
Review Article
Austin J Vet Sci & Anim Husb. 2023; 10(3): 1120.
Review on Discriminatory Tests of Immune Reaction due to Infection and Vaccination (DIVA Test)
Tsion B¹* and Fanose T²
1Sebeta, Oromia, Ethiopia Animal Health Institute, Department of Veterinary Microbiology, Ethiopia
2Bishofitu, Oromia Addis Ababa University College of Veterinary Medicine and Agriculture, Ethiopia
*Corresponding author: Tsion B Sebeta, Oromia, Ethiopia Animal Health Institute, Department of Veterinary Microbiology, Ethiopia
Received: January 21, 2023; Accepted: March 07, 2023; Published: March 14, 2023
Abstract
In concern for animal diseases, the goal of vaccination is to prevent or reduce clinical diseases associated with the infectious agent, but it can also be used as a means of managing or eradicating a disease from a particular region. Therefore it is essential to differentiate immune responses due to vaccination compared to natural infection. It was to satisfy this requirement that the term Differentiation of Infected from Vaccinated Animals (DIVA) was coined in 1999 by Jan T van Oirschot. DIVA principle is based on a DIVA vaccine producing an antibody response that is different from the antibody response produced by the wild-type virus. The DIVA approach has been applied and proposed successfully to various diseases. Although considerable progress has been made to evaluate which different DIVA strategies are most likely to be applicable in the field, considerably more work needs to be done. If properly applied, DIVA vaccination strategies promise to provide new tools for the control and eradication of diseases.
Keywords: Control; Diseases; DIVA vaccines; Eradication
Abbreviations: DIVA: Differentiation of Infected from Vaccinated Animals
Introduction
Vaccination has effects at the individual and population, levels. Efficacious vaccines reduce or prevent clinical signs without necessarily preventing virus replication. They may also increase the dose of virus needed to establish an infection and/or reduce the level and duration of virus shedding following infection. Vaccine effectiveness within a population is a function of its ability to reduce virus transmission. Transmission is best described by the reproductive ratio, R, which is defined as the average number of new infections caused by one infectious individual. By helping to reduce the R-value below 1, vaccination can be an effective adjunct in abbreviating an outbreak. Nevertheless, vaccination can also complicate serological surveillance activities that follow eradication, if the antibody response induced by vaccination is indistinguishable from that which follows infection. This disadvantage can be overcome by the use of DIVA vaccines and their companion diagnostic tests [1,2].
The term DIVA (differentiating infected from vaccinated individuals) was coined in 1999 van Oirschot of the Central Veterinary Institute, in the Netherlands [3]. It is now generally used as an acronym for differentiating infected from vaccinated animals. The term was originally applied to the use of marker vaccines, which are based on deletion mutants of wild-type microbes, in conjunction with a differentiating diagnostic test. The DIVA strategy has been extended to include subunit and killed whole-virus vaccines. This system makes possible the mass vaccination of a susceptible animal population without compromising the serological identification of convalescent individuals. The DIVA approach has been applied successfully to pseudo rabies and avian influenza eradication, and has been proposed for use in foot-and-mouth disease and classical swine fever eradication campaigns [4].
This document provides a short review on the design and use of DIVA tests to differentiate between vaccinated and infected animals and also its impact on disease control and eradication.
Principle of Diva Test
The general DIVA principle is that antibody response produced by vaccination can be differentiated from the antibody response elicited by natural infection (e.g. a modified wild type virus with a gene deletion resulting in the absence of a particular diagnostic antigen). It can be live or killed vaccines. The DIVA immune response can be detected by companion diagnostic tests such as enzyme linked immunosorbent assay. Companion serology based tests rely on seroconversion, and upon exposure to wild type virus the antibody response to DIVA vaccines will be masked by that of the wild type virus [5]. More recently, the use of genetic DIVA has been introduced, which is based on identifying genetic differences between live vaccines and the field viruses.
The ability to identify and selectively delete genes from a pathogen has allowed the development of “marker vaccines” that, combined with suitable diagnostic assays, allow Differentiating Infected from Vaccinated Animals (DIVA) by differentiation of antibody responses induced by the vaccine (no antibodies generated to deleted genes) from those induced during infection with the wild-type virus. Such DIVA vaccines and their companion diagnostic tests are now available or in development for several diseases including Infectious Bovine Rhinotracheitis (IBR), pseudorabies, Classical Swine Fever (CSF), and FMD.
A marker vaccine can be either positive or negative in that it can include antigens not found in the wild-type virus, which would be a positive-marker vaccine, or it can contain fewer antigens than the wild-type virus and would be a negative-marker vaccine. In either case, a companion diagnostic test needs to be available to allow the differentiation of the vaccinated animal to the naturally infected animal [6].
The main advantage of DIVA vaccines and their companions test is the possibility to distinguish between infected and vaccinated animals. Therefore, special restrictions, which are necessary for infected animals, can be loosened for vaccinated animals [8].
Diva Designing and Development Strategies
The DIVA strategy has been extended to include subunit and killed whole-virus vaccines. This system makes possible the mass vaccination of a susceptible animal population without compromising the serological identification of convalescent individuals. The DIVA approach has been applied successfully to pseudorabies and avian influenza eradication, and has been proposed for use in foot-and-mouth disease and classical swine fever eradication campaigns [9].
Suitable and simple tests for differentiating vaccinated animals from infected are required for DIVA designing. The simple test has to be defined to differentiate vaccine strain from field strain. Although mucosal route is the best and most warranted, one study is required to prove that which of the route gives best protection against natural way of infection. Suitable dosages defined in terms of colony forming units (cfu) per dose have to be determined. Live vaccines require a bit of nutrient to support the life and side by side there is need to keep the cfu at constant level therefore live vaccines need a suitable excipient when the vaccine is not a freeze dried one and when it is freeze dried it need a suitable freeze drying and re- suspension medium to be chosen after experimentation [7].
There are a number of different DIVA systems currently available or in the process of development and validation. One type is Heterologous Nuraminidase type DIVA. It is a DIVA strategy based on the use of an inactivated oil-emulsion vaccine containing the same Hemaglutinin subtype as the challenge virus, but a different NA [10].
Four different serologic tests have been described for the hNA DIVA strategy including the indirect immunofluorescent antibody test (iIFAT), a standard Neuraminidase Inhibition (NI) test, a modified NI test, and the ELISA test. For both the standard and modified NI tests, because they measure the reduction of NA activity they are designed to be used with any NA subtype. However, because of antigenic variation and the difficulty in running large numbers of samples with either test, both tests are used only experimentally, with little chance that they could be adapted to be a rapid, sensitive, and inexpensive test that can test large numbers of samples [11].
Another one is Non-structural 1 protein DIVA, a promising system based on the detection of antibodies against a specific antigen, the NS1 protein of AI, has been deemed a good DIVA candidate. The NS1 protein is synthesized in large amounts in infected cells but is not incorporated into the mature virions, and for this reason represents the ideal candidate to elicit a specific immune response [12].
It may be possible to differentiate the epitopes that induce antibodies in vaccinated and infected animals. Luminex is a florescence microsphere immunoassay technology where microspheres can be coupled with different antigens and the profile of antibodies can be assessed [6].
In endemic setting, NSP tests can be used to support sero-surveillance exercises that assess the prevalence of infection in livestock and wildlife, especially where the results for SP tests might be complicate by the presence of vaccine-induced antibodies. When used for ruminants, a limitation of these tests is that they are unable to distinguish between convalescent and carrier animals [13].
Vaccine DIVA functionality is often limited to large viruses with increased potential for gene deletion and removal of redundant expressed antigens. Therefore, for viruses with small genomes such as paramyxoviruses where gene deletion of neutralizing antigens may reduce vaccine efficacy, alternative approaches are required to provide DIVA functionality [14].
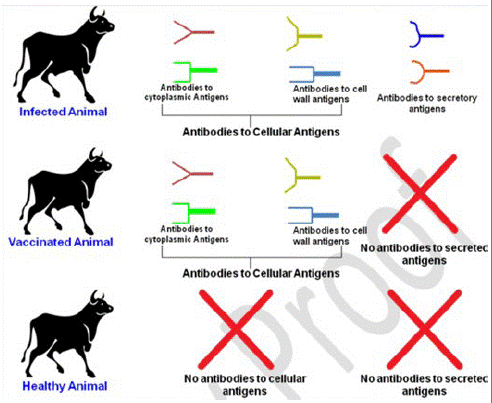
Figure 1: Scheme of the immune response that can be used to differentiate vaccinated and natural infected animals (Source: 7)
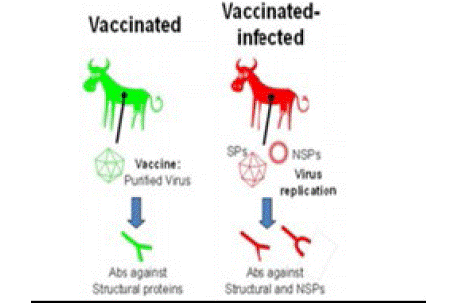
Figure 2: The principle of using Non-Structural Protein (NSPs) tests to differentiate between vaccinated and infected animals. Both structural (SP) and NSP antigens induce the production of antibodies in infected animals. In contrast, vaccinated animals that have not been exposed to replicating virus will only develop antibodies to the viral capsid antigens (Source: 13)
One approach is to design molecular DIVA vaccines that contain a marker nucleotide sequence differing from the wild type virus that can be employed in combination with PCR-based molecular diagnostics to differentiate between vaccine and wild virus strains. Successful differentiation of vaccinated from non-vaccinated animals using this technique requires concurrent vaccination and infection, with a narrow diagnostic window post-infection for detection of DIVA vaccine and viral genetic material. Furthermore, detection of vaccine genetic material only demonstrates exposure to the vaccine and not the successful generation of immune protection, limiting functionality in assessment of herd level immunity [5].
A potential approach that can meet these needs is based on the application of metabolomics to identify metabolites or ‘small molecules’ in biological samples that are signatures that correlate or provide some evidence of immune protection. These metabolites are often the end stage products of biological processes and therefore provide an accurate representation of an organism’s homeostatic status at time of sampling. Metabolomic analysis of bio-fluids has provided new insights to the understanding of the patho-physiological processes involved in disease establishment, development and diagnosis [15].
Use of Diva Vaccines for Disease Control and Eradication
In order to achieve the goal of eradication, DIVA vaccination strategies have been recommended and must be implemented. These systems, coupled with an appropriate monitoring system, enable the detection of field exposure in vaccinated flocks, and through this, infected flocks may be properly managed. There are a number of different DIVA systems currently available or in the process of development and validation [9].
Emergency vaccination using DIVA vaccines could be one control tool for disease outbreaks in densely populated livestock areas. DIVA vaccination might limit the number of culled animals in the process of disease eradication, thereby enhancing public acceptance for disease control measures and limiting economic damage. In contrast to conventional vaccination, DIVA vaccination should always be used as protective vaccination meaning that vaccinated animals are kept to the end of a normal production cycle and their meat eventually marketed [16].
The prophylactic use of vaccines against exotic viral infections in production animals was exclusively carried out in regions where the disease concerned was endemic. The DIVA vaccines allows for vaccination while still retaining the possibility of serological surveillance for the presence of infection [17].
IBR, caused by Bovine Herpesvirus type 1 (BHV-1) infection of cattle, and pseudorabies (Aujeszky's disease) in pigs have been identified internationally as being candidates for eradication from national herds, and so there has been an impetus for the development of DIVA vaccines and diagnostics. The demand for a marker (DIVA) vaccine for IBR in Europe was met by the development of a glycoprotein E (gE)-deleted vaccine using conventional methodology [18,19]. The gE protein is not essential for viral replication, but it plays a major role in intercellular spread, particularly along nerves. Specific diagnostic tests based on gE deletion have been developed using both gE-blocking Enzyme-Linked Immunosorbent Assay (ELISA) techniques and PCR amplification [20].
Deletion of the gE gene has also been used to enable a DIVA approach for an Aujeszky's disease vaccine [21]. The gene for thymidine kinase is also deleted in some formulations (e.g., Suvaxyn Aujesky), adding to the degree of attenuation [22].
Available Diva Vaccines for Farm Animals
DIVA vaccines were first used for the eradication of pseudorabies (Aujeszky disease) in pigs. Most of them are based on recombinant deletion mutants lack the gE envelope glycoprotein and thymidine kinase genes. The accompanying tests score pigs as seropositive for gE antibodies. DIVA vaccines against Infectious Bovine Rhinotracheitis (IBR) of cattle caused by the Bovine Herpesvirus 1 (BHV-1) work on a similar principle [23].
Successful DIVA technologies has been developed for animal vaccines like bovine rhinotracheitis (IBR), pseudo rabies, Classical Swine Fever (CSF) etc [24]. Strategies for developing DIVA based vaccines for other diseases like bovine tuberculosis [25,26], avian influenza [27], PPR [28] and bluetongue virus [29] are also under development.
Currently available Foot-and-Mouth Disease (FMD) vaccines are prepared from purified and chemically inactivated whole virus particles. The Non-Structural Proteins (NSP) were separated from virus particles. Serological assays detecting antibodies against NSP can therefore differentiate between infected and vaccinated animals [11].
Serological tests are widely used to monitor the immune status of animals exposed to infectious diseases. In contrast to SP tests such as the SPCE (Solid-Phase Completion ELISA), LPBE or VNT (Virus Neutralization Test), NSP ELISAs are not serotype specific and can therefore be used as generic screening tools. Therefore, in addition to their use to detect virus circulation in vaccinated livestock population, these tests are also used more generally for serological investigation, even when emergency vaccination is not practiced [17].
Currently, scientific research is in progress against many infectious diseases, such as, Salmonella infections in pigs, avian influenza, classical swine fever and Actinobacillus pleuropneumoniae [30,5].
Conclusion and Recommendation
Recently, the interest in DIVA vaccination has increased considerably. This is because of a greater understanding of how this technology can assist in disease control efforts and achieved by intensively vaccinating susceptible populations followed by its monitoring by the companion diagnostic test with the removal of the infected animals from the population. In absence of DIVA technology, vaccination programs cannot be implemented at national scale, as vaccinations often elicit immune responses indistinguishable from those generated by pathogens using standard test regimens. Although considerable progress has been made to evaluate which different DIVA strategies are most likely to be applicable in the field, considerably more work needs to be done. All the proposed DIVA strategies still have serious limitations that have prevented them from in the routine use. Therefore, additional development and validation of such a test be carried out on global scale, including longitudinal studies, to understand how quickly antibody can be detected and how long it persists, with many laboratories working in conjunction with one another. More data need to be gathered to understand the parameters that will affect our surveillance policies. So an effective strategy can be developed for combating the worldwide spread of various animal diseases.
References
- John Pasick. Application of DIVA vaccines and their companion diagnostic tests to foreign animal disease eradication. Animal Health Research Reviews. 2004; 5: 257-262.
- Maas A, Meens J, Baltes N, Hennig-Pauka I, Gerlach GF. Development of DIVA subunit vaccine against Actinobacillus pleuropneumoniae infection. Vaccine. 2006; 24: 3679-85.
- Oirschot JT. Diva vaccines that reduce virus transmission. Journal of Biotechnology. 1999; 73: 195-205.
- Patel JR, Heldens JG. Immunoprophylaxis against important virus disease of horses, farm animals and birds. Vaccine. 2009; 27: 1797–1810.
- Gray DW, Welsh MD, Mansoor F, Doherty S, Chevallier OP, et al. DIVA metabolomics: Differentiating vaccination status following viral challenge using metabolomic profiles. PLoS ONE. 2018; 13: e0194488.
- Javed R. Diva strategies for avian influenza. Mini-review. Int J Avian and Wildlife Biol. 2018; 3: 48-49.
- Jayaraman S, Jain M, Dhama K, Singh SV, Datta M, et al. Diva technology: indispensable tool for the control of johne‟s disease. Journal of Experimental Biology and Agricultural Sciences. 2016; 4.
- Kahrs RF. Viral Diseases of Cattle. 2nd ed. Iowa: Iowa State University Press. 2001; 324.
- Pasick J. Application of DIVA vaccines and their companion diagnostic tests to foreign animal disease eradication. Anim Health Res Rev. 2004; 5: 257-62.
- Uttenthal A, Satya P, Rasmussen TB, Paton DJ, Bernd H, et al. Strategies for differentiating infection in vaccinated animals (DIVA) for foot-and-mouth disease, classical swine fever and avian influenza. Expert Review of Vaccines. 2010; 9: 73-87.
- Chen SP, Lee MC, Sun YF, Yang PC. Application of non-structural protein ELISA kits in nationwide FMD surveillance in pigs to demonstrate virus circulation in Taiwan. Vet Microbiol. 2011; 152: 266-9.
- Avellaneda MG, Sylte C, Lee WD, Suarez LA. Heterologous neuraminidase subtype strategy for the differentiation of infected and vaccinated animals (DIVA) for avian influenza virus using an alternative neuraminidase inhibition test. Avian Dis. 2010; 54: 272-277.
- King DP, Ludi A, Wilsden G, Parida S, Paton DJ. The use of non-structural proteins to differentiate between vaccinated and infected animals. OIE Regional Commission, the Pirbright Institute, United Kingdom. 2015.
- Capua I, Terregino C, Cattoli G, Mutinelli F, Rodriguez JF. Development of a DIVA (Differentiating Infected from Vaccinated Animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza. Avian Pathol. 2003; 32: 47-55.
- Want EJ, Wilson ID, Gika H, Theodoridis G, Plumb RS, et al. Global metabolic profiling procedures for urine using UPLC-MS. Nature Protocols. 2015; 5: 1005-18.
- Clavijo A, Wright P, Kitching P. Developments in diagnostic techniques for differentiating infection from vaccination in FMD. Vet J. 2004; 167: 9-22.
- Lambrecht B, Steensels, MS Van Borm, G Meulemans, T Van den. Berg Development of an M2e-specific enzyme-linked immunosorbent assay for differentiating infected from vaccinated animals. Avian Dis. 2007; 51: 221-226.
- Kaashoek MJ, Moerman A, Madic J, Rijsewijk FAM, Quak J, et al. A conventionally attenuated glycoprotein E-negative strain of bovine herpesvirus type 1 is an efficacious and safe vaccine. Vaccine. 1994; 12: 439-44.
- Van Oirschot JT, MJ Kaashoek, FA Rijsewijk. Advances in the development and evaluation of bovine herpesvirus 1 vaccines. Vet. Microbial. 1996; 53: 43-54.
- Schynts F, E Baranowski, M Lemaire, E Thiry. A specific PCR to differentiate between gE negative vaccine and wildtype bovine herpesvirus type 1 strains. Vet. Microbiol. 1999; 66: 187-195.
- Pensaert M, G Labarque, H Favoreel, H Nauwynck. Aujeszky's disease vaccination and differentiation of vaccinated from infected pigs. Dev Biol. 2004; 119: 243-254.
- Ferrari M, A Brack, MG Romanelli, TC Mettenleiter, A Corradi, et al. A study of the ability of a TK-negative and gI/gE-negative pseudorabies virus (PRV) mutant inoculated by different routes to protect pigs against PRV infection. J Vet Med B Infect Dis Vet Public Health. 2000; 47: 753-762.
- Ganguly S, Padhy A, Para PA, Pandey AK, Praveen KP, et al. DIVA Vaccines: A Brief Review on its Novel Facets for the Eradication of Infections of Livestock and Poultry. World J Clin Pharmacol Microbiol Toxicol. 2015; 1: 22-23.
- Meeusen ENT, Walker J, Peters A, Pastoret PP, Jungersen G. Current status of veterinary vaccines. Clinical Microbiology Reviews. 2007; 20: 489-510.
- Milián-Suazo F, González-Ruiz S, Contreras- Magallanes YG, Sosa-Gallegos SL, Bárcenas-Reyes I, et al. Vaccination Strategies in a Potential Use of the Vaccine against Bovine Tuberculosis in Infected Herds. Animals. 2022; 12: 3377.
- Vordermeier HM, PJ Cockle, AO Whelan, S Rhodes, MA Chambers, et al. Effective DNA vaccination of cattle with the mycobacterial antigens MPB83 and MPB70 does not compromise the specificity of the comparative intradermal tuberculin skin test. Vaccine. 2001; 19: 1246-1255.
- Rahn J, Hoffmann D, Harder TC, Beer M. Vaccines against influenza A viruses in poultry and swine: Status and future developments. Vaccine. 2015; 33: 2414-2424.
- Liu F, Wu X, Liu W, Li L, Wang Z. Current perspectives on conventional and novel vaccines against peste des petits ruminants. Veterinary Research Communication. 2014; 38: 307-22.
- Calvo-Pinilla E, Castillo-Olivares J, Jabbar T, Ortego J, de la Poza F, et al. Recombinant vaccines against bluetongue virus. Virus Research. 2014; 182: 78-86.
- Fulton RW, Confer AW. Laboratory test descriptions for bovine respiratory disease diagnosis and their strengths and weaknesses: Gold standards for diagnosis, do they exist? Canadian Veterinary J. Revue Veterinaire Canadienne. 2012; 53: 754-61.