
Research Article
Austin J Vet Sci & Anim Husb 2024; 11(2): 1139.
Effects of Different Ratios of Linoleic to Linolenic Acids in Diets During Flushing Period on Blood Metabolites, Hormone Concentrations and Reproductive Performance of Zel Ewes
Samadi MS¹*; Deldar H²; Fouladi-Nashta AA³
1Department of Animal Science, Sari Agricultural Sciences and Natural Resources University, Iran
2Department of Animal Sciences, University of Guilan, Iran
3Reproduction Research Group, the Royal Veterinary College, UK
*Corresponding author: Samadi MS Department of Animal Sciences, Sari Agricultural Sciences and Natural Resources University, Iran. Tel: +981155272268; Fax; +981333690282 Email: m.sale.samadi@gmail.com
Received: January 22, 2024 Accepted: March 10, 2024 Published: March 18, 2024
Abstract
A total of 280 ewes (44 ± 3 kg and two years old) were fed to evaluate the effects of diets enriched in different ratios of omega-6 (linoleic acid; LA) and omega-3 (alpha-linolenic acid; ALA) Polyunsaturated Fatty Acids (PUFAs) on blood metabolites, some hormone concentrations and reproductive performance of Zel fresh ewes. Ewes were assigned to four experimental groups (n=70 per group) and within a group received one of four diets, including control (enriched beef tallow, Group1), LA to ALA ratio of 1:1 (Group 2), LA to ALA ratio of 5:1 (Group 3), and LA to ALA ratio of 10:1 (Group 4). This trial was continued approximately for 65 days. Blood samples were collected on days 14, 31, and 45 to measure plasma concentrations of blood metabolites. A significant increase in glucose and cholesterol showed in ewes group 2 compared to the Control group (P<0.05), but the triglyceride levels were significantly decreased (P<0.05). The concentrations of plasma 17-beta estradiol on day 31 were markedly higher for group 2 (P<0.05). LA to ALA ratio of 1:1 showed approximately 55 percent more plasma progesterone compared to Control (P<0.05). Plasma insulin concentrations increased in groups 3 and 4 compared to the Control group (P<0.05). Moreover, significant differences were observed among groups for pregnancy, lambing rates, and sex ratio of newborn lambs (P<0.05). In conclusion, ratios of 1:1 and 5:1 (LA: ALA) of fatty acids in diets improved fertility and reproductive performance.
Keywords: Ewe; Fatty acids; Nutrition; Blood parameters; Reproductive performance
Introduction
Flushing diets are commonly used in ewes with the lower body condition score to boost folliculogenesis, ovulation and increase the lambing rate [22]. It contain high energy density and fed at least two weeks before ram introduction during the breeding season. Increased level of dietary energy was shown to affect the ovarian function and follicular size [36]. Feeding energy-rich additives such as fat supplements modulates the recrudescence of hypothalamic and pituitary function and regulates the ovarian activity in females [27]. In addition, higher dietary fat ingestion have a direct impact on ovarian structures, which may lead to an increase in the size and number of the ovarian follicles [30]. The addition of fatty acids source into the diet could affect the concentrations of blood hormones [15]. The supplementation of polyunsaturated fatty acids to flushing diets had a significant impact on ewe reproductive performance through an increase in plasma concentrations of cholesterol and progesterone [1]. For instance, supplementing flushing diets with Calcium Salts of Fatty Acids (CSFA) in Afshari ewes increased plasma glucose and Blood Urea Nitrogen (BUN), and estradiol-17 β, which causes improvement of fertility, lambing rate, and the lambs’ birth weight. Several studies have also shown that Polyunsaturated Fatty Acids (PUFAs) of the omega-3 and omega-6 family, such as ALA and LA, which are usually found in feedstuffs derived from linseed and soybean, respectively, play an essential role in animal reproductive performance [31]. Despite the presence of evidence for the beneficial effects of PUFAs on folliculogenesis and reproductive performance in different animal species, the evidence for their impact on ovarian activity in sheep during breeding season are sparse. This study aimed to evaluate the effects of flushing diets enriched with three ratios of omega-6 (linoleic acid) / omega-3 (linolenic acid) fatty acids on reproductive performance, blood metabolites and hormones in Zel ewes during breeding season period.
Materials and Methods
Animals, Nutrition Program, and Experimental Design
A total of 280 ewes (44 ± 3 kg and two years old) were assigned to four experimental groups (n=70 per group), and received one of four diets, including the control diet (supplemented by beef tallow, Group1), LA to ALA ratio of 1:1 (Group 2), LA to ALA ratio of 5:1 (Group 3), and LA to ALA ratio of 10:1 (Group 4). The supplementation of different PUFA sources in the experimental diets was continued for 65 days (day 0= initiation of the feeding, Figure 1).
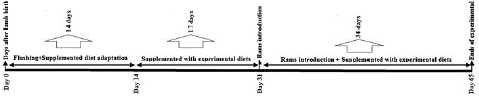
Figure 1: Experimental design. The ewes were assigned to four groups (n = 70 per group) and within a group received one of four diets include: 1) control (supplemented by tallow for fat source), 2) ratios 1 to 1 of LA to ALA, 3) ratios 5 to 1 of LA to ALA acids and 4) ratios 10 to 1 of LA to ALA acid.
To increase the accuracy in the contribution of each LA and ALA fatty acids in ewes' diet, the profile of fatty acid in the diet was determined (Table 1). Formulation of the experimental diets are provided in Table 2. These isoenergetic and isonitrogenous experimental diets contained approximately 30-gram fatty acids compared to the level of the saturated fat present in beef tallow for the control group. The amounts of fatty acid profiles of extruded soybean and extruded linseed diets were obtained from our previous study [26]. Based on former study results, a total fatty acid in extruded soybean and linseed were 18.88 and 36.7 percent, based on dry matter, respectively. These values were calculated according to the amounts (g) of fatty acid sources added to the experimental diets. Soybean extruded supplemented in experimental diets (except the control group) was 70, 145, and 155g for ratios 1:1, 1:5, and 1:10, respectively. On the other hand, 46, 8, and 0g extruded linseed were added to experimental diets, respectively. The flushing diets were formulated using SRNS software version 1.9.5566.0 and based on the requirements of a 35±1.2 kg ewe, with a body condition score of 2.5±0.25. The amount of dry matter for each ewe was 1450 g/day. The schematic protocol for the experimental diets and the length of the feeding period of the experimental groups is shown in Figure 1. Healthy two-years-old Zel rams were used for mating in this study. One ram was used per 15 ewes. The rams spent a period of three estrus cycles of the ewes (51 days) in the herd. All rams were fed with different diets from ewes.
Source of fatty acids
Fatty acid profiles (%)
C16:0
C18:0
C18:1
C18:2
C18:3
Palmitic acid
Stearic acid
Oleic acid
LA
ALA
Extruded Linseed
7.64
5.22
20.05
14.81
51.28
Extruded Soybean
9.65
4.43
21.64
50.21
4.9
Table 1: Amounts of different fatty acid sources in diet (% DM).
Ingredient (% DM)
Treatments
Control
Ratios of LA to ALA
1:01
5:01
10:01
Alfalfa Hay
27.61
26.67
25.71
25.8
Wheat – Straw
18.09
17.39
17.1
17.32
Barley Grain
15.17
13.43
14.92
14.68
Corn Dry
3.03
2.42
2.98
2.94
Soybean - Meal – 44
5.46
4.88
4.07
4
Rice Bran
27.91
27.22
25.71
25.8
Tallow
1.82
0
0
0
Soybean – Extruded
0
4.27
8.19
8.62
Linseed - Extruded
0
2.81
0.45
0
Calcium – Carbonate
0.61
0.61
0.57
0.56
Salt
0.3
0.31
0.28
0.28
Chemical components
Crude protein (% DM)
12.3
12.3
12.3
12.3
Dietary NDF (% DM)
39.2
39.4
41.2
41.3
Dietary Fat (% DM)
5.1
5.1
5.1
5.1
Table 2: Ingredient and chemical composition of experimental diets from week’s three to six.
Blood Metabolite and Hormone Analysis
Blood samples were collected from the jugular vein in EDTA tubes. Four blood samples were collected from each ewe (n=7/treatment) on days 0 (initiation of fat supplementation), 14, 31, and 45. The blood samples were centrifuged for 15 min at 2000g, and the blood plasma was stored at -20°C for future analysis. Plasma concentrations of progesterone, insulin, and estradiol were determined using ELISA (Diaplus, North York, Ontario, Canada). Intra-assay and inter-assay coefficients of variation were lower than 12%. Plasma glucose, cholesterol, and triglycerides were determined enzymatically using a spectrophotometer (Shimadzu 2100, Kyoto, Japan), according to the manufacturer’s instruction.
Reproductive Performance
The pregnancy rate, lambing rate, and sex ratios of lambs were measured after approximately five months. The pregnancy was confirmed by measuring plasma progesterone in the mated ewes. The pregnancy rate was determined based on the number of ewes had high plasma progesterone levels and the number of ewes that had mated with the painted back. The lambing rate was calculated by the number of lambs born to the number of mated ewes. The numbers of female and male lambs were recorded at birth to measure the sex ratio.
Statistical Analysis
Data were tested for normal distribution of the residuals using SAS software by the PROC UNIVARIATE procedure (SAS Institute, 2006). Data on blood metabolites and hormones were analyzed as repeated measurements using PROC MIXED of SAS software the following model:
Yijk = μ + ai + tj + (at)ij + Bk + eijk
Where μ is the population mean, ai is the treatment effect, tj is the effect of sampling day or time,(at)ij is the interaction effects of treatment and sampling day or time, Bk is a random effect of animal, and eijk is the residual error. Significance was declared at P<0.05. The binomially distributed reproductive data were analyzed using the logistic procedure of SAS. Significance and tendencies were declared at P<0.05, unless otherwise indicated.
Results and Discussion
The purpose of this study was to determine the effects of different proportions of essential fatty acids LA and ALA (linseed and soybean extruded as LA and ALA, respectively) in diets on blood metabolites, reproductive hormones, and reproductive performance. Unlike almost all previous investigations, in this study the relative amounts of ALA and LA were calculated. Formulation of each experimental diet based on the amount of each calculated fatty acid. In each experimental diet, the rations (1:1, 5:1, and 10:1) were formulated according to these amounts. The major aim of this procedure was to determine the probable effects the concentrations of each essential fatty acid on ewe’s fertility. The percentage of omega-3 and omega-6 fatty acid sources in experimental diets had shown variable effects on ruminant reproduction in most of previous studies.
Overall, the concentration of all metabolites in the blood plasma increased from day 14 (first sampling) compared to day 45 (fourth sampling). There were significant differences in plasma glucose concentrations between the experimental groups at all sampling times (P<0.05) (Table 3). In the first sampling on day 14, ewes in group 4 (ratio 10 to 1 LA to ALA) had the highest (51.09 mg/dl) concentrations of plasma glucose (P<0.05). In the second and third sampling times on days 31 and 45, respectively, the highest levels of glucose were observed in the experimental groups 5:1 LA to ALA (54.03 mg/dl) and 1:1 LA to ALA (77.01) (Table 3). In the present study, fresh ewes who received a control diet (beef tallow as the primary fat source) had the lowest concentration of plasma glucose in comparison to other groups in all three times of blood sampling. A possible reason for the decrease in glucose concentration could be related to the decreased feed intake caused by the presence of beef tallow, and the resultant decrease in the concentrations of propionate or metabolizable proteins in the rumen fluid [1]. Unlike our results, no significant differences were observed in plasma glucose concentration of ewes received diet supplemented with the PUFAs [12]. When the plasma glucose concentration rises, the uptake of glucose and cholesterol by the ovarian cells may increase [25].
Treatments
Metabolites
Control
1:01
5:01
10:01
SEM
P-Value
Glucose
Day 14
48.53b
50.02ab
49.78ab
51.09a
0.794
0.044
(mg/dl)
Day 31
50.71b
53.27ab
54.03a
53.06ab
1.509
0.023
Day 45
67.26b
77.01a
70.53ab
75.12ab
3.321
0.041
Cholesterol
Day 14
78.47
79.67
83.6
78.9
5.373
0.902
(mg/dl)
Day 31
91.99b
91.68a
97.27a
92.75ab
1.573
0.045
Day 45
100.83
101.55
103.53
104.68
4.2
0.91
Triglyceride
Day 14
17.76
17.98
18.33
18.71
0.84
0.862
(mg/dl)
Day 31
21.03
18.9
19.18
19.71
0.953
0.416
Day 45
25.24
19.7
23.25
20.09
1.814
0.123
Means in the same row with different superscript are significantly different (P<0.05).
Table 3: Plasma concentrations of metabolites in different treatment groups.
Plasma cholesterol in the second sampling time (day 14) in ewes receiving 5:1 LA to ALA ratio was significantly higher than in the other groups (p<0.05). There was no diet × sample day interaction on plasma cholesterol concentrations. In agreement with the results of the present study, the concentration of plasma cholesterol in ewes fed a flushing diet supplemented with calcium salts of fatty acids was higher compared to the control group [16]. It seems that the reason for the relative increase of cholesterol concentration in the blood plasma of treatment groups (supplemented with unsaturated fatty acids) as compared to the control group, could be due to the increased level of ruminal biohydrogenation of polyunsaturated fatty acids and then absorption in the small intestine. Our result is also consistent with the findings of Marcello et al. (1971), who showed that the increasing content of fatty acids in diets increases unsaturated fatty acids C16:1 and C18:1 concentrations in animals plasma [19]. Since cholesterol is considered a major component of lipoproteins, more fatty acids are used in cholesterol formation [13]. Cholesterol is an important precursor of progesterone in the Corpus Luteum (CL), and also plays a role in the synthesis of other steroid hormones in the follicular phase [7]. Feeding diets supplemented with n-3 fatty acids compared with n-6 fatty acids resulted in higher progesterone concentrations in follicular fluid in ewes [35].
Ewes in all experimental groups showed increased plasma triglyceride concentrations from 14 to 45 days of sampling (Table 3). The highest triglyceride concentration showed on day 45 for group 1 (25.24 mg/dl). However, the plasma triglyceride concentration did not differ among experimental groups (P>0.05). Increasing in triglyceride concentration may be due to decreased activity of the lipogenic enzyme in the animals’ livers and adipose tissues [13]. Moreover, increasing the accumulation of triglycerides in the liver of ruminants may be due to increased levels of Free Fatty Acids (FFAs) in the blood.
Plasma progesterone concentrations were similar across the experimental groups at the first sampling time. On the second and third sampling days, plasma progesterone concentrations were significantly higher in the experimental groups 4 (1.36 ng/dl) and 2 (4.00 ng/dl), respectively (P<0.05) (Table 4). There were no (P>0.05) diet × time interactions for concentrations of blood plasma progesterone. According to our results, the increasing trend of progesterone concentration in ewes’ blood plasma is shown in Table 4. This result was in agreement with the observations of Wonnacott et al. (2010) who reported that higher progesterone concentration in follicular fluid in ewes-fed diets supplemented with omega-3 and omega-6 fatty acids [35]. Moussavi et al. (2007) reported no significant difference in the plasma progesterone concentrations between the control group (no fat supplement in the diet) and cows fed fish meal and calcium salts of fatty acids during the luteal phase [21]. However, Walsh et al. (2011) showed that ruminants fed diets supplemented with PUFAs have a higher concentration of progesterone during the luteal phase of the estrus cycle [34]. Accordingly, Castaneda-Gutierrez et al. (2007) speculated that the increase in progesterone concentrations of plasma in cows supplemented with conjugated LA was due to an increase in IGF-I recycle [6] and the production of prostaglandin E2 from Steroidogenic Acute Regulatory (StAR) in the granulosa cells [18]. An increase in serum lipid concentrations is associated with an increase in the fat contents of small and large steroidogenic cells (estrogenic constituents) of ruminant corpus luteum. Therefore, an increase in intracellular lipids may be a reason for enhancing the level of progesterone precursors (Hawkins et al., 1995). On the other hand, the role of ALA in the prevention of PGF2a synthesis is clear, which has an optimal effect on progesterone concentration [14]. Higher levels of progesterone concentrations have been shown to support embryo viability and enhance the establishment of pregnancy [30].
Treatments
Hormones
Time
Control
1:01
5:01
10:01
SEM
P-Value
Insulin
Day 14
5.88
7.15
6.17
7.46
0.737
0.386
(μm/ml)
Day 31
8.17
8.11
7.19
9.41
0.941
1.331
Day 45
7.87b
8.46ab
10.13a
10.26a
0.925
0.239
Progesterone
Day 14
1.16
1.39
1.46
1.51
0.164
0.461
(ng/dl)
Day 31
1.24ab
0.97b
1.11ab
1.36a
0.125
0.039
Day 45
2.63b
4.00a
2.81ab
3.71ab
0.634
0.021
Eestradiol 17-β
Day 14
56.78
56.96
57.04
54.52
3.94
0.962
(pg/ml)
Day 31
70.15b
91.65a
75.46ab
81.87ab
6.63
0.048
Day 45
68.69
72.49
67.8
65.37
4.34
0.71
Means in the same row with different superscript are significantly different (P<0.05).
Table 4: Plasma concentrations of hormones in different treatment groups.
As shown in Table 4, the addition of different ratios (1:1, 5:1, and 10:1) of LA and ALA had no significant effect on ewes’ plasma estradiol 17-β hormone in all the experimental groups in the first and third sampling times. On the second sampling time, the highest plasma estradiol 17-β hormone concentrations (91.56 pg/ml) were observed in experimental group 2 with a ratio of 5:1 (LA to ALA). An interestingly marked increase in the concentration of estradiol 17-beta hormone was observed only on day 31 of sampling for the experimental diet with a ratio of 1 to 1 LA to ALA. Somchit et al. (2007) reported that plasma concentration of estradiol in ewes fed lupin as a source of supplementary fat in the diet was decreased, but this reduction was not significan [29]. Two papers have reported that nutritional supplementation with energetic sources decreased estradiol secretion [28,33]. In agreement with our study, serum estradiol concentration in ewes fed the fatty acid supplemented diets increased from the time of injection of PGF2a (day 0) to day 3 [2]. Increasing the availability of cholesterol may result in increased steroid component synthesis and improved energy balance by supplementing fat in the diet [1]. Downing et al. (1999) reported that glucose and insulin injections into the ovarian vessels reduced estradiol secretion [9]. The reason for reduced estradiol secretion might be due to the reduced availability of substrates for the production of estradiol or the loss of capacity of granulosa cells to convert these substrates to this hormone [29].
The pregnancy rate in the control group (57.14%) was lower than in the other experimental groups (Table 5). Also, the lambing rate of the control group (42.86%) was markedly lower than the other experimental groups (P<0.05). No significant differences were observed in lamb birth weight between experimental groups. However, supplementing sources of fats such as oil or oilseeds resulted in an improved lambing performance in sheep [8]. Adding supplemented fat to flushing diets in ewes may have a significant impact on the animal’s reproductive performance through an increase in plasma concentrations of progesterone [5].
Treatment
Pregnancy rate (%)
Lambing rate (%)
Sex ratio
(% male / female)Control
57.14b
42.86b
66.67/33.33ab
1:1
85.71a
85.71a
50/50b
1:5
71.43ab
75.14ab
75/25a
1:10
71.43ab
71.43ab
71.43/28.57a
SEM
0.175
0.184
0.262
P-Value
0.046
0.039
0.033
Means in the same columns with different superscript are significantly different (P<0.05).
Table 5: Pregnancy rates, lambing rates, and sex ratio of lambs in ewe with different treatment groups.
The sex ratio was significantly affected by experimental treatments (P<0.05). Ewes on a diet with 5:1 of LA to ALA produced the highest number of male offspring. Published data concerning the effect of food supplements on sex ratio are controversial and primarily relate to the diet composition during the mating and fertilization period [17]. Reports by Gulliver et al. (2013) showed that further research is required to determine whether the observed increase in the proportion (58.2 vs. 43.5%) of female-born lambs of the Merino × Border Leicester ewes [14]. The content of MUFA, PUFA, and glucose in diet could affect the sex ratio [20]. It seems that the effect of dietary fat supplementation on sex offspring is related to female parents; feeding male rats with fat supplemented diets did not affect the sex ratio of the offspring [3].
Due to the effect of fatty acids in each stage of ovulation and pregnancy, supplementing diets with omega-3 and omega-6 fatty acids could take place in the estrous, mating, and pregnancy stages in ewe’s herds. These effects might happen due to the impact of omega-3 and omega-6 fatty acids on the synthesis of steroid hormones, increased ovulation, and follicle size. There is a positive correlation between glucose and cholesterol concentrations in ovarian cells, which means that by increasing cholesterol absorption and increasing insulin concentration, glucose uptake increases [25]. The simultaneous absorption of glucose and cholesterol leads to the release of LH by influencing the gonadotrophic releasing hormones [10]. An increase in LH concentration leads to the development of larger ovarian follicles, yields higher estradiol concentration during the estrous cycle, and results in the formation of a larger corpus luteum, with aids in pregnancy establishment and continuity. Childs et al. (2008) fed beef heifers diets enriched in fish oil (omega-3 fatty acids), and even though no increase in serum progesterone was observed on day 7 of the estrous cycle [7]. They postulated that there was evidence for the more synthesis of progesterone during the whole estrous cycle due to the increase in the serum concentrations of cholesterol and the presence of a greater size corpus luteum.
Conclusion
In conclusion, the results of this study showed that ratios of 1:1 (LA: ALA) of fatty acids in diets of experimental groups more improve fertility and reproductive performance. The levels of plasma progesterone and estrogen in ewes who received LA and ALA at a ratio of 1:1 were significantly higher than in other experimental groups. Different ratios of LA to ALA acids in the diet during the flushing period had beneficial effects on reproductive postpartum in Zel fresh ewes. According to the results of our study, we suggested further studies to be carried out on this topic accompanied by measuring different amounts of omega-3 and omega-6 fatty acids in the diets.
References
- Abayasekara DR, Wathes DC. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. PLEFA. 1999; 5: 275-287.
- Abd El-Hamid IS, El-Din AN, Zaghloul AA, El-Bahrawy KA, Elshahawy II, Allam AM, et al. Effects of calcium salts of fatty acids rich in palmitic and oleic fatty acids on reproduction and serum biochemistry in Barki ewes. Small Rum Res. 2016; 144: 113-118.
- Alexenko AP, Mao J, Ellersieck MR, Davis AM, Whyte JJ, Rosenfeld CS, et al. The contrasting effects of ad libitum and restricted feeding of a diet very high in saturated fats on sex ratio and metabolic hormones in mice. Bio Rep. 2007; 4: 599-604.
- Butler ST, Pelton SH, Butler WR. Insulin increases 17β-estradiol production by the dominant follicle of the first postpartum follicle wave in dairy cows. Rep. 2004; 5: 537-545.
- Carroll DJ, Grummer RR, Mao FC. Progesterone production by cultured luteal cells in the presence of bovine low-and high-density lipoproteins purified by heparin affinity chromatography. J Anim Sci. 1992; 8: 2516-2526.
- Castañeda-Gutiérrez E, Benefield BC, De Veth MJ, Santos NR, Gilbert RO, Butler WR, et al. Evaluation of the mechanism of action of conjugated linoleic acid isomers on reproduction in dairy cows. J Dairy Sci. 2007; 9: 4253-4264.
- Childs S, Lynch CO, Hennessy AA, Stanton C, Wathes DC, Sreenan JM, et al. Effect of dietary enrichment with either n-3 or n-6 fatty acids on systemic metabolite and hormone concentration and ovarian function in heifers. Animal. 2008; 6: 883-893.
- Daghigh Kia H. Effect of fat supplementation in flushing diets on reproductive performance, blood metabolites and hormones in Ghezel breed ewes. J of Anim Sci Res. 2012; 2: 147-160.
- Downing JA, Joss J, Scaramuzzi RJ. The effect of a direct arterial infusion of insulin and glucose on the ovarian secretion rates of androstenedione and oestradiol in ewes with an autotransplanted ovary. J Endo. 1999; 3: 531-541.
- Downing JA, Scaramuzzi RJ. The effect of the infusion of insulin during the luteal phase of the estrous cycle on the ovulation rate and on plasma concentrations of LH, FSH and glucose in ewes. Theri. 1997; 3: 747-759.
- Evans E, Buchanan-Smith JG, Macleod GK. Postprandial patterns of plasma glucose, insulin and volatile fatty acids in ruminants fed low-and high-roughage diets. J Anim Sci. 1975; 5: 1474-1479.
- Green MP, Spate LD, Parks TE, Kimura K, Murphy CN, Williams JE, et al. Nutritional skewing of conceptus sex in sheep: effects of a maternal diet enriched in rumen-protected polyunsaturated fatty acids (PUFA). Rep Bio End. 2008; 1: 1-1.
- Grummer RR, Carroll DJ. Effects of dietary fat on metabolic disorders and reproductive performance of dairy cattle. J Anim Sci. 1991; 9: 3838-3852.
- Gulliver CE, Friend MA, King BJ, Clayton EH. The role of omega-3 polyunsaturated fatty acids in reproduction of sheep and cattle. Anim Rep Sci. 2012; 131: 9-22.
- Horoszewicz E, Pieniak-Lendzion K, Niedziolka R, Wójcik E. Fatty acids profile and physicochemical properties of muscle tissue from male kids and ram lambs offered feed supplemented with flaxseed. Acta Sci Polo. Zoot. 2011; 10.
- Khotijah L, Wiryawan KG, Setiadi MA, Astuti DA. Reproductive performance, cholesterol and progesterone status of Garut ewes fed ration containing different levels of sun flower oil. Pak J Nutr. 2015; 7: 388-391.
- Landete-Castillejos T, Gortázar C, Vicente J, Fierro Y, Garcia A, Gallego L. Age-related foetal sex ratio bias in Iberian red deer (Cervus elaphus hispanicus): are male calves too expensive for growing mothers?. Behavioral Eco Soci. 2004; 56: 1-8.
- Lemley CO, Butler ST, Butler WR, Wilson ME. Insulin alters hepatic progesterone catabolic enzymes cytochrome P450 2C and 3A in dairy cows. J Dairy Sci. 2008; 2: 641-645.
- Marchello JA, Dryden FD, Hale WH. Bovine serum lipids. I. The influence of added animal fat to the ration. J Ani Sci. 1971; 5: 1008-1015.
- Marei WF, Khalil WA, Pushpakumara AP, El-Harairy MA, El-Atta AM, Wathes DC, et al. Polyunsaturated fatty acids influence offspring sex ratio in cows. International Journal of Veterinary Science and Medicine. 2018; 6: 36-40.
- Moussavi AH, Gilbert RO, Overton TR, Bauman DE, Butler WR. Effects of feeding fish meal and n-3 fatty acids on ovarian and uterine responses in early lactating dairy cows. J Dairy Sci. 2007; 1: 145-154.
- Naqvi SM, Soren NM, Karim SA. Effect of concentrate supplementation on performance, ovarian response, and some biochemical profile of Malpura ewes. Trop Anim Health Prod. 2011; 43: 905-913.
- Nugroho P, Wiryawan KG, Astuti DA, Manalu W. Stimulation of follicle growth and development during estrus in Ettawa Grade does fed a flushing supplement of different polyunsaturated fatty acids. Vet World. 2021; 1: 11-22.
- Palmquist DL. Essential fatty acids in ruminant diets. In Proceedings of the 21nd Annual Ruminant Nutrition Symposium. 2010.
- Rabiee AR, Lean IJ. Uptake of glucose and cholesterol by the ovary of sheep and cattle and the influence of arterial LH concentrations. Anim Rep Sci. 2000; 64: 199-209.
- Samadi MS, Chashnidel Y, Dirandeh E, Deldar H. Effects of heat processing of soybeans and linseed on ruminal fatty acid biohydrogenation in situ. IJAAS. 2018; 4: 583-589.
- Santos JE, Bilby TR, Thatcher WW, Staples CR, Silvestre FT. Long chain fatty acids of diet as factors influencing reproduction in cattle. Rep Dom Anim. 2008; 43: 23-30.
- Scaramuzzi RJ, Campbell BK, Downing JA, Kendall NR, Khalid M, Muñoz-Gutiérrez M, et al. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Rep Nutri Dev. 2006; 4: 339-354.
- Somchit A, Campbell BK, Khalid M, Kendall NR, Scaramuzzi RJ. The effect of short-term nutritional supplementation of ewes with lupin grain (Lupinus luteus), during the luteal phase of the estrous cycle on the number of ovarian follicles and the concentrations of hormones and glucose in plasma and follicular fluid. Theri. 2007; 7: 1037-1046.
- Staples CR, Burke JM, Thatcher WW. Influence of supplemental fats on reproductive tissues and performance of lactating cows. J Dairy Sci. 1998; 3: 856-871.
- Thatcher WW, Staples RC. Using fats and fatty acids to enhance reproductive performance. In Proceedings of the 5th Mid-Atlantic Nutrition Conference. Timonium, MD: 2007 Mar 28. University of Maryland. 2007.
- Velazquez MA, Spicer LJ, Wathes DC. The role of endocrine insulin-like growth factor-I (IGF-I) in female bovine reproduction. Dom Anim End. 2008; 4: 325-342.
- Viñoles C, Forsberg M, Martin GB, Cajarville C, Repetto J, Meikle A. Short-term nutritional supplementation of ewes in low body condition affects follicle development due to an increase in glucose and metabolic hormones. Rep. 2005; 3: 299-309.
- Walsh SW, Williams EJ, Evans AC. A review of the causes of poor fertility in high milk producing dairy cows. Anim Rep Sci. 2011; 123: 127-138.
- Wonnacott KE, Kwong WY, Hughes J, Salter AM, Lea RG, Garnsworthy PC, et al. Dietary omega-3 and-6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Rep. 2010; 139: 57-69.
- Zachut M, Arieli A, Moallem U. Incorporation of dietary n-3 fatty acids into ovarian compartments in dairy cows and the effects on hormonal and behavioral patterns around estrus. Rep. 2011; 6: 833-840.
- Zamiri MJ, Rowghani E, Ghoreishi SM. Effects of calcium salts of fatty acids on follicular characteristics and several blood parameters in two fat-tailed sheep breeds. In Proceedings of the British Soc Anim Sci. 2002; Cambridge University Press. 2002.