
Research Article
Austin J Vet Sci & Anim Husb. 2023; 10(4): 1128.
Seroprevalence, Risk Factors, Detection and Isolation of Peste Des Petits Ruminants Virus from Sheep
Milkessa Gelana*
Oromia Agricultural Research Institute, Bako Agricultural Research Center, Animal Health Researcher, Bako, Ethiopia
*Corresponding author: Milkessa Gelana Oromia Agricultural Research Institute, Bako Agricultural Research Center, Animal Health Researcher, Bako, Ethiopia. Tel: +251910189839; +251937750429 Email: mikyabe143@gmail.com
Received: August 23, 2023 Accepted: October 02, 2023 Published: October 09, 2023
Abstract
Cross-sectional study, which combined multistage cluster sampling and simple random sampling, was employed with the objectives of investigating seroprevalence, associated risk factors, molecular detection and isolation of Peste Des Petits Ruminants Virus (PPRV) in sheep from October 2019 to August 2019 in selected districts of Horo Guduru Wollega Zone. A total of 387 serum samples were collected from 58 flocks comprised of 387 sheep population. Competitive Enzyme-Linked Immunosorbent Assay (cELISA) was used to detect the presence of PPRV specific antibodies in the sera of animals. Pearson’s Chi-Square and logistic regression analyses were used to see the association of PPR seroprevalence with potential risk factors. The flock-level overall seroprevalence of PPR was found to be 25.86%. An overall seroprevalence of 6.98% (95% CI: 4.65, 10.00) was recorded in the study areas. The seroprevalence of PPR in sheep was significantly higher in mid-highland than highland and lowland (P=0.029). Though the overall seroprevalence of PPR in this study was low, the seropositivity of sheep was due to natural infection indicating the PPRV infection has been circulating in the study areas. Therefore, regular vaccination should be given for sheep to control and prevent its further distribution and awareness should be created for farmers on identified potential risk factors.
Keywords: c-ELISA; Horo guduru wollega; Isolation; PPRV; PCR; Risk factors; Seroprevalence; Sheep
Introduction
Ethiopia is believed to have the largest livestock population in Africa. According to recent estimates, Ethiopia has 56.71 million cattle, 29.33 million sheep, 29.11 million goats, 1.16 million camels and 56.87 million poultry [1]. The livestock sub-sector accounts for 40% of the agricultural Gross Domestic Product (GDP) and 20% of the total GDP without considering the livestock contribution in terms of traction power, fertilizing and means of transport [2]. Sheep and goats contribute 25% of the meat domestically consumed with a production surplus mainly being exported as live animals. Both species also contribute 50% of the domestic needs in wool, about 40% of skins and 92% of the value of hides and skin exported. The annual production of sheep and goat meat is estimated at 56,560 and 28,650 tons respectively [3]. To fully utilize the untouched livestock potential and address food safety issues, the government has created the Ethiopia Livestock Master Plan (ELMP) in 2015. The animal health part of the plan calls for the establishment of a robust animal health information system; reduced production losses by controlling prioritized diseases; increased export earnings by reinforcing the quarantine, inspection and certification system; decreased impact of zoonotic diseases on public health by controlling them and ensuring safety of animal products, improved infrastructure, and addressing policy issues [4].
On the other hand, studies indicate that the current contributions of the livestock subsector which includes small ruminant production to the national economy to be limited and below the potential [5]. Despite its economic significance, investments in modern animal husbandry are limited, especially in the pastoral areas that are the sources of most animals destined for export markets. Value addition in the livestock sector is limited and exports remain dominated by live animals, thus hampering the sector’s potential to ease high unemployment in rural and urban areas. Inadequate veterinary services, feed shortages, poor infrastructure, insufficient financial services and low levels of technical inputs are well documented in the Ethiopian livestock sector [6]. Infectious diseases are among the major factors which limit the production and productivity of small ruminant resulting in significant negative socio-economic impacts [5]. Regardless of production and disease challenges in Ethiopia; farmers prefer to rear sheep and goats for their low cost of production, prolificacy, their adaptive capacity to the environment through dynamic feeding behavior and fast reproduction cycle and growth rate. The degree to which sheep and goats survive to marketable age is one of the key indicators of the efficiency of their production [7].
Peste des Petits Ruminants (PPR) is an acute, highly contagious, World Organization for Animal Health (WOAH-OIE) notifiable and economically important trans-boundary viral disease of sheep and goats associated with high morbidity and mortality [8]. The PPR virus (PPRV) belongs to the genus Morbillivirus in the family Paramyxoviridae. It is closely related to the rinderpest virus of bovine, distemper virus of dogs and other wild carnivores, human measles virus and Morbilliviruses of marine mammals [9]. Peste des petits ruminants epidemics can cause mortality of 50–80% in unvaccinated sheep and goats populations.
Based on the assumption that goats experience an outbreak every 5 years, estimated an annual sum ranging from 2.47£ per goat at a high loss and 0.36£ per goat at the lowest loss. Peste des petits ruminants is one of the important diseases affecting the productivity of small ruminants [10]. Regardless of declarations by the FAO and OIE of a 2030 target for PPRV eradication, the spread of PPR has been facilitated by inconsistent or very restricted vaccination strategies as well as porous borders of neighboring countries between which there is significant illegal cross border animal trade through long-standing traditional animal trading routes [11].
Peste des petits ruminants became one of the most economically important livestock diseases and currently, it is the global issue causing major economic losses in tropical and sub-tropical countries including Ethiopia. Studies so far conducted provide historical information about the frequency and distribution of PPR in Ethiopia. There was no work done so far concerning PPR antibodies prevalence in the Horo, Jimma Geneti, and Jimma Rare districts of Horo Guduru Wollega Zone.
A baseline survey conducted in 2016 in livestock and fish project sites of Horo Guduru Wollega Zone by Animal Health Research Group of Bako Agricultural Research Center in collaboration with International Livestock Research Institute (ILRI) researchers indicated extensive circulation of PPR virus among sheep and suggested further study needs to be conducted in identified districts of Horo Guduru Wollega Zone. Therefore; the initiation is raised from the economic importance of PPR and information gaps mentioned above-seeking research work in the described place.
Therefore; the objectives of this research work are:
To estimate the seroprevalence of peste des petits ruminants virus infection in sheep in the study areas.
To identify associated risk factors of peste des petits ruminants virus infection in sheep in selected districts of Horo Guduru Wollega Zone.
To detect the genome of the PPRV from specimens collected during the active cases of PPR in sheep in the study areas.
Materials and Methods
Description of the Study Area
The study was conducted in selected districts of Horo Guduru Wollega Zone of Oromia Regional State. Three districts with different agro-ecologies were selected purposively based on their small ruminant population and rearing history (Figure 3). Shambu town which is the seats and capital city of Horo Guduru Wollega Zone administration is located at about 315 km from Addis Ababa (9° 33 10 N latitude and 37° 03 56 E longitude) in the Oromia Regional State, West Ethiopia.
According to the Horo Guduru Wollega Zone Livestock and FISHERY BUREAU (HGWZLFB) report, the livestock population of the Zone is composed of 1,162,212 cattle, 279,487 sheep, 209,768 goats, 135,543 donkeys, 77,349 horses, 14,260 miles and 479,009 poultry [12]. Nearly half of the livestock population of the zone is cattle followed by poultry (Figure 2).
Figure 1: Map of the study areas up to peasant association (Kebele) level.
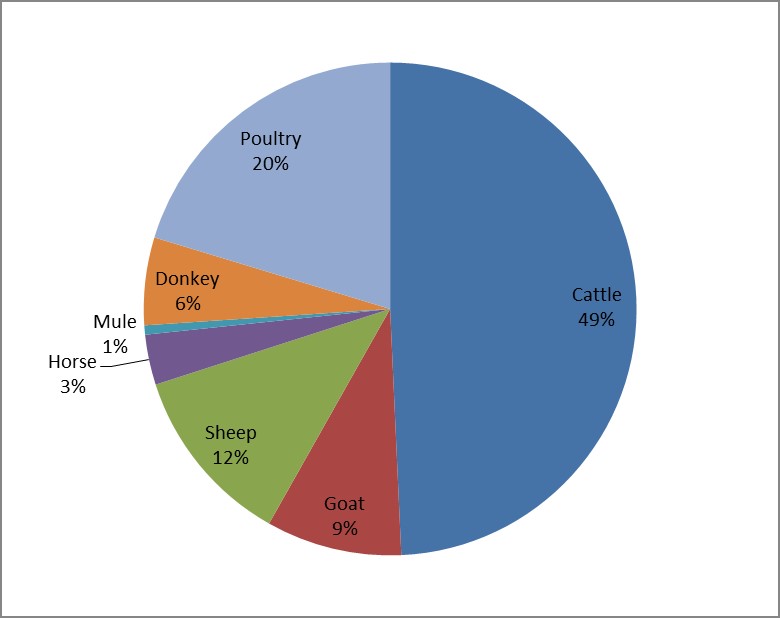
Figure 2: Livestock composition percentage of the Horo Guduru Wollega Zone.
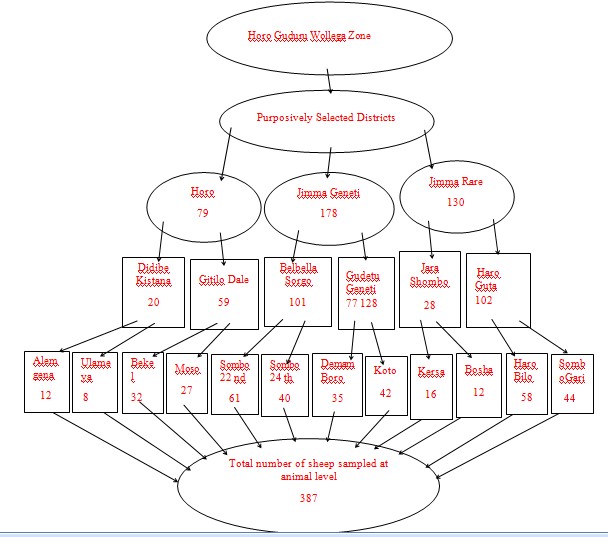
Figure 3: Schematic presentation of sample size and sampling procedures.
The Zone has an average annual rainfall of 1650 mm and the annual minimum and maximum temperatures are 18oC and 27oC, respectively. Mixed crop-livestock agriculture is the mainstay of the farming communities of the Zone. The Horo district, in which the capital city of the Zone is found, has one long rainy season that extends from March to mid-October with a mean annual precipitation of about 1800 mm [13]. Horo district has a total of 65,818 sheep and 9,076 goat population. The agro-ecologies of its specific study areas (Gitilo Dale and Didibe Kistana) are highland (dega) with an altitude of 2747 m and 2481 m above sea level respectively.
The other study area was Jimma Geneti district which has sheep and goat population of 50,050 and 41,501 respectively. The agro-ecologies of the selected PAs (peasant associations) of the district are lowland (kola) (Belbella Sorgo) with an elevation of 1,490 m above sea level and mid-highland (woina daga) with an altitude of 2,255 m above sea level (Gudetu Geneti). Jimma Rare district has 37,228 sheep and 9,432 goat population which Jara Shombo and Haro Guta were its selected PAs and exhibits mid-highland and highland weather conditions respectively [12].
Study Population and Study Animal
The study population included sheep found in the selected PAs reared in a mixed crop-livestock farming system, extensive and semi-intensive management system but have not been vaccinated against PPR so far. The study animal was sheep above six months of age from the identified PAs in each district.
Study Design
A cross-sectional study was conducted from October 2018 to August 2019 to estimate the seroprevalence and associated risk factors of PPR infection in sheep in selected districts of Horo Guduru Wollega Zone.
Sample Size Determination
Sample size was determined using Thrusfield formula by considering 5% absolute precisions and 95% confidence level as shown below [14]:
Where n is the required sample size,
P is the Previous PPR seroprevalence,
d² is the desired absolute precision.
An expected prevalence of 29.2% was used [7]. The Silti and Meskan districts of Siltie and Gurage zones, south regional state of Ethiopia where Hailegebreal 2018 conducted his study lies at the altitude ranges of 1500 up to 3100 m above sea level with three types of agro-ecologies which are similar with the current study. Likewise, all farmers included in both studies used a mixed-crop livestock farming systems. Accordingly, a sample size of 317 was calculated using the formula. However, the calculated sample size was increased to increase the precision and consider the clustering effect in multi-stage sampling design. Thus, 387 blood samples were collected from sheep in two purposively selected PAs of each three district. Two single-level clustered villages were also selected purposively within each PA. Then blood samples were collected from sheep as required at the animal level. The number of sheep to be sampled from each district and PA was proportionally allocated based on the flock size of that district and then down to the PA level. Thus, a total of 387 blood samples were collected accordingly.
Sampling Techniques
A multi-stage cluster sampling with a combination of simple random and purposive sampling were used as sampling techniques with hierarchical stages to reach the sampling units (Figure 3). Thus, the three districts were purposively selected from the Zone based on their agro-ecology and PPR vaccination history. Two PAs having different agro-ecologies were selected purposively within each district. Clustering was made on different villages of the selected PAs and single-level clusters were applied on villages that share common grazing areas and watering points. Then, two villages were selected by a simple random sampling method between clusters and samples were collected from a total of twelve villages to meet the required sample sizes at animal level.
Sample Collection
All sheep greater than six months were considered for sampling to rule out the transfer of maternal antibodies to lambs through breast milk. The blood samples were collected from the jugular vein using sterile vacutainer tubes and needles and kept at room temperature to clot down for 12 hours. The serum was harvested by using a pipette and stored in ice packs at +4oC until transported to the National Animal Health Diagnosis and Investigation Center (NAHDIC), Sebeta, Ethiopia. The samples were stored at -20oC in the deep freeze in the laboratory until the test is conducted.
Serological test for PPRV Specific Antibody
Monoclonal antibody-based Competitive Enzyme-Linked Immunosorbent Assay (cELISA) was used to test the serum samples as prescribed by the manufacturer and the Office International des Epizooties Terrestrial Manual [15]. The laboratory test was conducted at NAHDIC using PPR cELISA kits (IDvet innovative diagnostics, France). The kit detects specific antibodies against PPRV in the serum of sheep. The cELISA kit comprised PPR antigen (75/I) strain, anti-PPRV monoclonal antibody, anti-mouse conjugate, control sera, substrate, and chromogen with sensitivity and specificity of 92.2% and 98.4%, respectively [16]. The cELISA test result was read at 450-nanometer wavelength by using ELISA reader and the competition percentage (S/N %) value was calculated to decide the test as positive or negative [17].
S/N% = OD sample *100
ODNC
Where S/N % = competition percentage
OD sample = optical density of sample
ODNC = mean optical density of negative control
Samples presenting a competition percentage (S/N %) of less than or equal to 50% were considered positive. Samples presenting a competition percentage (S/N %) of greater than 50% and less than or equal to 60% were considered doubtful whereas samples presenting a competition percentage (S/N %) of greater than 60% are considered as negative.
The test is validated if:
¾The mean optical density of the negative control OD (ODNC) is greater than 0.7 (ODNC >0.70).
¾The mean optical density of the positive control (ODPC) is less than 30% of the ODNC (ODPC/ODNC <0.3).
The apparent prevalence was used to estimate the true prevalence by using the following formula.
True prevalence = AP +SP -1
Se + Sp -1
Where AP is apparent prevalence and Sp and Se are test specificity and sensitivity, respectively. The cELISA kit has a reported relative diagnostic sensitivity and specificity of 92.2% and 98.4%, respectively when compared with the VNT [16].
Molecular Detection and Isolation of Peste des Petits Ruminants Virus
Thirthteen swab samples were collected from clinically suspected cases of PPR sheep of the study areas. From 13 swabs, 11 nasal and 2 ocular swabs were collected from sheep. The swab samples were preserved using Virus Transport Media (VTM) reagent until it covers the tip of the swab. The samples were labeled and kept in icebox during the sample collection process and in the deep fridge at -20°C until it was transported to NAHDIC. The swab samples were stored at -80°C at NAHDIC until tested. Reverse transcription polymerase reaction was employed to detect the nucleic acid of PPRV. All the 13 samples were suspended in 500μl of 0.9% saline solution and total RNA was extracted from 100μl of the supernatant of sample on a BioRobot EZ1 automat (Qiagen) using the EZ1 Virus Mini Kit version 2.0 in the presence of DNase (Qiagen) according to manufacturer’s instructions. Afterward, the RNA was eluted with RNase free water. The extracted viral RNA was amplified. The master mix was prepared using the one-step RT-PCR kit (Applied Biosystems, Courtaboeuf, France) with PPRV specific primers (primer forwarded NP3 (10μm) and primer reversed NP4 (10μm)) (Eurogentec, Liege, Belgium). All reactions were run with Nigeria 75/1 vaccine strain as a positive control and nuclease-free water as the negative control. Reverse transcription polymerase reaction was carried out in GeneAmp PCR System 2720 (Applied Biosystems, Carlsbad, USA). Reverse transcription polymerase reaction products were separated by electrophoresis on a 1.5% agarose gel in 0.5% Tris-acetate-EDTA (TAE) buffer (Thermo scientific 50X TAE Buffer, Heidelberg, Germany) stained with 2μl of cyber safe which is used as an intercalating dye. Each well was loaded with 5μl of the PCR product and 1μl of blue 6X DNA loading dye (Promega, Madison, USA). Samples were separated along with a 100 base-pairs DNA ladder (Promega, Madison, USA) at 100 volts for 45 minutes. The agarose gel was visualized by ultraviolet fluorescence light using a gel documentation system (Biorad, Gel Doc TM EZ Imager).
The PPRV isolation and cell culture attempted to forward PPRV positive swab samples from RT-PCR test result to grow on Vero cells and see for the Cytopathic Effect (CPE) of the virus under microscope. But, the genome of PPRV was not detected on RT-PCR test from all 13 nasal and ocular swabs and all 13 swab samples were not submitted for cell culture and isolation because there was no PPRV genome detected by RT-PCR test result.
Assessment of Potential Risk Factors through a Questionnaire Survey
A semi-structured questionnaire was developed and field-tested on seven (5%) households keeping sheep before the standard survey. All owners of sheep from which blood samples collected were interviewed and all necessary information about associated risk factors of PPR in sheep were gathered. Epidemiological data related to associated risk factors of PPR such as district, Kebele (the lowest administrative level in Ethiopia), agro-ecology (highland, mid-highland and lowland), flock size (small and medium), sex (male and female), management system (extensive and semi-intensive), wind speed (medium and high), body condition score (BCS, 1-5) (1=extremely thin, 2= moderately thin, 3=moderate, 4=moderately fat, and 5=extremely fat) [18] but on the present study they were grouped as (normal and fat), grazing system (private, communal and mixed), age (young, adult and old) and presence or absence of wildlife contact and illegal cross-border small ruminant trade were assessed using a checklist. Study areas with an altitude of =2300 m above sea level were categorized as highland, while areas with an altitude between 1500–2300 m and =1500 m above sea level were grouped as mid-highland and lowland respectively. The minimum and the maximum numbers of sheep in the flocks of the study areas were 1 and 21 respectively with the average flock sizes of 5.29. Based on number of sheep per flock, sheep flock having up to 15 population size were categorized as small (=15) and more than 15 numbers were categorized as medium (=16) flock size. Similarly, [19] grouped as small (1-13), medium (14-26), and large flock sizes (=27) based on number of ewes per smallholder farmers' flock. Wind speed was categorized as medium and high based on respondents’ opinions. Sheep having BCS 2-3 were grouped as normal while sheep with BCS 4-5 were categorized as fat. Age was approximated and classified as young (7-12 months), adult (13–48 months) and old (=48 months). In-depth interviews of key informants were conducted to obtain an opinion from local livestock field officers as well as from local the district veterinary officers of the selected districts to ascertain whether PPR vaccine was given or not sheep of the study areas. So, a total of 14 key informants facilitated the process of data collection in the study sites; responded about small ruminants populations of the PAs and their vaccination history.
Data Management and Analysis
The data collected were entered, coded and organized into Micro-soft excel 2010 spread sheet program and analyzed using STATA version 11 (Stata Corp. College Station, USA). Descriptive statistics such as frequency, proportion, and percentage were used to summarize the results. The seroprevalence of PPR was calculated as the total number of seropositive samples for PPRV divided by total number of samples tested multiplied by 100. Questionnaire survey data were also entered into Micro-soft excels in 2010 and analyzed to see the association of potential risk factors with PPR seropositivity. Pearson’s Chi-Square test was employed to see the existence of differences among different categories. Univariable and multivariable logistic regression analyses were used to access the association of PPR seroprevalence with potential risk factors. Factors having P-value of =0.25 upon univariable logistic regression analysis, comparable frequencies and non-collinear to each other were forwarded for multivariable logistic regression analysis. For statistical significance, 95% confidence interval and P-value of 0.05 was considered.
Results
PPR Serostatus
Flock-level PPR seroprevalence: Flock-level overall seroprevalence of PPR was 25.86% by considering at least one infected animal in one flock.
Animal level PPR seroprevalence: From a total of 387 sheep, the overall seroprevalence of PPR was found to be 6.98%. The least seroprevalence of PPR in sheep was recorded in Horo districts (3.8%) followed by Jimma Rare (7.69%) whereas the highest prevalence was in Jimma Geneti (7.87%) (P>0.46). The disease showed a widespread spatial distribution covering 91.67% (11/12) of the studied villages in the selected districts. True PPR seroprevalence was adjusted based on its apparent prevalence, sensitivity, and specificity of cELISA kit. The flock-level true and apparent PPR seroprevalence was 4.53% and 6.98% respectively. Thus, apparent prevalence was higher than its true prevalence.
Identified Risk Factors
Flock-level identified risk factors: Potential risk factors significantly associated with the seroprevalence of PPR at flock-level in the multivariable logistic regression analysis were identified as the district, species, flock size, wind speed, water source, grazing system and sharing of common grazing land with other flocks. The seroprevalence of PPR was high (25.86%) in sheep flock out of which 15/58 sheep flocks were infected with PPRV.
Animal level identified risk factors: Different associated risk factors such as species, age sex, flock size, residential place, management system, and others were evaluated to see their association against PPR seroprevalence in sheep, but none of them were statistically significant except agroecology. The seroprevalence of PPR in sheep was higher in mid-highland than highland and lowland (P=0.024). Based on univariate logistic regression result of P-value =0.25 and collinearity matrix result; some independent variables like district, species, wind speed, animal source, and others were further subjected to multivariable logistic regression analysis but, they were not statistically significant (P>0.05). Among different associated risk factors evaluated with seroprevalence of PPR in sheep, the agroecology was the only statistically significant factor. The seroprevalence of PPR in sheep was higher in mid-highland than highland and lowland (P=0.010) (Annex D). Those sheep reared in mid-highland were 5.31 times more likely to be infected than those sheep reared in lowland (P=0.029, 95% CI=1.19, 23.77) (Table 1).
Variable
Category
Crude OR (95% CI)
P-value
District
Horo
1.0
Jimma Rare
2.11 (0.57, 7.92)
0.268
Jimma Geneti
2.16 (0.60, 7.75)
0.236
Agro-ecology
Lowland (= 1500)
1.0
Highland (= 2300 m)
1.96 (0.41, 9.30)
0.396
Mid-highland (1500-2300 m)
5.31 (1.19, 23.77)
0.029 *
Age
Adult (13 -48 months)
1.0
Young (6 – 12 months)
1.54 (0.63, 3.76)
0.346
Old (= 49 months)
1.62 (0.55, 4.74)
0.378
Sex
Male
1.0
Female
1.10 (0.37, 3.31)
0.859
Flock size
Small (= 15)
1.0
Medium (= 16)
1.54 (0.69, 3.43)
0.288
Residential place
Peri-urban
1.0
Rural
1.18 (0.27, 5.21)
0.829
Management system
Extensive
1.0
Semi-intensive
1.57 (0.68, 3.62)
0.291
Grazing place
Plain
1.0
Mixed
1.06 (0.45, 2.50)
0.886
Mountainous
1.14 (0.34, 3.81)
0.828
Grazing system
Private
1.0
Communal
4.71 (0.61, 36.12)
0.136
Mixed
6.03 (0.74, 48.89)
0.092
Wind speed
Medium
1.0
High
2.22 (0.89, 5.53)
0.086
Share common grazing land
No
1.0
Yes
1.49 (0.50, 4.43)
0.476
Share a common house with others
Yes
1.0
No
1.42 (0.52, 3.87)
0.489
Water source
Private
1.0
Shared
1.49 (0.50, 4.43)
0.476
Animal source
Purchased
1.0
Born at home
1.18 (0.52, 2.65)
0.695
Awareness on PPR
Yes
1.0
No
1.86 (0.24, 14.28)
0.552
Wild life contact
No
1.0
Yes
1.14 (0.50, 2.62)
0.750
Free movement of animal
No
1.0
Yes
1.27 (0.57, 2.85)
0.561
Presence of theft
No
1.0
Yes
2.66 (0.90, 7.88)
0.077
Introduction of a new animal into the flock
Yes
1.0
No
1.17 (0.51, 2.68)
0.716
Shifting of the animals from place to place for feed ("dereba")
No
1.0
Yes
3.12 (0.64, 15.22)
0.159
OR=Odds Ratio; CI=Confidence Interval; *=Significant
Table 1: Result of univariable logistic regression of risk factors of PPR in sheep.
Those variables forwarded for multivariable logistic regression analysis were district, agro-ecology, grazing system, wind speed, presence of theft and shifting of an animal from one place to another to search fodder during feed scarcity. No statistically significant differences attributed to PPR infection were observed using multivariable logistic regression analysis (P>0.05) (Table 2).
Variable
Category
Adjusted OR (95% CI)
P-value
District
Horo
1.0
Jimma Rare
2.10 (0.25, 5.75)
0.824
Jimma Geneti
2.62 (0.20, 13.18)
0.650
Agro-ecology
Lowland (= 1500 m)
1.0
Highland (= 2300 m)
1.40 (0.12, 16.97)
0.794
Mid-highland (1500–2300 m)
3.38 (0.64, 17.95)
0.154
Grazing system
Private
1.0
Communal
2.35 (0.26, 20.86)
0.444
Mixed
2.89 (0.30, 28.23)
0.361
Wind speed
Medium
1.0
High
2.16 (0.79, 5.90)
0.132
Presence of theft
No
1.0
Yes
2.01 (0.56, 7.18)
0.281
Shifting of an animal from place to place for feed
No
1.0
Yes
3.46 (0.49, 24.63)
0.216
OR=Odds Ratio; CI=Confidence Interval
Table 2: Results of multivariate logistic regression analysis of risk factors associated with PPRV infection at an animal level (sheep).
Detection and Isolation of PPRV
The RT-PCR test was employed to detect the nucleic acid of PPRV from swab samples collected from clinically PPR suspected sheep of the study areas. The agarose gel was visualized by ultraviolet fluorescence light using a gel documentation system (Biorad, Gel Doc TM EZ Imager). The test result showed all 13 swabs (11 nasal swabs and 2 ocular swabs) found to be negative to conventional-PCR test (Figure 4).
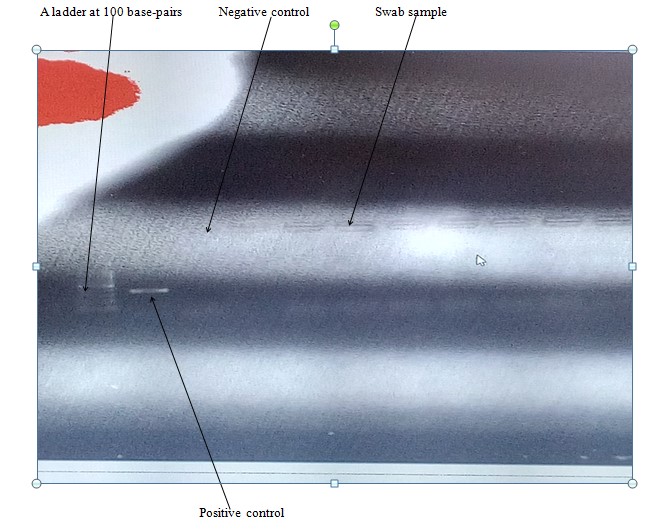
Figure 4: Detection of PPRV in swab (nasal and ocular swabs) samples collected from suspected clinical cases in sheep of the studied districts. No bands were observed in all samples tested.
All the 13 swabs samples were not forwarded for viral culture and isolation due to the absence of PPRV genome detection upon the RT-PCR test result.
Questionnaire Survey
The vast majority (91.10%) of the respondents live in rural areas while 8.90% of them live in the peri-urban areas. All sheep included in the study belong to the Horo breed. Similarly, all the farmers of the study areas exercise the sedentary farming system. Eighty percent of the respondents manage their sheep extensively while some of them (19.50%) practice a semi-intensive system of management. Seventy-seven percent of the respondents used communal grazing land for sheep and 22.8% kept their animal in private grazing land. More than half of the respondents (63.40%) claimed that there were no enough veterinary services near their residential place. On the other hand, about 36.60% of the sheep keepers of the study areas got enough veterinary services around their home. Nearly all respondents (96.70%) have no awareness about the transmission, control and prevention options of PPR in the study areas. Loaning of sheep ("locally called “ribi” ") to help poor farmers was practiced by all respondents of the study areas. Likewise, all respondents practiced culling of sheep due to different reasons like aging, disease problems, sex preference, off-color and etc. Peste des Petits ruminants is locally known (called) as “Mariye Somba” by small holder farmers of the study areas. According to respondents’ opinion; winter (39.90%), Spring (26.80%), Summer (25.20%) and Autumn (8.10%) were seasons when PPR became more prevalent respectively.
Discussion
Peste des Petits Ruminants is one of the priority diseases for which the FAO-OIE has set the goal of eradicating the disease by 2030. For effective control of PPR, accurate diagnostic techniques, timely vaccination of susceptible animals, and a full understanding of the disease epidemiology are imperative [20]. The overall seroprevalence of PPR recorded (6.98%) in sheep in this current study was nearly similar (6.1%) to the previous study of [21] conducted in Gewane district of Afar region. The overall seroprevalence of PPR in the current study slightly agrees with the finding of [3] who reported 6.4% in the analysis of a national serological survey of PPR in Ethiopia. Likewise, [22], in their study on antibody seroprevalence of PPR virus in camels, cattle, goats, and sheep in Ethiopia, found an overall PPR seroprevalence of 6.8% in sheep and goats which is in accord with the current findings. On the other hand, the overall seroprevalence found in the present study was higher than the apparent overall seroprevalence of 2.1% and 1.7% reported by [23] and [24] in Benchi Maji and Kafa Zones of South West Ethiopia, and Awash Fentale district in Afar region respectively.
The overall seroprevalence reported by [7] (29.2%) in selected districts of Siltie and Gurage Zones and that of [25] (48.43%) in East Shewa and Arsi Zones of Oromia regional state were higher than the current study. Previous serological investigation of PPR in Ethiopian small ruminants managed under pastoral and agro-pastoral systems [26] and seroepidemiology and spatial distribution of PPR virus antibodies in selected pastoral areas of Somali regional state [27] recorded much higher overall seroprevalence of 30.5% and 41%, respectively compared with the present study. An overall higher seroprevalence of PPR (40.2%) than the present study was recorded in selected districts of Afar region [10] and southern parts of Tigray region (46.53%) [28]. Similarly, the study conducted by [29] in small ruminants of eastern Amhara region bordering afar reported an overall seroprevalence of 28.1%, which is higher than the present finding. The difference in prevalence among different studies could be due to the difference in production systems, agro-ecology, flock size, and geographical locations; where free movement of animals and illegal cross-border animal trade could be practiced. The other possible reason for the higher prevalence reported by the above authors might be because those studies were at a country or region level while the current study was in selected districts of Horo Guduru Wollega Zone. Apart from Ethiopia, the result of the current study is also lower, compared to the findings of [30] (61.1%) in North-Eastern [31] (45.6%) and [32] (54.0%) in Sudan, [33] (28.5%) in Tanzania, [34] (62.0%) in Somalia, [35]in Libya (46.7%), [36] (22.3%) and [37] (55.2%) both in Pakistan, and [38] (20.57%) in Bangladesh. The reasons for the higher seropositivity of PPR in small ruminants in the reports of these scholars might be due to occurrence of PPR outbreak and sampling of clinically suspected animals. On the contrary, in the present study serum samples were collected from apparently healthy animals where there was no PPR outbreak. Additionally; the season of specimen collection, the difference in sample size and agro-ecology might have contributed to the difference in seroprevalence between the current and previous studies.
An overall flock-level seroprevalence of PPR in this study was found to be 25.86% which is higher than the finding of [23] who reported flock prevalence of 18.8%. A higher (98%) the flock-level overall seroprevalence of PPR than the present study was reported [27]. The difference in seropositivity might be most likely be due to the difference in PPR prevalence recorded and flock size since large flock sizes were kept in agro-pastoral and pastoral areas, unlike the current study areas where small flocks were kept under the sedentary farming system.
Sex wise seroprevalence of PPR in the present study was lower in males (4.76%) without a statistically significant difference when compared with the females (5.96%). In line with the present study, [39] observed no significant difference between sexes. In agreement with the current finding, [28] revealed the higher prevalence of 47.5% in female than the prevalence of 43.73% in male but there was no statistically significant variation between them. In contrast with the present study, [36] reported significantly higher proportions of seropositive female sheep and goats (25.6%) compared to male animals (5.1%). Likewise, [27] reported higher seroprevalence of 44% in female compared to the male counterpart (26%). This could be related to the physiological differences between female and male and the majority of male animals are not usually kept in a flock for a long period of time. They are often sold out for meat while the females remain in the flock for breeding purposes, which may indirectly account for the high seroprevalence of PPR in females in case recovered animals will have detectable levels of circulating antibodies in their serum. The present study also disagrees with that of [3] who indicated males be more prone to the disease due to higher chance of getting infection than females as they mixed and contacted with the other flocks for breeding purpose. Additionally, pregnancy, lambing in female animals lower the immune status, as a result, their ability to resist the challenge of the infection will be low [40]. Age-based seroprevalence of PPR in this study was higher in old age group animals (6.25%) followed by the young (5.96%) and adult ones (5.22%) even though it is not statistically significant. In contrast to the present study, PPR seroprevalence was increased as age increases in both sheep and goats [25,28,27]. This might have resulted from unstandardized age categorization differences among researchers.
Univariable logistic regression analysis showed a higher prevalence of PPR infection in sheep of mid-highland agro-ecology as compared with highland and lowland agro-ecologies. On the other hand, [27] indicated that sheep reared in the higher altitude (>1000 m above sea level) was found to be at lower risk for PPR compared with the low altitude (<500 m). This could be due to the recurrent drought and subsequent shortage of water and feed in lowland areas, which makes the animals travel long distances during the dry season in search of fodder and water. Arguably, the other possible reason might be the difference in altitude categorization between the two studies (Annex D).
The RT-PCR test using primers targeting the N and F genes indicated that all 13 swab samples were negative to PPRV. This might be due to either the clinically PPR suspected disease might be other disease resembling PPR or hosts’ immune system may overcome the virulence factor of the virus as it is stated by [41] immunization with F and or H induces protective humoral immunity probably through the production of neutralizing antibodies.
Conclusions and Recommendations
The overall seroprevalence of PPR in sheep of selected districts of Horo Guduru Wollega Zone was low (6.98%). The sheep included in this study were not vaccinated against PPR and seropositivity could result from field infection indicating active PPR virus circulation among the herds investigated. The estimated flock-level overall seroprevalence was high (25.86%). Infection with PPRV occurred in all the three studied districts of the Zone. The infection was found to be more prevalent in Jimma Geneti district than Horo and Jimma Rare districts. The study result showed that district, flock size, sharing of common grazing and grazing system were the determinant risk factors for PPR seroprevalence. Likewise; agro-ecology is the predictors of PPRV seropositivity. The attempt of detection of PPRV genome by RT-PCR from swabs of clinically PPR suspected sheep resulted in the absence of the nucleic acid of the virus.
Based on the above conclusions, the following recommendations are forwarded:
Farmers of the study areas should avoid the direct introduction of newly purchased sheep into their flock and free movement of animals. Responsible bodies should strengthen and enforce the implementation of the government policies on free animal movement control and regulation to enhance surveillance, monitoring and diagnosis of trans-boundary animal disease. Awareness should be created for sheep keepers on identified potential risk factors, to practice gradual culling of those seropositive animals, means of transmission, and control and prevention options of PPRV. Regular vaccination should be implemented for the sheep to prevent its further distribution to the neighboring districts of the study areas. A further detailed study exploring PPR seasonal occurrence should be conducted to facilitate the implementation of its control and prevention strategies. The Zonal as well as the districts veterinary officers should apply possible PPR intervention strategies focusing on identified risk factors.
Author Statements
Funding Source
Bako Agricultural Research Center.
Acknowledgments
I want to express my heartfelt thanks to Mr. Bayeta Sembeta who gave me technical support on the molecular aspect and facilitated the process of laboratory tests of samples at Sebeta National Animal Health Diagnostic and Investigation Center (NAHDIC). Many thanks to Oromia Agricultural Research Institute and Bako Agricultural Research Center for providing me financial support for my research work. I want to extend my thanks to Bako Agricultural Research Center researchers who participated and helped me during field data collection and questionnaire survey at study sites.
Conflict of Interests
Authors declare there is no conflict of interest.
References
- CSA (Central Statistical Agency). ’Federal Democratic Republic of Ethiopia, Agricultural Sample Survey’: report on livestock and livestock characteristics. Addis Ababa, Ethiopia. 2015.
- Fentie T, Fenta N, Leta S, Molla W, Ayele B, Teshome Y, et al. Seroprevalence, risk factors, and distribution of sheep and goat pox in Amhara region, Ethiopia. BMC Vet Res. 2017; 13: 385.
- Waret-Szkuta A, Roger F, Chavernac D, Laikemariam Y, Libeau G, Dirk UP, et al. Peste des petits ruminants (PPR) in Ethiopia: analysis of a national serological survey. BMC Vet Res. 2008; 34.
- Feyisa A. Feed the future innovation lab for livestock systems. Ethiopia: livestock disease management and food safety brief. The Manag Entity Univ Fla. 2016; 27.
- Kihu SM, George CG, Lily CB, Njenga MJ, Gidraph GW, Ndichu M, et al. Economic losses associated with peste Des petits ruminants in Turkana, Kenya. Pastoralism Res Policy Pract. 2015; 5: 9.
- Legese G, Fadiga M. Small ruminant value chain development in Ethiopia: situation analysis and trends [ICARDA/ILRI Project report]. Available from: https://hdl.handle.net/10568/52339. Nairobi, Kenya: International Center for Agricultural Research in the Dry Areas/International Livestock Research Institute; 2014.
- Hailegebreal G. Seroprevalence of peste Des petits ruminants in selected districts of siltie and Gurage zones, south region, Ethiopia. J Vet Sci Technol. 2018; 09: 529.
- Balamurugan V, Hemadri D, Gajendragad MR, Singh RK, Rahman H. Diagnosis and control of peste Des petits ruminants: A comprehensive review. Virol Soc. 2013; 25: 39-56.
- Khalafalla IA, Saeed K, Yahia H, Ali M, Abdurrahman BK, et al. An outbreak of peste des petits ruminants (PPR) in camels in Sudan. Acta Trop. Nov 2010; 116: 161-5.
- Gizaw F, Merera O, Zeru F, Bedada H, Gebru M. Seroprevalence and socioeconomic impacts of peste Des petits ruminants in small ruminants of selected districts of Afar, Ethiopia. J Vet Sci Technol. 2018; 9: 513.
- Baazizi R, Mana M, Brian DC, Khatima AO, Djamel K, Satya P. Peste des petits ruminants (PPR): A Neglected Tropical Disease in the Maghreb Region of North Africa and its Threat to Europe. PLOS ONE. 2017; 12: e0175461.
- HGWZLFB. Horo Guduru Wollega zone livestock and fishery bureau. Oromia: Shambu. 2017.
- CSA (Central Statistical Agency). “Federal Democratic Republic of Ethiopia. Central Statistical Agency agricultural sample survey. Addis Ababa, Ethiopia. 2009.
- Thrusfield M. Veterinary epidemiology. 2nd ed. Oxford: Blackwell Publishing Science. 2005; 117-98.
- OIE (Office International des Épizooties). World animal health information database. Paris, France: World Organization for Animal Health. 2012; 6.
- Zahur AB, Ullah A, Hussain M, Irshad H, Hameed A, Jahangir M, et al. Seroepidemiology of peste des petits ruminants (PPR) in Pakistan. Prev Vet Med. 2011; 102: 87-92.
- Libeau G, Préhaud C, Lancelot R, Colas F, Guerre L, Bishop DH, et al. Development of a competitive ELISA for detecting antibodies to the peste Des petits ruminants’ virus using a recombinant nucleoprotein. Res Vet Sci. 1995; 58: 50-5.
- Kenyon PR, Maloney SK, Blache D. Review of sheep body condition score in relation to production characteristics. N Z J Agric Res. 2014; 57: 38-64.
- Amare T, Goshu G, Tamir B. Flock composition, breeding strategies and farmers’ traits of interest evaluation of Wollo highland sheep and their F1 crosses. J Anim Sci Technol. 2018; 60: 14.
- Dayhum A, Sharif M, Eldaghayes I, Kammon A, Calistri P, Danzetta ML, et al. Seroprevalence and epidemiology of peste des petits ruminants in Libya. Transbound Emerg Dis. 2018; 65: e48-54.
- Dawit T. Seroprevalence study of peste des petits ruminants in Gewane District, Afar Regional State, Ethiopia [MSc thesis]. Debre Zeit, Ethiopia: Addis Ababa University. 2006.
- Abraham G, Sintayehu A, Libeau G, Albina E, Roger F, Laekemariam Y et al. Antibody seroprevalences against peste des petits ruminants (PPR) virus in camels, cattle, goats, and sheep in Ethiopia. Prev Vet Med. 2005; 70: 51-7.
- Tsegaye G, Yosef D, Feyissa B. Seroprevalence and associated risk factors of peste des petits ruminants (PPR) in sheep and goats in four districts of bench Maji and Kafa zones, south West Ethiopia. Glob Vet. 2018; 20: 260-70.
- Faris D, Yilkal A, Berhe G, Kelay B. Seroprevalence and seroconversion after vaccination against Peste des Petits Ruminants in sheep and goats from Awash Fentale District, Afar, Ethiopia. Prev Vet Med. 2012; 103: 157-62.
- Gari G, Serda B, Negesa D, Lemma F, Asgedom H. Serological investigation of peste Des petits ruminants in east shewa and Arsi zones, Oromia region, Ethiopia. Vet Med Int. 2017; 2017: 9769071.
- Megersa B, Biffa D, Belina T, Debela E, Regassa A, Abunna F, et al. Serological investigation of Peste des PetitsRuminants (PPR) in small ruminants managed under pastoral and agro-pastoral systems in Ethiopia. Small Ruminants Res. May 2011; 97: 134-8.
- Wondimagegne D. Seroepidemiology and Spatial Distribution of Peste des Petits Ruminants Virus antibodies in Some Selected pastoral Areas of Somali Regional State, Ethiopia [MSc thesis], Addis Ababa University, College of Veterinary Medicine and Agriculture. Ethiopia: Bishoftu. 2016.
- Afera B, Hussien D, Amsalu K. Seroprevalence of peste Des petits ruminants in goats of southern parts of Tigray region. Glob Vet. 2014; 12: 512-6.
- Biruk A. Epidemiology and identification of peste des petits ruminants (PPR) virus circulating in small ruminants of eastern Amhara region bordering Afar, Ethiopia. Available from: http://etd.aau.edu.et/handle/123456789/4319 [MSc thesis], Addis Ababa University. Ethiopia: Bishoftu. 2014.
- Bello AM, Lawal J, Dauda R, Wakil J, Marta Y, Lekko E, et al. Research for peste des petits ruminants (PPR) virus antibodies in goats, sheep, and gazelle from Bauchi and Gombe states, North-Eastern Nigeria. Direct Res J Agric Food Sci. 2016; 4: 193-8.
- Salih H, Elfadil A, Saeed I, Ali Y. Seroprevalence and risk factors of Peste des Petits Ruminants in sheep and goats in Sudan. J Adv Vet Anim Res. 2014; 1: 42-9.
- O.M. Ishag, I.K. Saeed, Y.H. Ali. “Peste des petits ruminants outbreaks in White Nile State, Sudan”. Onderstepoort J Vet Res. 2015; 82: 897.
- Birindwa BA. Serosurveillance, risk factors and molecular diagnosis of peste Des petits ruminants virus in south kivu, Democratic Republic of the Congo [DVM thesis]. Morogoro, Tanzania: Sokoine University of Agriculture. 2015.
- Njue S, Saeed K, Maloo S, Muchai J, Biaou C, Tetu K. Seroprevalence study to determine the effectiveness of Peste de Petits Ruminants vaccination in Somalia, Pastoralism. Pastoralism, and Practice. 2018; 8.
- Almeshay M, Abubaker D, Gusbi I, Eldaghayes R, Mohammed M, Abdunaser B, et al. An epidemiological study on peste Des petits ruminants in tripoli region. Vet Ital. 2017; 53: 235-42.
- M.A. aziz-ul-Rahman, Muhammad HR, Shumaila M, Muhammad S, Muhammad R, Muhammad M, et al. Evaluation of risk factors for peste des petits ruminants virus in sheep and goats at the Wildlife-Livestock Interface in Punjab Province, Pakistan”. Bio-Medical Research International. 2016; 2016.
- Abubakar M, Arshed MJ, Hussain M, Ali Q. Evidence of peste des petits ruminants in serology of sheep and goats from Sindh, Pakistan. Transbound Emerg Dis. 2011; 58: 152-6.
- Sarker S, Islam H. Prevalence and risk factors assessment of PPR in goats in Rajshahi, Bangladesh. Vet World. 2011; 4: 546-9.
- Swai ES, Kapaga A, Kivaria F, Tinuga D, Joshua G, Sanka P. Prevalence and distribution of Peste des petits ruminants virus antibodies in various districts of Tanzania. Vet Res Commun. 2009; 33: 927-36.
- Yash RP, Jignesh MP, Priti DV, Kalyani IH, Pramod SS, Pratik PP. Seroprevalence of peste des petits ruminants (PPR) in Navsari and Valsad districts of South Gujarat. Int J Curr Microbiol Appl Sci. 2017; 6: 221-8.
- Diallo A, Minet C, Le Goff C, Berhe G, Albina E, Libeau G, et al. The threat of peste Des petits ruminants: progress in vaccine development for disease control. Vaccine. 2007; 25: 5591-7.