity 1 (Figure 1). At 2 weeks follow-up visit, patient reported a marked reduction of his pain (VAS was rated as 1), and colour Doppler scans showed no, or few, remaining neovessels (Figure 1). Patient was able to return to running and his previous level of sport without any restrictions.
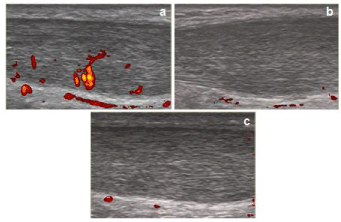
Figure 1: Longitudinal colour doppler scans of mid-portion of Achilles tendon
taken at baseline (a), at 15 minutes (b) and at 2 weeks (c) after dobesilate
injection. Note the tortuous and dilated immature neovessels before
treatment.
Discussion
No studies have been performed to our knowledge to evaluate antiangiogenic therapy in human Achilles tendinopathy. Considering the anti-angiogenic properties of Dobesilate [1], this study assesses the possible therapeutic effect of a local injection of Dobesilate in a patient with mid-portion Achilles tendinopathy. Our patient showed a good response to treatment suggesting that local administration of Dobesilate is of help in Achilles tendinopathies.
Symptoms of tendinopathy may include persistent pain, loss of range of motion, and dysfunction that can prevent a return to full activity. Furthermore, as a chronic connective disorder, tendon regeneration does not occur, resulting in a tissue structure that may incur further injury or rupture due to a failed healing repair response.
A number of options for the treatment of tendinopathies are ranging from rehabilitation protocols to injective treatment and surgery [2-4], but there is no efficient and safe treatment for this condition.
Neovascularization, the formation of new blood vessels from preexisting one, has been identified in painful tendons from sport activity [5-7], which demonstrated a strong link between hypervascularity and tendon dysfunction caused by overuse. Hypervascularity which is a feature of chronic changes in tendon is one of the causes of pain due to parallel migration of vascular innervation [3,7,8] and can also contribute to the disorganization of the tendon tissue, which lead to increased metabolic demand and, consequently, increased hypervascularity [8].
Since chronic tendon pathology appears to be a highly active process of ongoing angiogenesis, anti-angiogenic therapy could be a new approach for Achilles tendinopathy [8,10]. Angiogenesis is mediated by angiogenic factors as Fibroblast Growth Factor (FGF) and Vascular Endothelial Growth Factor (VEGF) which are highly expressed in degenerative Achilles tendons, whereas FGF and VEGF expression is nearly completely down regulated in healthy tendons [10-14]. It has been reported that neovessels are accompanied by small glutamate positive neural structures. This finding suggests that angiogenesis plays an important role in pain experienced during the degenerative tendon disease [7,13,14]. These findings are in accordance with clinical results showing that strategies to destroy neovessels (i.e. local administration of a sclerosing agent, polidocanol) produced pain improvement [14].
Recently, it has been reported that FGF is a nociceptive modulator [15,16]. Thus, the analgesic effect of Dobesilate injection in Achilles tendon may be related to inhibition of FGF-related nociception and also may be due to inhibition of nerve ingrowth that occurred in association to neovessels invasion during tendon angiogenesis.
FGF and VEGF are involved in several cellular signalling pathways that are important in regulation and maintenance of vasculature [17]. FGF signalling is critically required for maintenance of VEGF expression, and its inhibition has profound effects on VEGFdependent biological processes [18]. A great amount of animal and clinical data showed that FGF and VEGF are required for function, maintenance and stability of blood vessels. Furthermore since FGF and VEGF can induce vessel relaxation through a nitric oxide (NO) pathway [19-25], the early consequence of inhibition of FGF and VEGF is thought to reduce NO synthesis promoting vasoconstriction.
Anti-angiogenic therapies targeting FGF and VEGF can cause vessel disintegration and regression not only in many experimental setting, but also in human clinical trials [1,26-29].
Dobesilate is a dual antagonist of FGF and VEGF activities which inhibits some of their activities as such vessel relaxation and angiogenesis [19,27,29]. Thus Dobesilate may provoke spasm and destruction of neovessels in two consecutive steps. Since target inhibition of FGF in tissues undergoing pathological angiogenesis is safe without significant off-target effects on nondiseased tissues [30], dobesilate is an attractive drug for treating tendinopathies. A proposed mechanism of efficacity of dobesilate in Achilles tendinopathy is shown in (Figure 2).
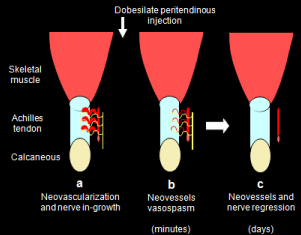
Figure 2: Simplified diagram of the proposed two-steps process implicated in
dobesilate effectiveness in Achilles tendinopathy. FGF and VEGF signalling
pathways promote vessel in-growth into tendon tissue and maintain vessel
survival and patency (a). In a first minutes step dobesilate injection promotes
neovessels spasm by elimination of FGF and VEGF vasorelaxationdependent
signals (b). Finally, dobesilate produced neovessels regression as
consequence of neutralization of FGF and VEGF survival signals in vascular
cells (c). Additionally, since FGF regulates pain and nerve invasion into the
tendon, the inhibition of both processes by dobesilate can contribute to pain
improvement in Achilles tendinopathy.
Therapeutic modulation of neoangiogenesis by influencing the level of FGF and VEGF might be a promising target for new approaches in degenerative tendon diseases.
Conclusion
Angiogenesis is associated with chronic overuse tendinopathies. We report here that local injection of Dobesilate, an antiangiogenic drug, in the mid-portion Achilles tendinopathy improved, as early after two weeks of treatment, leg function and pain concomitantly with a great decrease of tendon hypervascularity.
References
- Angulo J, Cuevas P, Cuevas B, youssef E, Fernandez A, Martinez-salamanca E, et al. Diacetyloxyl derivatization of the fibroblast growth factor inhibitor dobesilate enhances its anti-inflammatory, anti-angiogenic and anti-tumoral activities. J Transl Med. 2015; 13: 48.
- Sussmilch-Leitch SP, Collins NJ, Bialocerkowski AE, Warden JS, Crossely MK. Physical therapies for Achilles tendinopathy: systematic review and meta-analysis. J Foot Ankle Res. 2012; 5: 15.
- Alfredson H, Cook J. A treatment algorithm for managing Achilles tendinopathy: new treatment options. Br J Sports Med. 2007; 41: 211-216.
- Alfredson H. Chronic tendon pain-implications for treatment: an update. Curr Drug Targets. 2004; 5: 407-10.
- Zanetti M, Metzdorf A, Kundert HP, Zollinger H, Vienne P, Seifert B, et al. Achilles tendons: clinical relevance of neovascularization diagnosed with power Doppler US. Radiology. 2003; 27: 556-660.
- Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc. 2001; 9: 233-238.
- Knobloch K, Kraemer R, Jagodzinski M, Zeichen J, Meller R, Vogt PM. Eccentric training decreases paratendon capillary blood flow and preserves paratendon oxygen saturation in chronic achilles tendinopathy. J Orthop Sports Phys Ther. 2007; 37: 269-276.
- Walsh DA, Pearson CI. Angiogenesis in the pathogenesis of inflammatory joint and lung diseases. Arthritis Res. 2001; 3: 147-153.
- Savitskaya YA, Izaguirre A, Sierra L, Perez F, Villalobos E, Almazan A, et al. Effect of angiogenesis-related cytokines on rotator cuff disease: the search for sensitive biomarkers of early tendon degeneration. Clin Med Insights Arthritis Musculoskelet Disord. 2011; 4: 43-53.
- Pufe T, Petersen WJ, Mentlein R, Tillmann BN. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005; 15: 211-22.
- de Oliveira RR, Martins CS, Rocha YR, Braga AB, Mattos RM, Hecht F, et al. Experimental diabetes induces structural, inflammatory and vascular changes of Achilles tendons. PLoS One. 2013; 8.
- Nakama LH, King KB, Abrahamsson S, Rempel DM. VEGF, VEGFR-1, and CTGF cell densities in tendon are increased with cyclical loading: An in vivo tendinopathy model. J Orthop Res. 2006; 24: 393-400.
- Petersen W, Pufe T, Pfrommer S, Tillmann B. [Overload damage to the Achilles tendon: the importance of vascularization and angiogenesis]. Orthopade. 2005; 34: 533-542.
- Cook JL, Malliaras P, De Luca J, Ptasznik R, Morris ME, Goldie P. Neovascularization and pain in abnormal patellar tendons of active jumping athletes. Clin J Sport Med. 2004; 14: 296-299.
- Andres C, Hasenauer J, Ahn HS, Joseph EK, Isensee J, Theis FJ, et al. Wound-healing growth factor, basic FGF, induces Erk1/2-dependent mechanical hyperalgesia. Pain. 2013; 154: 2216-2226.
- Liu H, Wu QF, Li JY, Liu XJ, Li KC, Zhong YQ, et al. Fibroblast growth factor 7 is a nociceptive modulator secreted via large dense-core vesicles. J Mol Cell Biol. 2015; 7: 466-475.
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997; 18: 26-45.
- Murakami M, Nguyen LT, Hatanaka K, Schachterle W, Chen PY, Zhuang ZW, et al. FGF-dependent regulation of VEGF receptor 2 expression in mice. J Clin Invest. 2011; 121: 2668-2678.
- Cuevas P, Carceller F, Ortega S, Zazo M, Nieto I, Giménez-Gallego G. Hypotensive activity of fibroblast growth factor. Science. 1991; 254: 1208-1210.
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993; 90: 10705-10709.
- Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw. 2009; 20: 158-163.
- Rusnati M, Presta M. Fibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategies. Curr Pharm Des. 2007; 13: 2025-2044.
- De Smet F, Tembuyser B, Lenard A, Claes F, Zhang J, Michielsen C, et al. Fibroblast growth factor signaling affects vascular outgrowth and is required for the maintenance of blood vessel integrity. Chem Biol. 2014; 21: 1310-1317.
- Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, et al. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994; 89: 2183-2189.
- Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001; 280: 1375-1386.
- Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009; 6: 507-518.
- Fernandez IS, Cuevas P, Angulo J, Lopez-Navajas P, Canales-Mayordomo A, Gonzalez-Corrochano R, et al. Gentisic acid, a compound associated with plant defense and a metabolite of aspirin, heads a new class of in vivo fibroblast growth factor inhibitors. J Biol Chem. 2010; 285: 11714-11729.
- Cuevas P, Calvo M, Angulo J, Cuevas-Bourdier A, Gimenez-Gallego G. Efficacy of the fibroblast growth factor inhibitor 2,5-dihydroxyphenylsulfonate in basal cell carcinoma: a histopathological and inmunohistochemical study. Dermatolog Treat. 2011; 22: 348-352.
- Angulo J, Peiro C, Romacho T, Fernandez A, Cuevas B, Gonzalez-Corrochano R, et al. Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial proliferation, arterial relaxation, vascular permeability and angiogenesis by dobesilate. Eur J Pharmacol. 2011; 667: 153-159.
- Oladipupo SS, Smith C, Santeford A, Park C, Sene A, Wiley LA, et al. Endothelial cell FGF signalling is required for injury response but not for vascular homeostasis. Proc Natl Acad Sci USA. 2014; 111: 13379-13384.