
Review Article
Austin J Vet Sci & Anim Husb. 2023; 10(1): 1116.
Review on Ruminant Fasciolosis in Ethiopia
Girma A¹* and Hailu B²
1Yemalogi Welel Woreda Livestock and FisheriesDevelopment Office, Ethiopia
2Boneya BosheWoreda Livestock and FisheriesDevelopment Office East Wollaga, Oromia, Ethiopia
*Corresponding author: Girma A Adugna Girma Lema, Yemalogi welel Woreda Agriculture Office, Kellem Wollega, Oromia, Ethiopia
Received: December 30, 2022; Accepted: February 10, 2023; Published: February 17, 2023
Abstract
Ethiopia possesses the largest livestock population in Africa. Ruminant Fasciolosis is a serious problem in animal production in different areas of the world especially in Ethiopia. It is a wide spread trematodal disease affecting ruminants (cattle, sheep and goats) and also other species of animals. F. hepatica and F. gigantica are the parasitic species belonging to Genus Fasciola under the phylum platyhelminths. The fasciola disease has three phases of clinical sign acute, sub-acute and chronic forms. Fasciolosis is more apparent in young ruminant and is usually chronic in nature. Adult flukes in the bile ducts cause inflammation, biliary obstruction, distraction of liver tissue and anemia. Snails of family Lymnaeidae are main intermediate hosts having great role on the transmission of the disease and the infection is acquired through grazing on swampy pasture. The disease mostly diagnosed by prior knowledge of the epidemiology of the disease in a given environment; observation of clinical signs, information on grazing history, seasonal occurrence and standard examination of feces in the laboratory. The affected cattle should be effectively treated with narrow spectrum anthelmintic such as Triclabendazole in addition to reducing the population of the intermediate host to control the disease. Now a days, fasciolosis is recognized as emerging human disease over the world even if only few case reports of human fascioliasis are available in Ethiopia, as the disease mostly affects animals in the country. The disease causes a significant economic loss in ruminant production by inflecting direct and indirect loss at different parts of Ethiopia. To control and prevent the disease, the strategic destruction of snail population should be implemented throughout the country to break down the life cycle of liver fluke.
Keywords: Fasciola gigantica; Fasciola hepatica; Fasciolosis; Liver fluke; Ruminants
Abbreviations: CDC: Center for Disease Control; ELISA: Enzyme Linked Immune Sorbent Assay; ES: Execratory-ecretory; ETB: Ethiopian Birr; FAO: Food and Agriculture Organization; FH: Fasciola Hepatica; FH: Final Host; FG: Fasciola Gigantica; IH: Inter Mediate Host; GLDH: Glutamate Dehydrogenase; GGT: Gama Glutamate Transferase; LH: Lactate Hydrogenase; m.a.s.l: Meter Above Sea Level; PCR: Polymerase Chain Reaction; WHO: World Health Organization.
Introduction
Fasciolosis is an economically important disease of domestic livestock, particularly in cattle, sheep and goats. The disease is caused by digenean trematodes of the genus Fasciola commonly referred to as liver fluke. The two species most commonly implicated as the etiological agents of fasciolosis are F. hepatica or temperate liver fluke and F. gigantica or tropical liver fluke [40]. The ability of Fasciola to spread is related to the capacity of fasciolids to colonize and adapt to new environments, new definitive hosts as well as intermediate hosts [18]. Fasciola is commonly recognized as liver flukes and they are responsible for wide spread of morbidity and mortality in cattle characterized by weight loss, anemia and hypo proteinemia [69].
A study conducted by [34], reported up to 100% liver condemnation rates in slaughter slabs in Iringa region in Tanzania in cattle. In Ethiopia the prevalence of fasciolosis is as high as 83.08% in cattle, 62.7% in sheep and 17.2 % in goats. The variation in climate-ecological conditions such as altitude, rainfall and temperature, and livestock management system influences the prevalence of fasciolosis together with survival and distribution of the parasites as well as their intermediate host (snails) [35].
Diagnosis of ruminant fasciolosis is based on clinical sign, grazing history, seasonal occurrence, examination of faces by laboratory tests and post-mortem examination. In cattle, chronic form of the disease is more common and drugs like rafoxanide and nitroxynil other than triclabendazole are more effective. The disease can be controlled by reducing the population of the intermediate host or by using anthelmintic [2].
Fasciolosis is one of the most prevalent helminthic infections of ruminants in different parts of the world including Ethiopia. It causes significant morbidity and mortality. The prevalence and economic significance of fasciolosis in Ethiopia has been reported by several researchers. In recent years, small scale traditional irrigation schemes have been expanding in many parts of Ethiopia. Implementation of irrigated agriculture will create favorable habitat for fluke and snail vectors, thereby, influencing the occurrence of fasciolosis. Both F. hepatica and F. gigantica are found in Ethiopia and are transmitted by Lymnaea truncatula and L. natalensis, respectively [79].
The economic impact of Fasciolosis may vary greatly from year to year depending on the climate, management, level of infection, host immunity status and the age of animals [46,49]. In Ethiopia, Fasciolosis is mainly an animal disease, causing a great economic burden in the highland areas of the country [79]. Therefore, the objectives of this review are:
¾To review on ruminant fasciolosis.
¾To provide current information on epidemiology, diagnosis, treatment, prevention and control of ruminant fasciolosis.
Literature Review
General Description of Fasciolosis
Fasciolosis, a disease of the bile duct of domestic herbivorous animals, contributes to great economic and health losses in the cattle industry in many countries worldwide [52]. Fasciolosis is an economically important disease of domestic livestock, in particular cattle and sheep. The disease is caused by digenean Trematode of the genus Fasciola, commonly referred to as liver flukes. The two species most commonly implicated as the etiological agents of Fasciolosis are F. hepatica and F. gigantica. F. hepatica has a worldwide distribution but predominates in temperate zones while F. gigantica is found on most continents, primarily in tropical regions. Both F. hepatica and F. gigantica are transmitted by the snails of the family Lymnaesidae [66].
The disease is a worldwide zoonosis caused by Fasciola spp. and is often neglected despite its common occurrence in endemic areas and it is caused by two species of parasitic flatworms or trematodes that mainly affect the liver [40,71,74]. Liver fluke infection caused by Fasciola hepatica and F.gigantica remains economically significant parasite of livestock and is emerging zoonotic infection. Millions of human population is infected with fascioliasis and about 180 million are at risk of fascioliasis according to WHO estimation. Its prevalence is growing in human population and has been reported from 70 different countries of world [46].
Etiology and Morphology of Fasciolosis
Fasciolosis is caused by Digenean trematode of the Genus Fasciola consisting of two species usually implicated in causing the disease namely F. hepatica and F. gigantic [40,71,74].
The morphology of fasciola is helping us to classify them at the species level. There are different structures found between species of fasciola being, Fasciola hepatica is a leaf shaped, with broad and cone shaped anterior projection. The tegument is armed with sharp spines. The young fluke at the time of entry in to the liver is 1-2 millimeter (mm) in length and lancet like when it has become fully mature in the bile ducts. The eggs have an indistinct operculum and develop only after the eggs have been laid [42]. The eggs of fasciola have yellowish brown shell with a small knob at their posterior ends. Fasciola gigantica is larger than Fasciola hepatica and can reach up to 7.5cm length. It has leaf like, the anterior end with very short conical shape. Fasciola gigantica eggs are larger than those of Fasciola hepatica, measuring 190 x 100 micrometer (μm) as from measured report of [65].
Host Range
Intermediate host: Intermediate host of Fasciolosis is determined by the number of infected lymnaeid snails in thegrazing area. The disease is seasonal pattern in regions where snails are active for only part of the year. Some lymnaeid snails have more aquatic habitat than others but most are restricted to damp or wet environments. In general, non acidic, low lying swampy areas with slow moving water and irrigated areas are highly suitable for infection to takes place. Snails burrow in to the soil to survive dry periods and release cercaria when free water is present [53]. The snails of the genus Lymnae are the IHs for the genus Fasciola. The epidemiology of Fasciolosis depends on the ecology of the snail. Lymnae species most important in transmission of F. hepatica Fasciolosis is L. truncatula, widespread in Australia. Other species, which have been incremented in the transmission of Fasciolosis, include L. viator and L. diaphone (South America), L. celummella (USA, Australia, Central America and Netherland) and L. humilis in Northern America [62]. The most important IHs of F. gigantic is L.natalensis and L. auricular [37]. In Ethiopia the results of the malacological survey demonstrated the existence of Lymnaea natalensis, Lymnaea truncatula, Biomphalaria pfeifferi and Bulinus species around Kemissie [3].
Final hosts
Infection of the final host occurs by ingestion of encysted metacercariae on herbage, or less commonly by ingestion of suspended metacercariae in drinking water. Once ingested, the young flukes encyst in the small intestine, penetrate the gut wall and traverse the abdominal cavity to reach the liver capsule and the liver tissue. The immature flukes migrate in the liver parenchyma for 6-8 weeks before entering a bile duct where they mature and commence egg production [28].
Fasciola hepatica occurs in the bile ducts of the sheep, goat, cattle, other ruminants, pig, hare, rabbit, beaver, coypu, elephant, horse, dog, cat, kangaroo and man. In the unusual hosts, such as man and the horse, the fluke may be found in the lungs, under the skin or in other locations. The fluke is cosmopolitan in its distribution and is the cause of fascioliasis (liver fluke disease, liver rot), especially in sheep and cattle [62]. Hosts for F. hepatica are most mammals including man, sheep and cattle being most important. For F. gigantica affects a wide range of domestic animals and is found in lowland areas replacing F. hepatica [70].
Life Cycle
Knowledge of the life cycle of a parasite may contribute to control strategies focusing either on the mammalian host or the vector. Infected mammals including cattle, sheep, buffaloes, donkeys and pigs but also horses, goats, dromedaries, camels, llamas and other herbivores pass ovulated eggs in stool into fresh-water sources. Since the fasciola worm lives in the bile ducts of such animals, its un embryonated eggs reach the intestine with bile and are voided with feces. Fresh water is required for the development of intermediate stages of the fasciola species in the snail. The ciliated miracidium hatches from the egg. It bores a snail in the genus Lymnae and develops into a sporocyst. The next developmental stages are redia and cercaria which later vacate the snail [46].
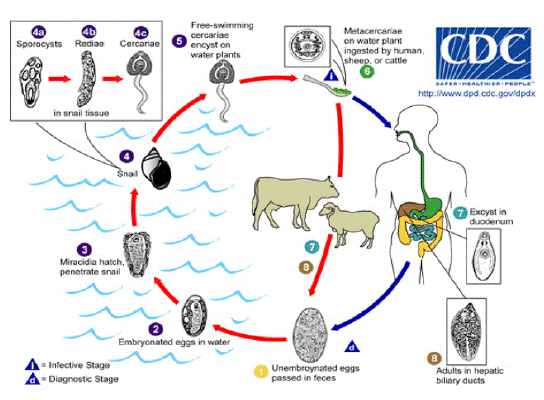
Figure 1: Life cycle of fasciola.
Source: http:// www.dpd.cdc.gov / dpdx / HTML / Fasciolosis.htm
Immature eggs are discharged in the biliary ducts and in the stool (1). Eggs become embrionated in water (2); Eggs release miracidia (3), which invade a suitable snail intermediate host (4), including many species of the genus Lymnae. In the snail the parasites undergo several developmental stages sporocysts (4a), rediae (4b), and cercariae (4c), the cercariae are released from the snail (5), and encyst as metacercariae on aquatic vegetation or other surfaces. Mammals acquire the infection by eating metacercariae. Human can become infected by eating vegetables.
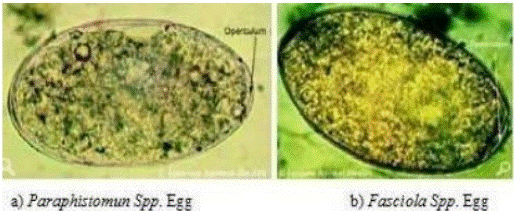
Figure 2: Rumen and Liver Fluke Eggs.Source: (Sloss et al., 1994)
The cercaria can infect the definitive mammalian host, including humans passively when the host drinks infected water or it can encyst on leaves and the mammalian host becomes infected when it eats leaves containing the metacercariae. The ingested metacercariae excyst in the duodenum and migrate into the peritoneal cavity and finally reach the liver. They bore through the liver capsule and in about 12 weeks enter the bile ducts where they start to lay eggs. Infected persons develop hyperplasia of the bile ducts. Clinically patients lose appetite, have nausea and diarrhea. Urticaria, acute epigastric pain, jaundice, eosinophilia and hepatomegaly are common findings. Generally, Fasciola has two-host life cycle and comprises of four different phases. Adult flukes reside in bile duct of definitive host; eggs are passed onto the ground in feces. Transit period of egg development in water of appropriate physicochemical characteristics and temperature of 15-25°C usually take 2-3 weeks. Miracidium hatch from egg and attempt to find an intermediate host (snail), which after penetrate in intermediate host, replicate asexually in to sporocyst, redial and cercarial form in snail, that takes approximately 5 to 7 weeks depending on temperature (if high temperature then this time is curtail) of environment [46].
In wet conditions, these cercariae emerge and swim to vegetation where they encyst into metacercariae; this shedding process requires a temperature range of 9-26°C. Animals become infected by ingestion of these metacercariae that excyst, releasing juvenile flukes in small intestine. These juvenile flukes penetrate the wall of small intestine (duodenum), migrate through the peritoneal cavity over a period of 7 days and then penetrate through liver capsule. Juvenile flukes migrate through hepatic parenchyma for approximately 6 to 8 weeks before entering the bile ducts where they mature. Egg production begins as early as 8 weeks after infection. Thus the entire life cycle (egg shed to next generation egg shedding) is completed in 18-24 weeks (4.5 to 6 months) [57].
Transmission of Fasciolosis
Once an animal is infected with fascioliosis, it will pass eggs from the feaces of the animals. On entering the water, the eggs hatch into miracidia. The miracidia locate water snails by chemotaxys. Sporocyst produce rediae and rediae may produce second generation rediae. Cercariae emerge from the snails. Encystment of Cercariae on vegetation occurs at the edge of water. Sheep and cattle become infected by ingesting metacerceriae while grazing. People normally catch the disease more often in situations where livestock live in the same general areas where food is grown. Most humans catch it from underwater food plants like watercress. Infection can potentially be avoided by cooking these plants fully before eating them. In some areas, eating these plants raw is relatively customary, and fasciliasis in humans is more common in those areas [73].
Pathogenesis and Clinical Sign
Pathogenesis
The development of fasciolosis infection in definitive host is divided into two phases: the parenchymal (migratory) phase and the biliary phase. The parenchymal phase occurs during migration of flukes in the liver parenchyma and is associated with liver damage and hemorrhage. During the parenchyma stage of the infection, liver damage caused by the migrating flukes compromise liver function, which in ruminant is reflected in a decline in plasma albumin concentrations, attributed partly to reduced rate of synthesis and partly to an expansion of the plasma volume [8,58].
Early infection, during fluke migration, there is hyperproteinemia, hyperglobulinemia, and hypoalbuminemia. The hypo-albuminemia is associated with plasma volume expansion caused by liver damage and reduced albumin synthesis. When excysted juvenile flukes penetrate the intestinal wall then flukes migrate within the abdominal cavity and penetrate the liver or other organs. F. hepatica has a strong predilection for the tissues of the liver [58]. The biliary phase occurs when the parasite is in the bile ducts, and results from the hematophagic activity of the adult flukes and from the damage to the mucosa, by their cuticles spines and in biliary ducts, flukes mature, feed on blood, and produce eggs. Hypertrophy of biliary ducts associated with obstruction of the lumen occurs as a result of tissue damage [67].
In general adverse effect of fasciolosis is caused by three ways: Mechanical (obstruction of parenchyma and blood vessels of the liver by immature flukes burrowing through the liver and irritation of the epithelia lining of the bile ducts by the adult); toxic (by secretary and excretory product of the fluke); and loss of blood resulting from hemorrhage in the liver (acute form) and hematophagous feeding habits of the flukes [32]. Light infection due to F. hepatica may be asymptomatic. However they may produce hepatic colic with coughing and vomiting, generalized abdominal rigidity, head ache and sweating, irregular fever, diarrhea, pipe clay and anemia [8].
Clinical sign
The clinical features of Fasciolosis can have acute, sub-acute and chronic forms. Acute Fasciolosis occurs as disease outbreak following a massive, but relatively short-term, intake of metacercaria [42]. Several clinical syndromes are acute Fasciolosis in sheep most often occurs as sudden death without other apparent clinical abnormality. It is usually seen in the summer and autumn but may occur at any time when sheep have the opportunity to graze heavily contaminated herbage. If the disease is observed clinically in sheep it is manifested by dullness, weakness, lack of appetite, pallor and edema of mucosa and conjunctiva and pain when pressure is exerted over the area of the liver. It is rarely occurs in cattle [53]. Sub acute Fasciolosis is caused by ingestion of a moderate number of metacercariae and is characterized by anemia, jaundice and ill-thrift. The migrating fluke causes extensive tissue damage, hemorrhage and in particular liver damage. The result is severe damage, anemia, liver failure and death 8-10 weeks [42]. Chronic Fasciolosis does not become apparent until several weeks after the danger of acute disease has receded. It occurs when the parasite reaches the hepatic bile duct [65].
Animals become anemic and anorectic, as the adult fluke becomes active within the bile duct and signs may include dependent edema or swelling under the jaw (‘bottle jaw’). Affected animals are reluctant to travel. Death eventually occurs when anemia becomes severe. Additional stress upon anemic animals, such as droving, may lead to collapse and death. Cattle typically present with signs of weight loss, anemia and chronic diarrhea [70].
Diagnosis
Diagnosis of fasciolosis both in animals and in man may involve considerations of various aspects such as history, clinical findings and general epidemiology of the disease. Confirmation in all cases can be made by either faecal examination or recovery of the worms at postmortem examination. Currently serological and molecular techniques are developing by various researchers. Analysis of the enzyme and haematological profiles are also known to give important clues as to the presence or absence of fasciolosis in animals [29,38].
History and clinical manifestations
Infection with F. hepatica is usually associated with herds and flocks grazing in wet, marshy lands. On the other hand, F. gigantica uses water snails as intermediate host. Therefore, infection with this species is associated with livestock grazing around the snail-infected watering places, which may be seasonally not dated [50,64]. In acute case of fasciolosis sudden death and severe anemia occur due to migrating young flukes through the liver; however no fluke’s eggs are passed in the feces. Sub-acute case cause signs of rapid loss of condition, severe anemia, high fluke egg count and death occurs 12-30 weeks after infection and in chronic fasciolosis gradual wasting, severe anemia with as cites, bottles jaw and very high fluke egg count which may lead to death more than 20 weeks after infection [70].
Faecal examination
It is used to know the presence or absence of a parasite or whether an animal is infected or not. Under field condition, the methods most commonly used are sedimentation and floatation [5,28,29]. Sedimentation procedure concentrates both faeces and eggs at the bottom of a liquid medium, usually water, and detects most parasite eggs or cysts that have high or specific gravity, like trematode (fluke) eggs [5,29].
Chronic fasciolosis is diagnosed by finding eggs in the feces by using sedimentation technique. Examination employing sedimentation technique. Fasciola eggs have high specific gravity and sedimentation is preferred to floatation [60]. The oval operculated golden eggs of F. hepatica appear in the feces 10 weeks after infection, while F. gigantica eggs only appear 15 weeks after infection. Excretion of fluke eggs shows considerable day to day and within day variation and the distribution of eggs in feces are irregular; single fecal egg count assay may lead to incorrect conclusion [32].
Serological method
Serological diagnosis of parasitic infection has been further extended for the detection of antigen of parasitic infections, which is circulating in the serum or blood. The test for antibodies (ELISA) is highly specific and sensitive but remains positive for five months after treatment with or without the presence of flukes. The test can be particularly useful for the early detection of F hepatica infection [82].
In vivo diagnosis of mild and prepatent infection is possible serologically. For example, detection of antibodies by ELISA in serum or milk is available and particularly useful for diagnosis of infection in cattle in an individual or herd basis. A rise in antibodies can be detected by two weeks after infection and keeps rising until week six [60; Radostits et al., 2007).
Liver enzyme test
Liver enzyme determination is used in the diagnosis of fasciolosis that changes in serum enzymes are indicators of hepatic metabolism impairment [26]. It is assumed that certain liver tissue cells contain characteristic enzymes, the presence of which in the blood indicates the probable site of tissue damage by immature or adult Fasciola species. A rise in the level of serum hepatic enzyme Gamma Glutamyl Trasnspeptidase (GGT) and Glutamate Dehydrogenase (GLDH) were shown to be indicative of liver damage due to obstruction of the biliary tract and liver damage by parasites [38]. Hence, estimation of serum levels of enzyme released by damaged liver cells is used as supplement to faecal examination to justify the presence of infection with fasciolosis [70]. Serum activities of GLDH and GGT are used as markers of the different stages of Fasciola infection in ruminant, indicating the presence of cell necrosis caused by juvenile migrating flukes and bile duct lesions associated with mature helminths respectively [23].
Molecular method
The PCR method is a proper method for most epidemiological surveys. PCR with DSJF/DSJ3 primers were used to identify F.hepatica eggs from fecal samples of naturally infected various domestic ruminants and accordingly, PCR was found to be more powerful diagnostic tool to detect fasciola infection. PCR-RFLP tests showed that F.hepatica was dominant species in animals and no evidence F. gigantica was observed [31,76].
Post mortem examination
Post mortem parasite count provides a more precise assessment of parasite burden than parasite egg count. It is used for the detection and identification of the adult and immature forms of parasites. This method is more suitable for differential parasite count under field condition or laboratory conditions using simple, easily obtainable inexpensive equipments [29]. Liver is examined for the assessment of worm burden, particularly Fasciola species and Dicrocoelium species [68]. Lesions related to fasciolosis are specific, which is localized in the liver forming chronic cholangitis [82].
Scientific classification
Kingdom
Animalia
Phylum
Platyhelminthes
Class
Trematode
Subclass
Digenia
Order
Echinostomida
Suborder
Distomata
Family
Fasciolidae
Genus
Fasciola
Species
F.hepatica, F. gigantica
Source: [70]
Table 1: The taxonomical classification of Fasciola.
No
Region
Species of parasite
Prevalence/%/
References
1
Sheno municipal abattoir, Oromia region
Mixed(F.hepatica and F.gingantica)
74
[41]
2
Addis Ababa abattoir enterprise, AA
Both
18.75
[7]
3
Guduru and AbayChomaan Districts
Both
32.6
[19]
4
Addis Ababa Abattoir Enterprise
Both
18.8
[12]
5
Areka municipal abattoir ,southern ethiopia
Both
30
[45]
6
Hawassa Municipal abattoir, southern Ethiopia
Both
28.63
[1]
7
Hossana Municipal abattoir, Southern Ethiopia
Both
30.5
[16]
8
Woreta, Northwestern Ethiopia
Both
41.41
[69]
9
Addis Ababa Abattoir, Ethiopia
Both
20.3
[6]
10
Dessie Municipal Abattoir, South Wollo Zone, Ethiopia
Both
25.2
[9]
11
Nekemte Municipal abattoir
Both
21.9
[51]
12
Hosanna Municipal Abattoir, Southern Ethiopia
Both and mixed
30.47
[10]
13
Hosanna Municipal Abattoir, Southern Ethiopia
Both
30.47
[11]
14
ELFORA Export Abattoir, Bishoftu
Both
21.13
[35]
15
Andassa Livestock research center in north- west of Ethiopia
Both
60.42
[77]
16
Kombolcha Industrial Abattoir, Ethiopia
Both
28
[30]
17
bedele, ethiopia
Both
32.53
[79]
Table 2: Provides review of a comprehensive data on the prevalence of fasciolosis in postmortem and coprological examination in different parts of the Ethiopia.
The detection of adult flukes in the liver at necropsy is the most reliable method to confirm fasciolosis. Prevalence studies should be based on abattoir survey other than coproscopic investigation [33]. Acute fasciolosis is characterized by a badly damaged, swollen liver. The peritoneal cavity may contain an excess of blood-stained serum. The liver capsule shows many small perforations, sub capsular hemorrhage and the parenchyma shows tracts of damaged tissue and much more friable than the normal. Chronic fasciolosis is characterized by the presence of leaf-like flukes in grossly enlarged and thickened bile ducts particularly in the ventral lobe of the liver. Calcification of bile duct walls is a common finding in cattle. The hepatic parenchyma is extensively fibrosed and the hepatic lymph nodes are dark brown in color (Radostits et al., 2007).
Treatment
For the treatment of acute Fasciolosis, it is essential to choose a product highly effective against the juveniles that damage the liver parenchyma. For chronic disease a compound active against adult fluke is required (Radostits et al., 2007). Triclabendazole (Fasinex) is considered as the most common drug due to its high efficacy against adult as well juvenile flukes. It is effective against adult F. hepatica at a dose rate of 7.5 mg/kg in sheep and 10 mg/kg in cattle. It is ovicidal and well kills any F. hepatica eggs present in the bile duct or the alimentary tract at the time of treatment. Clorsulon is supplied in combination with ivermectin for combined fluke and around warm control in cattle. Nitroxynil is given sub cutaneausly at 10 mg/kg and has good efficacy against the adult fluke but the dose has to be increase by up to 50% to obtain adequate control of acute disease (Radostits et al., 2007).
Prevention and Control
Several control methods against ruminant fasciolosis are available and can either be used independently and as a combination of two or more of them. These methods involve reduction in the number of intermediate snail hosts by chemical or biological means, strategic application of anthelmintics, reduction in the number of snails by drainage, fencing and other management practices and reduction in the risk of infection by planned grazing management [70].
Controlling intermediate host (snail)
Control of parasitic diseases is crucial to improve the productivity of the animals. In most fasciolosis endemicareas, the control of the intermediate snail host population offers a good opportunity for the reduction of transmission and is generally effective when combined with one or more other methods such as chemotherapy or environmental sanitation (Solomon and Alemu, 2019; [81]. Although eradication of the snail hosts is the most effective method of total fluke controls this, however, is often very difficult in low-lying, wet areas with a mild climate. Snails multiply extremely rapidly and hence eradication is almost impossible in irrigation areas. There are different types of snail poison available that are safe for stock but need care and precision in their application. Other use full methods of fluke control include biological control of the intermediate host, fencing the water logged area and soon [43,81].
The use of molluscicides for the control of snail intermediate hosts is a potential tool for the control of fluke infections. Before considering chemical control of snails, it should be noted that many habitats are topographically unsuitable for the use of molluscicides and it is often very difficult to apply them effectively. Whereas, they are not species-specific, may destroy edible snails highly valued as food in some communities and expensive [81].
A great number of chemicals have been used as molluscicides in the past, but at present Niclosamide (Bayluscide or mollotor) and copper sulfate are used in different part of African Countries. Molluscicidal properties have been demonstrated in extracts from a variety of plants. A substance ‘Endod’ or toxinsderived from the fruits of shrubs Phytolacca dodecandra [13]. Substance such as ‘Endod’ might provide means of snail control less costly to developing countries than synthesized by molluscides but the production naturally molluscides on a commercial scale has yet to achieved [21,25,63] from their finding they indicated that ‘Endod’ used for the control of fasciola transmitting snails particularly L. truncatula and L.natalensis.
Controlling by use of chemicals (Chemotherapy)
Effective control of most trematode infections is based on strategically applied chemotherapy [81]. Combination of chemotherapy, intermediate host control, sanitation and Environmental manipulation are believed to be more efficient but very expensive. Chemotherapy with drugs remains the most cost-effective way of treating parasitic diseases, and is usually at the heart of any major control campaign. Compared to environmental engineering, drug treatment is very cheap [25]. The drugs to be used against flukes should ideally destroy the migrating immature flukes as well as adults in the bile ducts. Several drugs are now available for the treatment of fasciolosis, which are against the adult flukes, and the parenchymal stages. These include Rafoxanide, Nitroxynil, Brotanide, Closantel and Albendazole. Diamphentide that kills all immature flukes even a day old once and the Triclbendazole (TCBZ) are highly effective against all stages of fluke. It is one of the widely used drugs worldwide for the control of fasciolosis [25,63]. Chemotherapy normally reduces the prevalence and intensity of infection as measured by fecaleggcounts [27].
Environmental sanitation
Draining swamps, building sewage systems and providing clean water supplies are used to control water-borne /including snail borne/ helminths but it is very expensive compare to chemotherapy [25,81]. Strategies for the treatment and prophylaxis of infections with Fasciola are developed based on Epidemiological data. Effective treatment during the prepatent period for an extended duration could eliminate fasciola infection or reduce contamination of pasture to a very low level, requiring less frequent treatments for a considerable time [36,81].
Immunity and immunization
It has been suggested that natural immunity is expressed both during the migratory parenchymal and adult bile duct stages of the infection. This is considered to be related to the distribution and amount of connective tissue in the hosts liver parenchyma. Cattles are more resistance because of the relatively large amount of connective tissue in their liver possibly the connective tissue helps to trap young migrating flukes. Immunity to F. hepatica has been demonstrated and antibodies can be found in the blood of infected animal. Observation in the field indicated that older animals become resistant to infection [70]. F. hepatica has a number of survival mechanisms for evading host immune responses, including changing its surface antigen during migration, releasing a proteolytic enzyme that can cleave immunoglobins and modulating the host immune response (Radostits et al., 2007).
Status of Ruminant Fasciolosis in Ethiopia
Currently in Ethiopia, Fasciola hepatica is wide spread in areas with altitude of 1200-2500 masl. Both Fasciola species co-exist in area with latitude ranging between 1200-1800 masl. [78]. The snail intermediate hosts of Fasciola species in Ethiopia are principally two, which are Lymnaea truncatula and Lymnaea natalensis. The former one is the intermediate hosts of F. hepatica and the later for F. gigantica. Lymnaea natalensis are widely distributed in low land (kola), in irrigated canals, and pockets of water with vegetation, Lymnaea truncatula are usually encountered in medium altitude (Weyna dega) and a high land (dega), also the prevalence of the disease is mostly encountered in these areas [76].
Public Health Importance of Fasciolosis
Fasciolosis occasionally affects humans, thus Considered as a zoonotic infection [47,74] but it is a disease of sheep, goat, and cattle mainly [4]. The study shown that human fasciolosis to be an important public health problem [17]. The incidence of human cases has been increasing in countries of five contents. It has been reported from countries in Europe, America, Asia, Africa and Oceania [22,39]. A global analysis show that the expected correlation between animal and human fasciolosis only apparent a basic level. High prevalence in human infections is not found in areas where fasciolosis is a great veterinary problem. For instance, in South America, hyper endemics and mesoendemics are found in Bolivia and Peru where the veterinary problem is less important, while in countries such as Uruguay, Argentina and Chile, human fasciolosis only sporadic or hypoendemic [40,59].
F. hepatic may be acquired by man, but not directly from cattle. The person must ingest the metacercaria in order to become infected [39]. The most common transmission route is ingestion of watercress although; depending up on the geographic location and a variety of edible aquatic plants can be vehicles of transmission [56]. The symptoms in Humans vary depending on whether the disease is chronic or acute. During the acute phase, the immature worms begin penetrating the gut, causing symptoms of fever, nausea, swollen liver, skin rashes, and extreme abdominal pain. The chronic phase occurs when the worms mature in the bile duct, and can cause symptoms of intermittent pain, jaundice, and anemia [75]. Global estimate prevalence is between 1.7 and 2.4 Million human infection worldwide and a further 180 Million at risk of infection [56].
Human fasciolosis has been reported from countries in Europe, America, Asia, Africa and Oceania. The incidence of human case has been increasing in the 51 countries of 5 continents. A person must ingest the metacercaria to become infected. Human acquire infection through ingestion of metacercaria that are attached to certain aquatic plant and vegetable. In addition experimental studies suggested that human consuming raw liver dish from liver infected with juvenile flukes could become infected [2].
Conclusion and Recommendations
Ruminant fasciolosis is an economically important parasitic disease causing great loss of revenue through reduction in productivity of animal in terms of lowered growth rate, meat and milk production, fertility, feed efficiency and draught power in Ethiopia. The disease aggravation depends on distribution of Lymnaeid species snails which are the intermediate hosts of the fluke in areas where the cattle and sheep raised. The young parasite that developed on small intestine of definitive host can cause mechanical damage to the liver tissue up on its migration for feeding that result on bacillary hemoglobinuria and other abnormalities in the cattle. The diagnosis may be conducted by demonstration of fasciola egg from fecal sample in the laboratory and examination of infected animal liver after slaughter. The disease prevention and control involve controlling snail or intermediate hosts, programed use of anthelmintics, facilitating environmental sanitation and good management practices of herds and their grazing conditions. Many reports in Ethiopia show that fascioliasis is mainly an animal disease, causing a great economic burden in the highland areas of the country. There are only a few reported cases of the disease in humans that reported from the areas where the animal fasciolosis are fierily reported.
Therefore, the Ruminant fasciolosis in Ethiopia has significant impact on the economy of the country. So that, the following recommendations are forwarded based on the above conclusion;
¾Control of snails (intermediate host for Fasciola species) is highly recommended to control and prevent the disease.
¾Every animal owner should be able to regularly treat their animals with the appropriate anthelmintics and awareness should be created on the prevention and control methods of fasciolosis.
¾Government bodies should have created effective policies in collaboration with veterinarians to control and prevent the disease throughout the country.
¾Solid epidemiological investigations should be conducted on the current status of ruminant fasciolosis in the country.
References
- Abebe R, Abunna F, Berhane M, Mekuria S, Megersa B, et al. Fasciolosis: Prevalence, financial losses due to liver condemnation and evaluation of a simple sedimentation diagnostic technique in cattle slaughtered at Hawassa Municipal abattoir, southern Ethiopia. Ethiopian Veterinary Journal. 2010; 14: 39-52.
- Admassu B, Shite A, Kinfe G. A Review on Bovine Fasciolosis. European Journal of Biological Sciences. 2015; 7: 139-146.
- Ameni G, Erko B, Bogale T. Preliminary study on the major bovine trematode infections around Kemissie, northeastern Ethiopia and treatment trial with praziquantel. Bull Animal Heallth and Production Afr. 2001; 49: 62-67.
- Andrews SJ. The life cycle of F. hepatica (3rd ed.) Wallingford: CABI publishing. 1999; 1-30.
- Antonia M, Coceicao P, Rule M Durao, Isabel H Costa, Jose M, et al. Evaluation of single sedimentation method, modified McMaster for diagnosis of bovine fasciolosis. Journal of Veteterinary Parasitology. 2002; 105: 337-343.
- Aragaw K, Negus Y, Denbarga Y, Sheferaw D. Fasciolosis in slaughtered cattle in Addis Ababa abattoir, Ethiopia. Global Veterinarian. 2012; 8: 115-118.
- Bayu Y, Asmelash A, Zerom K, Ayalew T. Prevalence and economic importance of liver parasites: Hydatid Cyst, Fasciola species and Cysticercus tenuicolis in sheep and goats slaughtered at Addis Ababa abattoir enterprise in Ethiopia. Journal of Veterinary Medicine and Animal Health. 2013; 5: 1-7.
- Behm CA, Sangster NC. Pathology, pathophysiology and clinical aspects. In: Dalton JP (Ed), Fasciolosis. CAB International Publishing, Wallingford. 1999: 185-224.
- Belay E, Molla W, Amare A. Prevalence and Economic Losses of Bovine Fasciolosis in Dessie Municipal Abattoir, South Wollo Zone, Ethiopia. Journal of Biological Sciences. 2012; 4: 53-59.
- Betebo T. Prevalence of Fasciolosis in Cattle Slathered at Hosanna Municipal Abattoir, Southern Ethiopia. International Journal Advanced Research of Biolological Science. 2017a; 4: 70-76.
- Betebo T. Prevalence of Fasciolosis in Cattle Slathered at Hosanna Municipal Abattoir, Southern Ethiopia. International Journal Advanced Research of Biolological Science. 2017b; 4: 70-76.
- Birhanu A, Tesfaye R, Derso S. Prevalence and Associated Risk Factors of Fasciola Infection in Small Ruminants Slaughtered at Addis Ababa Abattoir Enterprise, Ethiopia with Reference to Diagnostic Value of Its Coprological Examination. African Journal of Basic & Applied Sciences. 2015; 7: 181-186.
- Brown DS. Fresh water snails of Africa and their Medical importance (2ndEd.), Taylorand FrancisLtd, London. 2005; 169-487.
- Centers for Disease Control and Prevention. Parasites and health Fascioliasis: http://www.dpd.cdc.gov/dpdx / HTML / Fasciolosis.htm. 2005.
- Centers for Disease Control and Prevention. Fasciolosis: DPDx- Laboratory Identification of parasitic disease of Public Health concern. 2013.
- Chakiso B, Menkir S, Desta M. On Farm Study of Bovine Fasciolosis in Lemo District and its economic loss due to liver condemnation at Hossana Municipal abattoir, Southern Ethiopia. International Journal of Current Microbiological Applied Science. 2014; 3: 1122-1132.
- Chen MG, Motti KE. Progress in assessment of morbidity due to Fasciola hepatica infection: A reviews of recent literature. Tropical Disease Bulletin. 1990; 87: 1-3.
- Chhabra MB, Singla LD. Food-borne parasitic zoonoses in India: Review of recent reports of human infections. Journal of Veterinary Parasitolology. 2009; 23: 103-110.
- Daksa G, Abdisa M, Desalegn J. Abattoir Survey on Prevalence of Bovine Fasciolosis in Guduru and Abay Chomaan Districts. World Journal of Agricultural Sciences. 2016; 12: 111-118.
- Dalton JP, Spithil TW. Progress in Development of Liver Fluke Vaccines. Parasitology Today. 1998; 14: 224-228.
- Eguale T, Tilahune G. Molluscicidal effects of Endod (Phytolacca dodecndra) on Fasciola transmitting snails. SINET: Ethiopian Journal of Science. 2002; 25: 275-84.
- Esteban JG, Bargues MD, Mas Coma S. Geographical Distribution, diagnosis and treatment of human fascioliasis: a review. Res Rev Parasitol. 1998; 58: 13-42.
- Ferre I, Ortega-Mera L, Rojo-Vazquez FA. Serum and bile antibody responses (IgG and IgA) during subclinical Fasciola hepatica infection in sheep, Spain. Journal of Veterinary Parasitology. 1997; 68: 261-267.
- Food agriculture organization of united nation. Disease of domestic animal caused by flukes; epidemiology, diagnosis and control of fasciola , paramphistome, dicrolium, eurytrema and Schistosome interaction in ruminants, in development countries (FOA) UN, Vialedelle termedicaracalla, Rome, Italy. 1994; 49.
- Gaasenbeek CPH, Moll L, Cornelissen JB, Vellema P, Borgsteede FH. An experimental study on Triclabendazole resistance of Fasciola hepaticain sheep. Veterinary Parasitolology. 2001; 95: 37-43.
- Galtier P, Coulet M, Sutra JF, Biro Souvour B, Aluinerie M. Fasciola hepatica: Mebendazole and Thiabendazole pharmacokinetics in sheep. Journal of veterinary parasitology. 1994; 79: 166-176.
- Hansen DS, Clery DG, Estuningsih SE, Widjajanti S, Partoutomo, et al. Immune responses in Indonesianth in tailand Merino sheep during a primary infection with Fasciola gigantica:Lack of a specific IgG2 antibody response is associated with increased resistance to infection in Indonesian sheep. International Journal of Parasitology. 1999; 29: 1027-35.
- Hanson J, Perry B. The Epidemiology, Diagnosis and Control of Helminth Parasites of Ruminants, A hand book, Printed by the International Livestock Center for Africa, Addis Ababa, Ethiopia. 1994; 72.
- Hendrix CM. Diagnostic Veterinary Parasitology. Second edition. USA: Mosby, Inc. 1998; 108-136.
- Ibrahim N, Wasihun P, Tolosa T. Prevalence of bovine fasciolosis and economic Importance due to liver condemnation at Kombolcha Industrial Abattoir, Ethiopia. International Jornal of veterinary medicine. 2010; 8: 2.
- Imani Baran A, Cheraghi SH, Katiraee F. Molecular Determination of Fasciola Spp. Isolates from Domestic Ruminants Fecal Samples in the Northwest of Iran. Iranian journal of parasitology. 2017; 12: 243-250.
- Kassai T. Veterinary Helmintology. University of science, Oxford: Butterworth Heinemann. 1999; 9.
- Kaufmann J. Parasitic Infection of Domestic Animals. A diagnostic manual: 1sted. Berlin: Birhouser Verlag. 1996; 90-92.
- Keyyu JD, Kassuku AA, Msalilwa LP, Monrad J, Kyvsgaard NC. Crosssectional prevalence of helminth infections in cattle on traditional, small-scale and large-scale dairy farms in Iringa district, Tanzania, Veterinary research communications. 2006; 30: 45-55.
- Kitila DB, Megerssa YC. Pathological and serum biochemical study of liver fluke infection in ruminants slaughtered at ELFORA Export Abattoir, Bishoftu, Ethiopia. Global Journal of Medical Research. 2015; 48.
- Malone JB, Gommes R, Hansen J, Yilma JM, Slingenberg J. A geographic information system on the potential distribution and abundance of Fasciola hepatica and F. gigantic in east Africa based on Food and Agriculture Organization databases. Veterinary Parasitolology. 1998; 78: 87-101.
- Maqbool A, Hayat CS, Tanveer A, Hashmi HA. Epidemiology of Fasciolosis in Buffaloes under Different Management Conditions. Veterinarski Arhiv. 2002; 72: 221-228.
- Martinez-Moreno A, Jimeney A, Lugue V, Moreno T, Radondo E, et al. Liver pathology and immune response in experimental Fasciola hepatica infection of goats. Journal Veterinary Parasitolology. 1999; 82: 19-33.
- Mas Coma S, Barguest MD, Esteban JG. Human fasciolosis Dalton, J.P. ed. LABI publishing, walling ford, UK. 1999; 411-34.
- Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. International Journal of Parasitology. 2005; 35: 1255-1278.
- Mekonnen Y, Mekonnen A. Factors influencing the use of maternal healthcare services in Ethiopia. Journal of health, population and nutrition. 2017; 374-382.
- Asrat Michael. Infectious Prevalence of Ovine Fasciolosis in Irrigation Schemes along The Upper Awash River Basin and Effect of Strategic Anthelmintic Treatment in Selected up Stream Areas. M.Sc. Thesis, Department of Biology, School of Graduate Studies, Addis Ababa University, Addis Ababa. 2004.
- Mitchael A, Yilma J. Implication Awash River basin area, in the partial fulfillment for the attainment of the Degree of Master of Science in Biology department, biomedical Science in AAU, Ethiopia. 2001.
- Mitchell GB. Treatment and Control of liver fluke in sheep and cattle. Technical notes November, Sac 2003. West mains roads, Edinburgh. 2003.
- Moje N, Mathewos S, Desissa F, Regassa A. Cross-sectional study on bovine fasciolosis: prevalence, coprological, abattoir survey and financial loss due to liver condemnation at Areka Municipal Abattoir, Southern Ethiopia. Journal of Veterinary Medicine and Animal Health. 2015; 7: 33-38.
- Nyindo M, Lukambagire AH. Fascioliasis: an ongoing zoonotic trematode infection. Biomedical Medical research. 2015.
- Okewole E, Aogndip GAT, Adejimmi JO, Olaniyan AA. Clinical evaluation of chemoprophylactic regime against ovine hementheasis in fasciola endemic farm in Ibadan, Nigeria. Israel Journal of Veterinary Medicine. 2000; 5691: 15-28.
- Olsen O. Animal parasites: their life cycle and ecology, (3rd Ed.), Royal University, Park press, London. 1991; 120-128.
- Ortiz P, Scarcella S, Cerna C, Rosales C, Cabrera M, et al. Resistance of Fasciola hepatica against Triclabendazole in cattle in Cajamarca (Peru): a clinical trial and an in vivo efficacy test in sheep. Veterinary parasitology. 2013; 195: 118-121.
- Payne WJ. An Introduction to Animal Husbandry in the Tropics. Fourth edition. ELBS, UK, Longman, British. 1990; 238-258.
- Petros A, Kebede A, Wolde A. Prevalence and economic significance of bovine fasciolosis in Nekemte municipal abattoir. Journal of Veterinary Medicine and Animal Health. 2013; 5: 202-205.
- Qureshi AW, Tan veer A, Maqbool A, Niaz S. Seasonal and monthly prevalence pattern of Fasciolosis in buffaloes and its relation to some climatic factors in northeastern areas of Punjab, Pakistan. Journal of Veterinary Research, Shiraz University. 2012; 13: 39.
- OM, Gay CC, Hinchclitt KW, Constable PD. Veterinary Medicine, a Text Book of the Disease of Cattle, Horses, Sheep, Goats, and Pigs. 10th (Edn.), Elsevier, New York. 2007; 1516-1579.
- Rahman AK, Islam SK, Talukder MH, Hassan MK, Dhand NK, et al. Fascioliasis risk factors and space-time clusters in domestic ruminants in Bangladesh" Parasites Vectors. 2017; 10: 228.
- Rahmato D. Water resource development in Ethiopia: Issue of sustainability and participation. Ethiopian Institute of Agricultural Research. 1999; 49.
- Ramajo V, Oleaga A, Casanueva P, Hillyer GV, Muro A. vacination of sheep against Fasciola hepatica with homologous fatty acid binding proteins. Veterinary Parasitology. 2001; 97: 35-46.
- Rehman TU, Khan MN, Sajid MS, Javed MT. Slaughter house based epidemiology and estimation of economic losses of bovine fascioliasis in tehsil sargodha. Pakistan Journal of Science. 2013; 65: 467-472.
- Richter J, Fraise S, Mull R, Millan JC. Fasciolosis: son graphic abnormalities of the bilary tract and evolution after treatment with Triclabendazole. Tropical Medicine of International Heath. 1999; 11: 774-781.
- Rokin MB, Moreover MJ, Kia EB. Comparison, of Adult somatic and cysteine proteinase antigens of Fasciola gigantica in Enzyme linked Immunosorbent Assay for diagnosis of bovine Fasciolosis. DIE seminar on biotechnology, proceeding November, Tehran, Iran. 2003; 9-13.
- Sloss MW, Kemp RL, Zajac AM. Veterinary clinical parasitology. 6 ed. London: Blackwell publishing. 1994; 90-92.
- Smyth D. Introduction to Animal Parasitology. 3rd edition, Cambridge University press, UK. 1994; 203-212.
- Soulsby EJ. Fascioliasis, Helminthiasis, Arthropods and protozoa of domestic animals, 7th ed. Lea and Febiger Philadelphia. 1982; 40-52.
- Spithill TW, Dalton JP. Progress in development of liver fluke vaccine. Parasitology. 1998; 14: 224-228.
- Spithill TW, Smooker PM, Copman DB. Fasciola gigantica: Epidemiology, control, immunology and molecular biology. In: Fasciolosis (edited by Dalton, J. P.). Dublin City University. CAB International Publishing, UK. 1999; 465-466.
- Taylor MA, Coop RL, Wall RL. Veterinary parasitology. 3rd ed. oxford Black well Publishing. 2007; 85-87.
- Terefe D, Wondimu A, Gachen D. Prevalence, gross pathological lesions and economic losses of bovine Fasciolosis at Jimma Municipal Abattoir, Ethiopia. Journal of Veterinary Medicine and Animal Health. 2012; 4: 6-11.
- Tesfaheywet Z. Helminthosis of Sheep and Goats in and around Haramaya, Southeastern Ethiopia. Journal of Veterinary Medicine and Animal Health. 2012; 4: 48-55.
- Theodoridis Y, Duncan JL, MacLean JM. Pathphysiological studies on Dicrocoelium dendreticum infection in sheep. Journal of veterinary parasitology. 1991; 39: 61-66.
- Tsegaye B, Abebaw H, Girma S. Study on coprological prevalence of bovine fasciolosis in and around Woreta, Northwestern Ethiopia. Journal of Veterinary Medicine and Animal Health. 2012; 4: 89-92.
- Urquhart GM, Amour J, Duncan JL, Dunn AM, Jennings FW. Veterinary Parasitology 2nd Edn, Oxford, Longman Scientific and Technical Press, UK. 1996; 100-109.
- Urquhart GM, Amour JL, Duncan JL, Dunn AM, Jennings FW. Veterinary Parasitology. 3rd (Edn.) Black Well Science, Hoboken. 2007; 103-133.
- Urquhart GM, Armour JL, Duncan JL, Dunn AM, Jennings FW. Veterinary parasitology. Oxford: Longman Scientific. 1994; 98-109.
- Usip LP, Banga ES, Doho HJ, Amadi E, Utah E. Varying Periods of Infection. Tropical Animal Health and Production. Research Journal of Food Science and Technology. 2014; 3: 54-75.
- World Health Organization. Control of Food Borne Trematode Infections. Technical Report Series. 1995; 849: 1-157.
- World Health Organization. Human Fascioliasis, 2016; 11: 149-158.
- Yadeta B. Epidemiology of bovine and ovine fasciolosis and distribution of snail intermediate host in western shoa DVM, FVM Thesis, AAU. 1994; 35.
- Yeneneh A, Kebede H, Fentahun T, Chanie M. Prevalence of cattle flukes infection at Andassa Livestock Research Center in north-west of Ethiopia. Veterinary Research Forum. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. 2012; 3: 85-89.
- Yilma JM, Malone JB. Geographic information systems forcost model for strategic control of Fasciolosis in Ethiopia. Veterinary Parasitology. 1998; 78: 103-23.
- Yitagezu A, Tefera W, Mahendra P. Prevalence of bovine fasciolosis and its economic impact in Bedele, Ethiopia. Haryana Veterinarian. 2015a; 54: 7-10.
- Yitagezu A, Tefera W, Mahendra P. Prevalence of bovine fasciolosis and its economic impact in Bedele, Ethiopia. Haryana Veterinarian. 2015b; 54: 7-10.
- Yohannes E. Prevalence and gross pathological lesion of bovine fasciolosis in Mekele municipal abattoir. DVM thesis, School of veterinary collage of Agriculture and Veterinary Medicine. Jimma, Ethiopia. European Journal of Animal Science and Technology. 2008; 1: 39-47.
- Zheng HJ, Tao Zheng-Hou CW, Pessens WF. Comparison of dot ELISA with Sandwich ELISA for the detection of circulating antigens inpatient with Bancroftiasis. Journal of Tropical Medicine and Hygiene. 1990; 42: 546-549.