
Special Article - Vascular Imaging
Austin J Vasc Med. 2016; 3(1): 1015.
Vascular Ultrasound and Cardiovascular Risk Assessment
Kozakova M¹ and Palombo C²*
¹Department of Clinical and Experimental Medicine, University of Pisa, Italy
²Department of Surgical, Medical, Molecular Pathology and Critical Care Medicine, University of Pisa, Italy
*Corresponding author: Carlo Palombo, Department of Surgical, Medical, Molecular Pathology and Critical Care Medicine, University of Pisa, Italy
Received: January 05, 2016; Accepted: February 02, 2016; Published: February 03, 2016
Abstract
Vascular ultrasound is able to detect endothelial dysfunction, arterial structural remodeling and increased arterial stiffness. These alterations have been shown to be associated with established and emerging cardiovascular risk factors and with incident cardiovascular events. Therefore, vascular ultrasound has been proposed to evaluate the role of different risk factors in the initiation and progression of atherosclerotic process, to study vascular aging and the relationship between arterial stiffness and atherosclerosis, to assess the efficacy of life-style and therapeutic interventions, and to improve the estimation of individual cardiovascular risk. The present paper provides a critical overview of the clinical evidence appraising the association of flow-mediated dilation, carotid and femoral intima-media thickness and plaque presence as well as local arterial stiffness with cardiovascular risk factors and cardiovascular events.
Keywords: Ultrasound; Biomarkers; Carotid artery; Cardiovascular risk; Intima-Media thickness; Arterial stiffness; Flow-Mediated dilation
Abbreviations
CV: Cardiovascular; D: Disease; US: Ultrasound; FMD: Flow- Mediated Dilation; CHS: Cardiovascular Health Study; MESA: Multi-ethnic Study on Atherosclerosis; NRI: Net Reclassification improvement; IMT: Intima-Media Thickness; CCA: Common Carotid Artery; ARIC: Atherosclerosis Risk in Communities Study; ICA: Internal Carotid Artery; 3-D: 3-Dimensional; FA: Femoral Artery; PWV: Pulse-Wave Velocity; CF: Carotid-Femoral
Introduction
Cardiovascular (CV) Disease (D) is the leading cause of death worldwide. In Europe, CVD is responsible for over 4 millions death per year, and causes 42% and 51% of deaths among men and women, respectively, compared with 23% and 19% for all cancers [1]. The medical cost of CVD has continuously increased in the past years, and in 2012 the overall CVD cost in Europe was estimated to be of €196 billion. In the next 20 years this cost is expected to further escalate.
Yet, CVD is largely preventable, and an early detection of individuals at increased risk followed by the implementation of life-style and therapeutic interventions can prevent disability and death, improve quality of life and reduce the global health-care cost. However, the accurate and cost-effective identification of subjects at risk is still a challenge. Various risk scores (Framingham Risk Score, SCORE Charts, and others) have been developed to guide the preventive strategies [2], yet these scores provide estimation of a population-based risk rather than quantification of the individual risk. Furthermore, a substantial part of population belongs to intermediate risk, where it is not clear whether and when an aggressive prevention strategy is beneficial and cost effective.
The use of CV biomarkers in conjunction with risk scores is expected to refine the risk stratification of an individual subject and to guide the preventive/therapeutic strategy. A “biomarker” was defined by the National Institutes of Health as a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” [3]. Therefore, biomarkers can be used to detect the burden of subclinical disease in order to apply preventive measures, and they should also enable to monitor the response of subclinical disease to preventive interventions. Yet, robust pathophysiologic, epidemiologic and therapeutic evidence is necessary to validate any new biomarker. According to a scientific statement from the American Heart Association, biomarker should differ between subjects with and without outcome, should predict the development of future events over and above established risk markers, and its use should improve clinical outcomes when tested in a randomized clinical trial [4].
Vascular measures might be particularly informative for the assessment of CV risk, as they detect organ damage in different parts of vascular bed, are measurable in a non-invasive way, and reflect both aging process and adverse impact of established CV risk factors, like plasma lipids, smoking, high blood pressure, diabetes, inflammation [5].Nowadays, several vascular biomarkers have been proposed. A position paper from the European Society of Cardiology Working Group on peripheral circulation suggests that the choice of vascular biomarker, or their combination, should depend on the clinical setting and present comorbidities, and may differ for each individual patient [5]. The widespread clinical use of biomarkers depends also on their cost-effectiveness, ease of use, clear methodological consensus and availability of reference values. From this perspective a vascular Ultrasound (US) is an interesting method, as it allows the composite evaluation of vascular structure and function in a non-invasive way at a relatively low cost (Table 1). Vascular ultrasound can be used to assess endothelial function, geometry and stiffness of elastic arteries (like carotid artery) and muscular arteries (like brachial and femoral artery).
Description
Method of measurement
Formulas
Clinical Utility
Recommendation/
Level of evidence
for CV prevention
Reference
values
FMD
response of BA to
reactive hyperemia
B-mode US
RF-based US
direct
measurement
in intermediate risk subjects NRI=2.4% for CHD [17]
III/B
No
Carotid IMT
thickness of the near/far wall of CCA/bulb/ICA
B-mode US
RF-based US
direct
measurement
CCA IMT in intermediate risk subjects NRI=3.6% for MI or stroke [34]
ICA IMT in general populationNRI=7% for CHD [36]
IIa/A
Yes
Carotid Plaques
number
thickness
area
volume
B-mode US
direct
measurement
IMT+plaque in general population NRI=9.9% for CHD [41]
IMT+plaque in intermediate risk subjects NRI=21.7%for CHD [41]
ICA plaque in general population NRI=7.3% for CVD [42]
carotid plaque score in general population NRI=10.2% for CVD [43]
IIa/A
No
Local stiffness
changes in vessel diameter/area to distending pressure
local PWV
B-mode US
RF-based wall tracking
distensibility:
DD/(DP*D)
compliance:
DD/DP
beta index:
ln(Ps/Pd)*D / (DD)
elastic modulus:
(DP*D)/ DD
Young’s modulus:
(DP*D)/ (DD*h)
Bramwell-Hill equation [71]
--
--
Yes
Table 1: Vascular biomarkers obtained by ultrasound.
Endothelial function
The vascular endothelium is a single layer of cells situated at the interface between the blood and the vascular wall and releasing a wide range of factors that play a central role in the maintenance of vascular health, as they regulate vascular tone, vascular growth, inflammation, cell adhesiveness and coagulation [6]. A loss of normal endothelial function is believed a key event in the initiation of atherosclerotic process [7]. Endothelial function can be measured by different techniques, yet the most widely applied technique is brachial artery Flow-Mediated Dilation (FMD). FMD is a nitric oxide-mediated process using high resolution US to monitor the response of arterial diameter to increase in blood flow, which represents a physiologic stimulus for endothelial nitric oxide release [8].
FMD has been shown to be adversely associated with CV risk factors, like hypertension, diabetes, dyslipidemia, smoking, inflammation and abdominal obesity [9-13]. The prognostic value of FMD for future CV events has been also tested. In the elderly population of the Cardiovascular Health Study (CHS) [14] as well as in the population-based cohort of the Multi-ethnic Study of Atherosclerosis (MESA) [15], FMD resulted an independent predictor for CV events, and in a recent meta-analysis of 23 studies including 14 753 subjects, for each 1% increase in FMD the overall CVD risk decreased by 13% in diseased population and by 4% in asymptomatic population [16]. However, the above-mentioned studies also demonstrated that FMD added little to the prognostic accuracy of traditional CV risk factors/scores. In the intermediate risk population of the MESA, the Net Reclassification Improvement (NRI) of FMD for incident coronary heart disease was only 2.4% (Table 1), being lower than that of carotid intima-media thickness (IMT; 10.2%) [17].
FMD has been also used to evaluate the effect of life-style and pharmacologic interventions on endothelial function, and numerous studies have demonstrated that endothelial dysfunction may improve with exercise, weight loss, vitamins, statins or antihypertensive treatment [18-21].
Although the concept of FMD is simple, the correct and reproducible measurement requires significant expertise, and published guidelines suggest a minimum of 100 supervised scans before the independent scanning, and at least 100 scans per year to maintain competency [22]. Additional methodological issues, like the level of inflation pressure, duration of ischemia, localization of inflation and the time point at which the effect of hyperemia on artery is measured, should be standardized and respected by all centers using this method [22-23].
Therefore, at the moment, FMD seems to be above all an interesting research tool that can be employed to study the role of emerging CV risk factors in the development of atherosclerosis and to monitor the effect of interventions and treatment on endothelial function, as the method is non-invasive, does not use pharmacologic stimuli, can be repeated several times and has an adequate reproducibility in expert laboratories [24]. On the other hand, its widespread clinical use for the risk stratification is not currently recommended by guidelines [25-26].
Carotid/Femoral artery IMT and plaques
Arterial wall is histologically constituted of three layers; 1) the inner layer, called tunica intima and composed of a monolayer of endothelial cells supported by internal elastic lamina; 2) the middle layer, called tunica media and consisting of longitudinal smooth muscle cells surrounded by connective tissue with elastic lamellae; 3) the outermost layer, called tunica adventitia and formed by connective tissue with collagen fibers.IMT is a combined measure of tunica intima and tunica media, and may be assessed by high-resolution US in different segments of extracranial carotid tree or in large muscular arteries as the distance between the intima-luminal and the medialadventitial interfaces of the arterial wall. With aging and the presence of CV risk factor, IMT increases and loses its double-line aspect (Figure 1). Plaque, a focal intima-media thickening, appears first in the plaque prone areas [27], i.e. areas with a particular hemodynamic pattern, like arterial branching, that is characterized by a low and oscillating shear stress and a cyclic reversal flow (Figure 2A). It is unclear, whether thickened intima-media complex and the presence of plaques represent two different stages or two different phenotypes of atherosclerotic process.
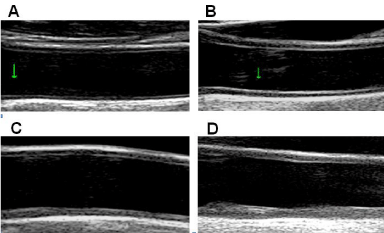
Figure 1: A) Thin intima-media complex in adolescent; B) Increased thickness
of intima-media complex in elderly healthy subject; C) Diffuse thickening of
intima-media complex with structural changes leading to the loss of a 3-layer
aspect; D) Irregular and markedly thickened CCA wall - development of the
plaque at the posterior wall.
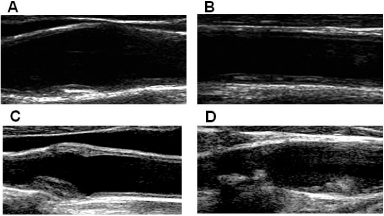
Figure 2: A) Small fibrotic plaque at carotid bifurcation (atherosclerotic
prone area); B) Flat, lipid-rich plaque at the posterior wall of CCA; C) Fibrotic
concentric plaque at carotid bulb; D) Irregular fibrotic plaque at carotid bulb
and calcified plaque with an irregular surface at the origin of internal carotid
artery (acoustic shadowing).
Numerous large prospective studies, like Kuopio Ischemic Heart Disease study, Atherosclerosis Risk in Communities (ARIC) study, CHS, Carotid Atherosclerosis Progression Study, Rotterdam Study, have demonstrated the association between carotid IMT and incident CVD risk [28-32]. A meta-analysis of 16 studies including 36.984 participants showed an increase in CV events risk of 16% per 0.1 mm difference in baseline common carotid artery (CCA) IMT [33]. However, the studies evaluating the predictive value of carotid IMT over and above established risk scores have been largely negative [17,31], and in a meta-analysis of 14 population-based studies (45.828 participants), the NRI of CCA IMT over Framingham risk score was small, being 0.8% for overall cohort and 3.6% for intermediate risk subjects [34] (Table 1). Still, the meta-analysis was affected by the variability in the IMT methodologies and reporting (number of projections, number of segments, mean or maximal IMT value), and the evaluation was limited only to CCA IMT, while IMT in carotid bifurcation or Internal Carotid Artery (ICA) was not included. It seems that IMT in different carotid segments could be associated with different CV risk factors and events. In the British Regional Heart Study, CCA IMT was strongly associated with risk factors for stroke (above all blood pressure) and with prevalent stroke, whereas bulb IMT and/or plaques were more associated with ischemic heart disease risk factors(smoking, plasma fibrinogen) and prevalent ischemic heart disease [35]. In the MESA, only the mean of maximum IMT in ICA, but not in CCA, has significant NRI (7.0%) for coronary heart disease events [36] (Table 1). The differences between carotid segments can be explained by the fact that the atherosclerotic process tends to develop initially in the areas of arterial branching (Figure 2A), and that increase in CCA IMT may represent not only the atherosclerotic process, but also arterial remodeling reflecting smooth muscle cells hypertrophy and hyperplasia responding to increase in wall tensile stress and pulsatile load [37-38], above all in hypertensive patients.
Carotid plaque (Figure 2 A-D), when compared to IMT, has been shown to have a higher diagnostic accuracy for the prediction of future coronary artery disease events [39], and the presence of carotid plaques predicted CV mortality independently of SCORE stratification [40]. In the ARIC population followed for 15.2 years, adding plaque and carotid IMT (an average of values obtained in 3 segments of both the left and right sides) to traditional risk factors improved coronary heart disease prediction (NRI= 9.9% in the entire population and 21.7% in the intermediate risk groups) [41]. Improvement of CVD risk estimation over Framingham risk score and traditional risk factors was observed for the plaque presence in the ICA (NRI=7.3%) and for the total plaque score (NRI=10.2%) in the Framingham Offspring Study cohort and in Chinese population [43], respectively (Table 1).
Altogether the published data indicate that extensive US examination of extracranial carotid tree is required to fully assess the degree of atherosclerotic burden. Indeed, the American Society of Echocardiography consensus statement specifies that carotidartery ultrasonography for CVD risk prediction should be based on a thorough scan to detect the presence of plaques, followed by measurement of IMT in the CCA [44], and Mannheim consensus recommends to image each carotid segment from multiple angels as atherosclerotic process is distributed asymmetrically within the vessel wall [45].
In healthy subjects, an average IMT ranges between 400-750 μm (according to age; Figure 1 A and B), IMT progression rate is small - between 1-32μm per year, and the differences between the 25th and 75th percentile are < 1 mm [46-49]. Consequently, a high degree of precision is required for IMT measurement. A higher accuracy and better reproducibility of measurements can be achieved by applications of automated edge-tracking algorithms [50], or by the use of Radiofrequency (RF)-based echo tracking technology allowing automatic measurement of IMT directly from unprocessed RF signal (Figure 3) [51].
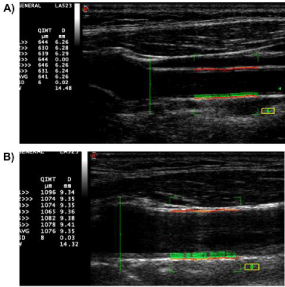
Figure 3: Far-wall CCA IMT is automatically measured within the ROI (green
rectangle), and the values of IMT (QIMT) and diameter (D) are displayed
beat-to-beat on the screen, together with averaged value (AVG) and standard
deviation (SD).
A) Far-wall CCA IMT of healthy middle-aged subject (641μm); B)Far-wall
CCA IMT of patient with dyslipidemia and coronary heart disease (1076 μm).
The definition of abnormal IMT values is also important, as carotid IMT increases with age and differs between men and women [47-48]. It is generally recommended to consider an abnormal carotid IMT when greater than the 75th percentile for age and sex [5,44]. Several age- and sex-specific reference intervals for CCA IMT as measured by B-mode US [52,53] or by RF-based echo tracking system were published [47].
The definition and quantification of carotid plaque varied in different studies. Mannheim consensus defined plaque as a focal thickening that encroaches into the lumen by 0.5 mm or by 50% of the surrounding IMT, or when IMT is >1.5 mm [45]. Plaque burden has been quantified through plaque number, plaque thickness, plaque area [54] or plaque volume as assessed by 3-D US [55]. Some investigators suggested a characterization of plaque composition, by means of densitometric gray-scale analysis (Figure 4) [56,57], in order to distinguish the less echogenic lipid-rich carotid plaques, which are associated with a higher risk of ischemic stroke, from stable more echogenic fibrotic plaques [58].
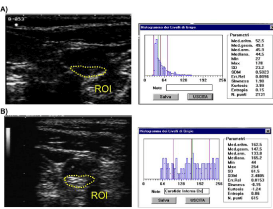
Figure 4: (A) Video-densitometric (gray-scale) analysis of carotid plaques;
(B) Histograms of gray levels (256 levels) of echolucent lipid-rich plaque and
echogenic fibrotic plaque.
The IMT in muscular Femoral Artery (FA) seems to provide information similar as or complementary to carotid IMT. FA IMT has been shown to be associated with CV risk factors [59,60],extent of coronary atherosclerosis [61] and peripheral artery disease [62], and patients with peripheral artery disease and hypo-echogenic femoral plaques had a 7.24-fold increased CV risk compared with patients with hyper-echogenic plaques after adjustment for possible confounders [63].
Altogether, a detailed US examination of extracranial carotid tree and/or femoral artery, which includes measurement of IMT at different segments, quantification of carotid plaque burden and evaluation of plaque composition, seems to provide clinically useful information on CV risk, above all in intermediate risk subjects (Table 1).
Local arterial stiffness
Arterial stiffness results from a degenerative process affecting mainly the extracellular matrix of elastic arteries under the effect of aging and risk factors. Changes in the mechanical properties of the vessel wall related to arterial stiffening may activate number of mechanisms involved also in the process of atherosclerosis [64]. In fact, arterial stiffening and atherosclerosis could be viewed as two synergic processes that may potentiate each other in the development of vascular changes underlying CVD [65]. Several noninvasive methods are now available to estimate large artery stiffness in the clinical setting, including segmental Carotid-Femoral (CF) Pulse Wave Velocity(PWV) and local arterial stiffness, namely carotid and femoral.
Carotid-femoral PWV, an estimate of aortic stiffness, represents a gold standard method for arterial stiffness assessment [66,67] and is measured directly, as a ratio of distance between two measurement points divided by the time required for the pressure propagation to travel this distance. Number of studies has demonstrated the association between cfPWV and CVD events [68,69], and in a large meta-analysis including 16 studies and 17 635 participants [70], the age-, sex- and risk factors adjusted hazard ratios per 1SD change in logcf-PWV were 1.23, 1.28 and 1.30 for coronary heart disease, stroke and CVD, respectively. In addition, cf-PWV improved risk prediction in the intermediate risk group by 13%.Carotid-femoral PWV is usually measured by tonometry (Complior, SphygmoCor); however, Doppler US can be also used, assuming that the pulse wave propagation corresponds to the flow wave propagation of spectral Doppler [71,72].
Local arterial stiffness is estimated as systo-diastolic changes in arterial diameter/area over systo-diastolic changes in distending pressure (local pulse pressure). RF-based US technology, thanks to its high frame rate and spatial resolution, is capable to track the movement of the arterial wall throughout the cardiac cycle with a great accuracy [73]. From the real-time distension curves (Figure 5), maximum and minimum arterial diameters are measured, and different indices of arterial stiffness can be calculated, including local PWV (Table 1) [74-76]. Local distending pressure used in these calculations is estimated converting the distension curve to pressure curve by a linear conversion factor and assuming that the difference between mean arterial pressure and diastolic pressure is invariant along the arterial tree [77].
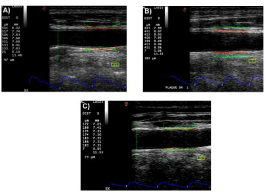
Figure 5: Distension values (DIST), diameter (D) and distension curves of
CCA in: A) Healthy young subject (distension=533 μm); B) Middle-Aged
subjects (distension=411 μm); C) Diabetic patient (distension=183 μm).
An assessment of local arterial stiffness on a larger scale became feasible only recently, with the introduction of RF-based echo tracking systems, and studies evaluating the association of local stiffness with risk factors and CVD events are still limited. Carotid stiffness has been shown to be associated with blood pressure, plasma lipids, smoking, C-reactive protein, diabetes and physical activity [78-80]. A recent analysis in the Hoorn Study population followed for 7.6 years has demonstrated that both carotid and femoral distensibility indices are independently associated with incident CV events and all-cause mortality [81].Indeed, the hazard ratios per 1 SD for CV events and all cause mortality, respectively, were 1.22 and 1.51 for lower carotid distensibility, and1.39 and 1.27 for lower femoral distensibility; these hazard ratios were comparable with those obtained in the same population for a higher cfPWV (1.56 and 1.13, respectively). The age- and sex-specific percentiles of carotid and femoral stiffness in a healthy population were estimated and the z-scores were established, which enables the comparison of carotid/femoral stiffness values between different patients and helps the interpretation of these measures [82,83].
Therefore, an assessment of local carotid and femoral stiffness by means of US is feasible and seems promising; however, this approach has to be further validated in prospective studies in order to determine its clinical utility in CV risk prediction.
The role of vascular biomarkers in pediatric populations
Assessment of CV risk in pediatric populations gets increasing interest. Although the CV events are rare in children, epidemiologic studies have demonstrated that CV risk factors are already identifiable in childhood and are predictive of adulthood CVD, type 2 diabetes mellitus and hypertension [84]. Moreover, current evidence suggests that childhood and adolescence are particularly vulnerable periods of life to the effects of cardiometabolic risk factors and later development of atherosclerosis, hypertension and diabetes [85]. To slow down the progression of atherosclerosis, and thus to reduce the future burden of CVD, preventive strategies should be developed [86]. A systematic screening of children and adolescents at high cardiometabolic risk (like obese children, children with dyslipidemia and type 1 diabetes, off-springs of diabetic parents), including also the assessment of preclinical vascular damage, may help to apply targeted early lifestyle interventions and follow their effect.
Several studies have shown that CV risk profile in childhood may influence vascular biomarkers in adulthood. In the Cardiovascular Risk in Young Finns Study, the number of risk factors measured in 12- to 18-year-old adolescents were directly related to CCA IMT measured in young adults at ages 33 through 39 years (P<0.001 for both men and women), and remained significant after adjustment for contemporaneous risk variables [87]. Data from the three large population-based prospective studies (the Bogalusa Heart Study, the Cardiovascular Risk in Young Finns Study and Childhood Determinants of Adult Health Study) have demonstrated that dyslipidemic lipoprotein levels in adolescence (age 12 to 18 years) are associated with increased carotid IMT in adulthood (age 29 to 39 years), and that adolescent dyslipidemic status is more strongly associated with high adult IMT than changes in dyslipidemic status during follow-up [88].
A major current trend that may significantly increase the future burden of CVD is a considerable increase in the prevalence of childhood obesity. Obesity in childhood increases the probability of being obese as adult, represents a risk for adulthood CV morbidity and mortality [89,90], and is associated with wide spectrum of vascular changes. Endothelial dysfunction, the first step towards atherosclerosis, has been described in childhood obesity [91-93]. However, data regarding the impact of obesity on carotid structure and function in children are not conclusive; CCA IMT has been reported as increased or unchanged [89,94-97], whereas arterial stiffness has been found to be increased, unchanged or even decreased [94,98-100].These discrepancies could reflect the fact that morphological and functional characteristics of large arteries in pediatric population are particularly influenced by age, body size, and sexual maturation, as adolescence brings an onset of hormonal changes and growth that are likely to affect the vasculature [101]. During a natural growth and development arterial diameter and distensibility increase [102], and this pattern could be amplified in obese youths, where increased arterial distension compensates for larger luminal diameter and higher blood pressure [103]. Yet, longerlasting adaptation to obesity-induced increase in hemodynamic load may induce structural changes within the arterial wall leading to its stiffening; the time course of this pathological adaptation is not known. In a population of 14-year old children followed for 5 years, cfPWV at baseline was significantly lower in obese as compared to lean subjects [94]; yet during a follow-up period obese subjects had significantly higher increase in cfPWV than lean ones (25% vs 3%; P<0.0001 vs<0.05, respectively) [104].
CV prevention and risk estimation in pediatric cohorts is challenging, and the interpretation of vascular measures is more demanding than in adults, as an adaptive remodeling of vessel wall in response to physiological development must be considered. A large study including 1051nonobese and nonhypertensive children aged 6 to 18 years, has tried to establish the sex-specific reference charts for CCA IMT and distensibility, and to explore the impact of developmental changes in body size and blood pressure on these measures [101].
Conclusion
Vascular US is capable to detect, during a single examination, the structural and functional changes of elastic and muscular arteries, which increases the probability to detect early organ damage, helps to understand the pathophysiology of vascular impairment, may improve the assessment of individual CV risk and facilitate the decision-making regarding the life-style or therapeutic interventions. Nowadays, vascular US is largely used for studying the impact of established and emerging risk factors and therapeutic interventions on arterial wall, and thus, on initiation, progression and regression of arteriosclerotic and atherosclerotic processes. In the next future, vascular US could become an interesting tool for the assessment of individual CV risk due to its common availability, non-invasiveness, absence of radiation and low cost. There is no evidence that single vascular measures/biomarkers are superior; in contrast the use of different measures could provide complementary information and improve the risk assessment. However, the widespread use of vascular biomarkers in CV risk assessment and CV prevention requires a clear standardization of US methodologies [44,45] and definition of ageand sex-specific reference intervals for different vascular measure in large populations [47,52,53,82,83].
References
- Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular Disease in Europe 2014: Epidemiologic Update. Eur Heart J. 2014; 35: 2950-2959.
- Willis A, Davies M, Yates T, Khunti K. Primary prevention of cardiovascular disease using validated risk scores: a systematic review. J R Soc Med. 2012; 105: 348-356.
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001; 69: 89-95.
- Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council. Criteria for Evaluation of Novel Markers of Cardiovascular Risk: A Scientific Statement from the American Heart Association. Circulation. 2009; 119: 2408-2416.
- Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, et al. The Role of Vascular Biomarkers for Primary and Secondary Prevention. A Position Paper from the European Society of Cardiology Working Group on Peripheral Circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015; 241: 507-532.
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007; 115: 1285-1295.
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003; 23: 168-175.
- Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011; 18: 775-789.
- Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart. 2013; 99: 1837-1842.
- Watts GF, O'Brien SF, Silvester W, Millar JA. Impaired endothelium-dependent and independent dilatation of forearm resistance arteries in men with diet-treated non-insulin-dependent diabetes: role of dyslipidaemia. Clin Sci (Lond). 1996; 91: 567-573.
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Salvetti A. Endothelial dysfunction in hypertension. J Nephrol. 2000; 13: 205-210.
- Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993; 88: 2149-2155.
- Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000; 102: 994-999.
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007; 115: 2390-2397.
- Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009; 120: 502-509.
- Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013; 168: 344-351.
- Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012; 308: 788-795.
- Dod HS, Bhardwaj R, Sajja V, Weidner G, Hobbs GR, Konat GW, et al. Effect of intensive lifestyle changes on endothelial function and on inflammatory markers of atherosclerosis. Am J Cardiol. 2010; 105: 362-367.
- Habib P, Scrocco JD, Terek M, Vanek V, Mikolich JR. Effects of bariatric surgery on inflammatory, functional and structural markers of coronary atherosclerosis. Am J Cardiol. 2009; 104: 1251-1255.
- Asselbergs FW, van der Harst P, van Roon AM, Hillege HL, de Jong PE, Gans RO, et al. Long-term effects of pravastatin and fosinopril on peripheral endothelial function in albuminuric subjects. Atherosclerosis. 2008; 196: 349-355.
- Yazici D, Yavuz DG, Unsalan S, Toprak A, Yüksel M, Deyneli O, et al. Temporal effects of low-dose ACE inhibition on endothelial function in Type 1 diabetic patients. J Endocrinol Invest. 2007; 30: 726-733.
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. International Brachial Artery Reactivity Task Force. Guidelines for the Ultrasound Assessment of Endothelial-Dependent Flow-Mediated Vasodilation of the Brachial Artery: A Report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002; 39:257-265.
- Charakida M, Masi S, Lüscher TF, Kastelein JJ, Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation. Eur Heart J. 2010; 31: 2854-2861.
- Charakida M, De Groot E, Loukogeorgakis SP, Khan T, Lüscher T, Kastelein JJ, et al. Variability and Reproducibility of Flow-Mediated Dilatation in a Multicentre Clinical Trial. Eur Heart J. 2010; 31: 2854-2861.
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (Version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted By Representatives of Nine Societies and by Invited Experts). J Eur Heart. 2012; 33: 1635-1701.
- Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010; 122: 2748-2764.
- Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006; 47: C7-12.
- Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb. 1991; 11: 1245-1249.
- Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997; 146: 483-494.
- O'Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992; 23: 1752-1760.
- Lorenz MW, Schaefer C, Steinmetz H, Sitzer M. Is Carotid Intima Media Thickness Useful for Individual Prediction of Cardiovascular Risk? Ten-Year Results from the Carotid Atherosclerosis Progression Study (CAPS). Eur Heart J. 2010; 31: 2041-2048.
- Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee D . Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997; 96: 1432-1437.
- Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, et al. Carotid Intima-Media Thickness Progression to Predict Cardiovascular Events in the General Population (The PROG-IMT Collaborative Project): A Meta-Analysis of Individual Participant Data. Lancet. 2012; 379: 2053-2062.
- Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012; 308: 796-803.
- Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, et al. Carotid Plaque, Intima Media Thickness, Cardiovascular Risk Factors, and Prevalent Cardiovascular Disease in Men and Women: The British Regional Heart Study. Stroke. 1999; 30: 841-850.
- Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013; 2: e000087.
- Chironi G, Gariepy J, Denarie N, Balice M, Megnien JL, Levenson J, et al. Influence of hypertension on early carotid artery remodeling. Arterioscler Thromb Vasc Biol. 2003; 23: 1460-1464.
- Kozakova M, Palombo C, Paterni M, Anderwald CH, Konrad T, Colgan MP, et al. Body composition and common carotid artery remodeling in a healthy population. J Clin Endocrinol Metab. 2008; 93: 3325-3332.
- Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012; 220: 128-133.
- Sehestedt T, Jeppesen J, Hansen TW, Wachtell K, Ibsen H, Torp-Pedersen C, et al. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. J Eur Heart. 2010; 31: 883-891.
- Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010; 55: 1600-1607.
- Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011; 365: 213-221.
- Xie W, Wu Y, Wang W, Zhao D, Liang L, Wang M, et al. A longitudinal study of carotid plaque and risk of ischemic cardiovascular disease in the Chinese population. J Am Soc Echocardiogr. 2011; 24: 729-737.
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Disease Risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. J Am Soc Echocardiogr. 2008; 21: 93-111.
- Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012; 34: 290-296.
- Mackinnon AD, Jerrard-Dunne P, Sitzer M, Buehler A, von Kegler S, Markus H . Rates and determinants of site-specific progression of carotid artery intima-media thickness: the carotid atherosclerosis progression study. Stroke. 2004; 35: 2150-2154.
- Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S. Reference Intervals for Common Carotid Intima-Media Thickness Measured with Echotracking: Relation with Risk Factors. J Eur Heart. 2013; 34: 2368-2380.
- Kozàkovà M, Palombo C, Morizzo C, Nolan JJ, Konrad T, Dekker JM, et al. Gender-Specific Differences in Carotid Intima-Media Thickness and Its Progression over Three Years: A Multicenter European Study. Nutr Metab Cardiovasc Dis. 2013; 23:151-158.
- Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014; 7: 1025-1038.
- Polak JF, Pencina MJ, Herrington D, O'Leary DH. Associations of edge-detected and manual-traced common carotid intima-media thickness measurements with Framingham risk factors: the multi-ethnic study of atherosclerosis. Stroke. 2011; 42: 1912-1916.
- Hoeks AP, Willekes C, Boutouyrie P, Brands PJ, Willigers JM, Reneman RS. Automated detection of local artery wall thickness based on M-line signal processing. Ultrasound Med Biol. 1997; 23: 1017-1023.
- Howard G, Sharrett AR, Heiss G, Evans GW, Chambless LE, Riley WA, et al. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC Investigators. Stroke. 1993; 24: 1297-1304.
- Ciccone MM, Balbarini A, Teresa Porcelli M, Santoro D, Cortese F, Scicchitano P, et al. Carotid artery intima-media thickness: normal and percentile values in the Italian population (camp study). Eur J Cardiovasc Prev Rehabil. 2011; 18: 650-655.
- Mathiesen EB, Johnsen SH, Wilsgaard T, Bønaa KH, Løchen ML, Njølstad I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromsø Study. Stroke. 2011; 42: 972-978.
- Ainsworth CD, Blake CC, Tamayo A, Beletsky V, Fenster A, Spence JD. 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke. 2005; 36: 1904-1909.
- Baroncini LA, Pazin Filho A, Murta Junior LO, Martins AR, Ramos SG, Cherri J, et al. Ultrasonic Tissue Characterization of Vulnerable Carotid Plaque: Correlation between Video densitometric Method and Histological Examination. Cardiovasc Ultrasound. 2006; 4: 32.
- Puato M, Faggin E, Rattazzi M, Paterni M, Kozàkovà M, Palombo C, et al. Study Group on Arterial Wall Structure . In vivo noninvasive identification of cell composition of intimal lesions: a combined approach with ultrasonography and immunocytochemistry. J Vasc Surg. 2003; 38: 1390-1395.
- Grønholdt ML, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation. 2001; 104: 68-73.
- Wallenfeldt K, Hulthe J, Bokemark L, Wikstrand J, Fagerberg B. Carotid and femoral atherosclerosis, cardiovascular risk factors and C-reactive protein in relation to smokeless tobacco use or smoking in 58-year-old men. J Intern Med. 2001; 250: 492-501.
- Paul TK, Srinivasan SR, Chen W, Li S, Bond MG, Tang R, et al. Impact of multiple cardiovascular risk factors on femoral artery intima-media thickness in asymptomatic young adults (the Bogalusa Heart Study). Am J Cardiol. 2005; 95: 469-473.
- Lekakis JP, Papamichael C, Papaioannou TG, Stamatelopoulos KS, Cimponeriu A, Protogerou AD, et al. Intima-Media Thickness Score from Carotid and Femoral Arteries Predicts the Extent of Coronary Artery Disease: Intima-Media Thickness and CAD. Int J Cardiovasc Imaging. 2005; 21:495-501.
- Okumura N, Saito M, Tanaka T, Matsumoto M, Tzusuki M, Murohara T. Not Carotid but Femoral Artery Intima-Media Thickness Predicts Peripheral Artery Disease in Patients with Coronary Artery Disease. (Abstract) Circulation. 2014; 130: A11040.
- Schiano V, Sirico G, Giugliano G, Laurenzano E, Brevetti L, Perrino C, et al. Femoral plaque echogenicity and cardiovascular risk in claudicants. JACC Cardiovasc Imaging. 2012; 5: 348-357.
- Gil-Ortega M, Martín-Ramos M, Arribas SM, González MC, Aránguez I, Ruiz-Gayo M, et al. Arterial stiffness is associated with adipokine dysregulation in non-hypertensive obese mice. Vascul Pharmacol. 2016; 77: 38-47.
- Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016; 77: 1-7.
- The Reference Values for Arterial Stiffness Collaboration. Determinants of Pulse Wave Velocity in Healthy People and in the Presence of Cardiovascular Risk Factors: ‘Establishing Normal and Reference Values’. J Eur Heart. 2010; 31: 2338-2350.
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013; 31:1281-1357.
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006; 113: 664-670.
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010; 121: 505-511.
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014; 63: 636-646.
- Calabia J, Torguet P, Garcia M, Garcia I, Martin N, Guasch B, et al. Doppler ultrasound in the measurement of pulse wave velocity: agreement with the Complior method. Cardiovasc Ultrasound. 2011; 9: 13.
- Jiang B, Liu B, McNeill KL, Chowienczyk PJ. Measurement of pulse wave velocity using pulse wave Doppler ultrasound: comparison with arterial tonometry. Ultrasound Med Biol. 2008; 34: 509-512.
- Palombo C, Kozakova M, Guraschi N, Bini G, Cesana F, Castoldi G, et al. Radiofrequency-based carotid wall tracking: a comparison between two different systems. J Hypertens. 2012; 30: 1614-1619.
- Bramwell JC, Hill AV. The Velocity of the Pulse Wave in Man. Proc R Soc Lond B. 1922; 93: 298-306.
- Paini A, Boutouyrie P, Calvet D, Tropeano AI, Laloux B, Laurent S. Carotid and aortic stiffness: determinants of discrepancies. Hypertension. 2006; 47: 371-376.
- Kozakova M, Morizzo C, Guarino D, Federico G, Miccoli M, Giannattasio C, et al. The impact of age and risk factors on carotid and carotid-femoral pulse wave velocity. J Hypertens. 2015; 33: 1446-1451.
- Van Bortel LM, Balkestein EJ, van der Heijden-Spek JJ, Vanmolkot FH, Staessen JA, Kragten JA, et al. Non-invasive assessment of local arterial pulse pressure: comparison of applanation tonometry and echo-tracking. J Hypertens. 2001; 19: 1037-1044.
- Caviezel S, Dratva J, Schaffner E, Schindler C, Zemp Stutz E, de Groot E, et al. Sex-specific associations of cardiovascular risk factors with carotid stiffness--results from the SAPALDIA cohort study. Atherosclerosis. 2014; 235: 576-584.
- Van Dijk RAJM, Nijpels G, Twisk JWR, Steyn M, Dekker JM, Heine RJ, et al. Changes in Common Carotid Artery Diameter, Distensibility and Compliance in Subjects with a Recent History of Impaired Glucose Tolerance: A 3-Year Follow-Up Study. J Hypertens. 2000; 18: 293-300.
- Kozakova M, Palombo C, Mhamdi L, Konrad T, Nilsson P, Staehr PB, et al. Habitual physical activity and vascular aging in a young to middle-age population at low cardiovascular risk. Stroke. 2007; 38: 2549-2555.
- van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, et al. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol. 2014; 63: 1739-1747.
- Engelen L, Bossuyt J, Ferreira I, Van Bortel LM, Reesink KD, Segers P, et al. Reference Values for Arterial Measurements Collaboration. Reference Values for Local Arterial Stiffness. Part A: Carotid Artery. J Hypertens. 2015; 33:1981-1996.
- Bossuyt J, Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S, et al. Reference values for local arterial stiffness. Part B: femoral artery. J Hypertens. 2015; 33: 1997-2009.
- Morrison JA, Glueck CJ, Wang P. Childhood risk factors predict cardiovascular disease, impaired fasting glucose plus type 2 diabetes mellitus, and high blood pressure 26 years later at a mean age of 38 years: the Princeton-lipid research clinics follow-up study. Metabolism. 2012; 61: 531-541.
- Agirbasli M, Tanrikulu AM, Berenson GS. Metabolic Syndrome: Bridging the Gap from Childhood to Adulthood. Cardiovasc Ther. 2016; 34: 30-36.
- Celermajer DS, Ayer JG. Childhood risk factors for adult cardiovascular disease and primary prevention in childhood. Heart. 2006; 92: 1701-1706.
- Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003; 290: 2277-2283.
- Magnussen CG, Venn A, Thomson R, Juonala M, Srinivasan SR, Viikari JS, et al. The Association of Pediatric Low- And High-Density Lipoprotein Cholesterol Dyslipidemia Classifications and Change in Dyslipidemia Status with Carotid Intima-Media Thickness in Adulthood: Evidence from the Cardiovascular Risk in Young Finns Study, the Bogalusa Heart Study, and the CDAH (Childhood Determinants of Adult Health) Study. J Am Coll Cardiol. 2009; 53: 860-869.
- Raitakari OT, Juonala M, Viikari JS. Obesity in childhood and vascular changes in adulthood: insights into the Cardiovascular Risk in Young Finns Study. Int J Obes (Lond). 2005; 29 Suppl 2: S101-104.
- Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014; 56: 369-381.
- Montero D, Walther G, Perez-Martin A, Roche E, Vinet A. Endothelial Dysfunction, Inflammation, and Oxidative Stress in Obese Children and Adolescents: Markers and Effect of Lifestyle Intervention. Obes Rev. 2012; 13: 441-455.
- Desideri G, De Simone M, Iughetti L, Rosato T, Iezzi ML, Marinucci MC, et al. Early activation of vascular endothelial cells and platelets in obese children. J Clin Endocrinol Metab. 2005; 90: 3145-3152.
- Bruyndonckx L, Hoymans VY, Van Craenenbroeck AH, Vissers DK, Vrints CJ, Ramet J, et al. Assessment of endothelial dysfunction in childhood obesity and clinical use. Oxid Med Cell Longev. 2013; 2013: 174782.
- Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging. 2008; 28: 287-293.
- Juonala M, Raitakari M, S A Viikari J, Raitakari OT. Obesity in youth is not an independent predictor of carotid IMT in adulthood. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2006; 185: 388-393.
- Freedman DS, Dietz WH, Tang R, Mensah GA, Bond MG, Urbina EM, et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004; 28: 159-166.
- Melo X, Santa-Clara H, Pimenta NM, Carrolo M, Martins SS, Minderico CS, et al. Body composition phenotypes and carotid intima-media thickness in 11-13-year-old children. Eur J Pediatr. 2014; 173: 345-352.
- Cote AT, Phillips AA, Harris KC, Sandor GG, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015; 35: 1038-1044.
- Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity (Silver Spring). 2012; 20: 165-171.
- Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, Girardet JP. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001; 358: 1400-1404.
- Doyon A, Kracht D, Bayazit AK, Deveci M, Duzova A, Krmar RT, et al. Carotid artery intima-media thickness and distensibility in children and adolescents: reference values and role of body dimensions. Hypertension. 2013; 62: 550-556.
- Gardner AW, Parker DE. Association between arterial compliance and age in participants 9 to 77 years old. Angiology. 2010; 61: 37-41.
- Kozakova M, Morizzo C, Bianchi V, Marchetti S, Federico G, Palombo C. Hemodynamic Overload and Intra-Abdominal Adiposity in Obese Children: Relationships with Cardiovascular Structure and Function. Nutr Metab Cardiovasc Dis. 2016; 26: 60-66.
- Dangardt F, Chen Y, Berggren K, Osika W, Friberg P. Increased rate of arterial stiffening with obesity in adolescents: a five-year follow-up study. PLoS One. 2013; 8: e57454.