
Review Article
Austin J Vet Sci & Anim Husb. 2024; 11(2): 1142.
Review on Avian Corona Virus in Ethiopia
Tadesse Mihret¹*; Omima Adam²
University of Gondar College of Veterinary Medicine and Animal Science, Department of Clinical Medicine, Ethiopia Omima Adam, Animal Resources Research Corporation, Sudan
*Corresponding author: Tadesse Mihret University of Gondar College of Veterinary Medicine and Animal Science, Department of clinical medicine Po. Box 196. Gondar, Ethiopia. Tel: +251932274818 Email: mihretadesse8@gmail.com,
Received: February 05, 2024 Accepted: March 25, 2024 Published: March 29, 2024
Abstract
The extremely contagious disease known as Infectious Bronchitis (IB) affects the urogenital and respiratory tracts of chickens. It is brought on by the Infectious Bronchitis Virus (IBV), a member of the Coronaviridae family. Because this virus is infectious, it causes significant economic losses. Mortality, stunted growth, and high condemnation rates in meat type birds are among the economic ramifications for the poultry sector. The performance of both meat type and egg laying birds has also been shown to be impacted by decreasing egg output, lower internal and exterior egg quality, and decreased hatchability in layers and breeders. Furthermore, kidney injury is caused by certain nephro pathogenic strains. Secondary infections have the potential to worsen the illness and raise morbidity and death rates. IBV is a single stranded RNA virus that can change a great deal through genetic recombination and spontaneous mutation, giving rise to new variations. Since the virus was initially identified in 1937, nearly every continent has seen its presence. Furthermore, it is currently recognized that the majority of nations have their own native IBV versions. The rise of variant strains of IB is a major challenge to control, even with the use of currently available live and inactivated vaccinations. This study summarizes the state of IBV research as of right now.
Keywords: Infectious bronchitis; Coronaviridae; RNA virus
Introduction
Ethiopia is predicted to have 56.5 million chickens overall. Despite the large number of chickens, the sector's economic contribution remains out of proportion due to multiple productions, reproduction, and infrastructure limitations. It is well known that the two main causes of death in the country are disease and predators. Newcastle disease caused the greatest percentage of flock deaths overall (57.3%), followed by coccidiosis (9.4%), fowl pox (31.6%), and predator loss (1.7%) [1,2].
Lesions in the reproductive, urogenital, and respiratory organs are the most common sign of infectious bronchitis, an acute, highly contagious viral disease that affects chickens [3]. All ages of hens are susceptible to the illness, but young birds are particularly at risk because resistance increases with age. Following avian influenza, the condition causes significant economic losses in the chicken industry due to poor performance, lower egg output and quality, and mortality that can be severe in the presence of nephropathogenic strains or when secondary infections emerge; it is thought that IBV infection causes the third-highest losses among all livestock diseases [2,4]. IBV was found in other US states after the first serotype was identified in the US in 1931, and a wide range of IBV genotypes and serotypes were reported worldwide [5]. Despite international efforts to control it up to this point, infectious bronchitis remains an issue for the chicken business. This is mostly because to the high mutation rate, rapid generation period, and wide genetic diversity of IBV [6]. The efficiency of natural or vaccine immunity is limited by the continual emergence of genetic diversity and the selection process among IBV variants [2].
One of the main viral infections that endanger chicken productivity in Africa is IBV. 1950 saw the first reports of it in Africa, from birds exhibiting respiratory issues in Egypt. The illness, which is considered an epidemic virus, is the most common viral respiratory infection in chickens on the continent and affects both vaccinated and unprotected poultry birds [7]. Although the prevalence and types of strains of the disease have not been well explored, it is widespread in Ethiopia [8].
Therefore, the main objective of this seminar paper is:
To highlight about the infectious bronchitis disease in chickens
To review its clinical managements.
To describe the prevention and control options to tackle the spread of the disease
The Disease
Avian Infectious Bronchitis (IB) is a contagious disease of birds caused by Corona virus and leading to economic loss in chicken operations. The infection was first described in 1931 in the USA as a respiratory disease of chicks. The virus is a corona virus and is prevalent in all countries with an intensive poultry industry. The infection has a significant economic impact; in broilers, production losses are due to poor weight gains, condemnation at processing and mortality. In laying birds, losses are due to suboptimal egg production and downgrading of eggs [9].
Etiology
Infectious bronchitis virus is a corona virus that causes infectious bronchitis affecting domestic chickens. It replicates in respiratory tissues as well as other epithelial tissues such as the kidneys, gonads, and bursa [10]. However, virologists have found it difficult to pinpoint exactly which tissues and animal species these viruses replicate in, as it has been seen that they can bypass host barriers, like in the case of SARS (severe acute respiratory syndrome). Gamma Coronavirus Classical and variant strains Massachusetts serotype (Classical) have a complex construction and consist of an envelope and a nucleocapsid. They are spherical, or kidney-shaped, or pleomorphic. The surface projections are distinctive club-shaped peplomers that evenly cover the surface. The nucleocapsid is cylindrical; 2 nm in diameter. The genome is not segmented and consists of a single molecule of linear positive-sense single-stranded RNA. The viral genome encodes structural proteins and non-structural proteins [1].
The virus is a member of the Coronaviridae family and its 27.6 kb single-stranded positive sense RNA genome has the following genomic arrangement and: fifty untranslated regions 30UTR: 1a/1ab S 3a 3b E M 5a 5b. While the non-structural viral proteins are encoded by two polyproteins, the structural viral proteins include the Membrane (M), small Membrane (E), Nucleoprotein (N), and Spike (S). The spike protein, among others, is the subject of extensive research due to its genetic diversity and biological function [10].
The virus has five structural proteins that are named S, M, N, HE and E. Surface glycoprotein (or spike, S), which S protein is responsible for attachment to cells, hemagglutination and membrane fusion, Integral membrane protein (M) which spans the virus envelope three times, Nucleocapsid protein (N) , Hemagglutinin-Esterase protein (HE), which forms short surface projections, and have receptor binding, hemagglutination and receptor destroying activities and Envelope protein (E), small, envelope-associated protein unlike (-) sense RNA viruses polymerase enzymes are not present in the nucleocapsid [9].
Epidemiology
Transmission
Infectious bronchitis is spread horizontally through direct contact with chickens (between diseased and vulnerable birds) or indirectly (by wild birds, water, and other materials) [11]. Furthermore, it may persist asymptomatically in minute concentrations in the ceacal tonsils of the digestive system for a long time. Protection may not be effective even if immunization has been used for several years [9].The affected birds shed the virus in respiratory secretion and faeces. The virus spreads rapidly among chickens in a flock through inhalation of virus droplets produced by infected chickens. Infection is also transmitted by aerosols, contaminated feed and water, contact with animals or material.Movement of live birds is considered as a potential source for the introduction of IBV. Affected birds recover within 14 days. However, a small number of chicks may become latently infected with erratic shedding of virus for a prolonged period of time via both faeces and aerosol [10]. There is no carrier state and vertical transmission. No vectors are also involved in the spread. Incubation period is 24 – 48 hours and severity of the disease is influenced by strain of the virus, age of bird, immune status of bird, cold stress and coinfection with other diseases like Mycoplasma and E. coli [12].
Geographic Distribution
Infectious bronchitis is widely distributed geographically and has been identified in parts of Africa, Asia, Australia, Europe, and the Americas [13-15]. It is one of the most endemic viral respiratory infections of chickens in Africa, where it is widespread in both vaccinated and unvaccinated poultry birds and is regarded as an epidemic virus [16,17]. The most significant IBV variations (793B, 4/91, and CR88) may be found in European nations as well in North Africa, where variants have received less attention, may represent a reservoir for these variant [18]. In fact, the 793B form, which originated in North Africa, has been discovered to have been present in France since 1985 [19]. The IBV variation 793B has been reported in Ethiopia by Hutton et al, [20] in 2017 and by Tegegne et al [8] in 2020.
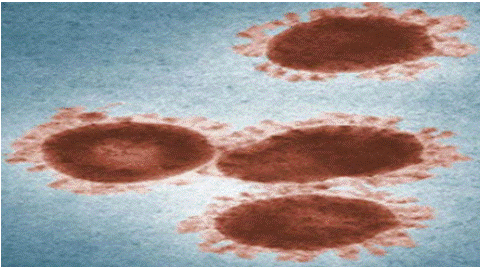
Figure 1: Structure of the avian corona virus source (© Public Health Image Library from the Centres for Disease
Control and Prevention CDC).
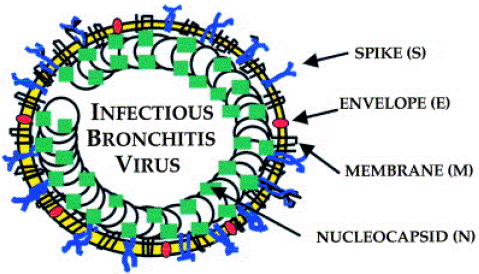
Figure 2: Structural proteins of the virus source (https://doi.org/10.1016/S0145-305X (99)00072-5).
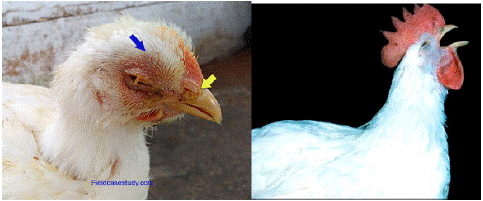
Figure 3: Nasal discharge, wet eyes, swollen sinuses and gasping [32].
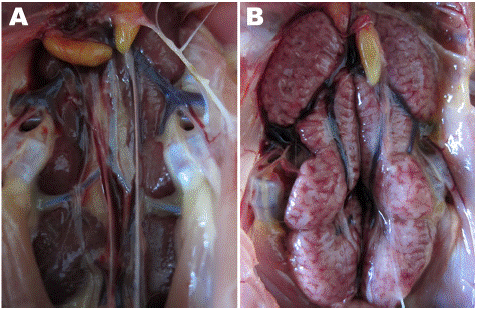
Figure 4: Normal kidneys A versus swollen kidneys B source [15].
Seroprevalence and Associated Risk Factors
The study carried out by Birhan et al [21] in northern Ethiopia indicated a seroprevalence of 24.6% and the study by Shiferaw et al [22] in Debrezeit, Ethiopia discovered a prevalence of 94.5 %. The study conducted by Tesfaye et al [23] and by Yonas et al [24] were found 70.6 % and 64.7 % seroprevalence of IB in Hawassa, and Ada'a Districts of Ethiopia, respectively.
The prevalence varied significantly among chickens of different ages. According to the study by [25], young chickens had the higher prevalence than adults. All age groups of chicks are susceptible to IBV; young chicks are more vulnerable than older ones [4]. Additionally, as chickens get older, their resistance to diseases is stronger as well. The prevalence of the disease is higher in exotic breeds than the prevalence of in local breed. This might be due to exotic breeds limited resistance to disease and other environmental stresses [26].
An investigation of the prevalence of IB in chicken farms indicated that intensive farming had higher seroprevalence than its extensive equivalents as indicated in commercial poultry farms in Nigeria by Oyejide, et al. [27], in Jordan by Rousson, et al. [28] and in Ethiopia by Hutton et al. [20]. Higher seroprevalence was found in layers and dual-purpose chickens as compared with broilers [29]. This could be explained by layer and dual-purpose birds spending more time on the farm than other birds, which leads to their re-infection if there is no effective way to manage it [30] and the longer the chickens were exposed to the virus, the higher the seroprevalence of IB became [31].
A higher seroprevalence was obtained in females than in males as indicated study of local chickens in live bird markets in Sokoto State, Nigeria, with a higher prevalence in females than in males. Due to differences in the activity of humoral- and cell-mediated immune responses between the sexes, this may be due to the presence of a less effective immunological response in males than in females [25].
Clinical Signs
The virulence of the virus and the organ or system implicated determines the severity and clinical aspects of IB infection. Although clinical indications of simple infection persist less than seven days and have a mortality rate of 5% to 25%, chronic infection can last for several weeks [19]. Respiratory rales (gurgling and snicking) and ocular discharge are associated with moderate morbidity and low flock mortality. With deformed shells, mature flocks produce fewer eggs [32]. The infection in young chickens is characterised by gasping, coughing and nasal discharge with wet eyes and swollen sinuses. The chicks appear depressed and may huddle near the heat source. Food consumption and weight gain are also reduced. In adult laying flocks, the respiratory symptoms of gasping and coughing are usually followed by a drop in production Pullets in good condition may suffer only a slight drop in production and regain normal production within few weeks. Production of misshapen soft-shelled eggs with inferior internal quality is often observed. Secondary bacterial infection due to E.coli complicate the disease scenario and lead increased condemnation of birds [12].
Diagnosis Methods
Although IBV is a virus that causes significant economic losses in poultry, there are no distinct clinical indications of IB. When there are clinical concerns in the field, it is vital to employ methods to identify IBV. IB can be diagnosed in general by identifying the virus (or components of it) or particular antibody reactions. Serological tests involving the Virus Neutralization Test (VNT) and Hemagglutination Inhibition (HI) test for detection and serotyping of Infectious Bronchitis Virus can be used to make various diagnoses. The gold standard test is VNT, while cross-neutralization assays are utilized for variation detection [33]. IgM IgG-specific ELISA is most commonly used in the field for monitoring antibody response because it is more sensitive and simpler. Choosing the proper test for diagnosis and then interpreting the results can be complicated and complex. An immunofluorescence assay or the isolation and identification of the causative virus utilizing egg inoculation or tissue culture techniques can be used to confirm the diagnosis. RT PCR is used to quickly diagnose IB when suitable laboratory resources are available. Retrospective diagnosis is achievable using an ELISA or SN assay to demonstrate a significant increase in circulating antibody in paired acute and recovery-phase samples [32].
Post mortem lesions like airsacculitis excessive mucus in the trachea, haemorrhagic tracheitis, severely congested lungs with exudates, perihepatitis, pericarditis, swollen kidneys and uroliths are also used to diagnose the disease [12].
Treatments
There is no specific treatment for infectious bronchitis as there is no effective treatment for viral diseases. Antibiotics against secondary bacterial pathogens can be provided in the case of mixed infections. In the event of nephropathogenic IB, 72mEq sodium and/or potassium might be given in the drinking water, with one-third in the citrate or bicarbonate salt form. To limit IB losses, extra heat sources, excellent ventilation, preventing overcrowding, and controlled feed consumption can be implemented [33].
Prevention and control
Due to the enormous number of IBV strains and the rapid evolution of new strains, control and management techniques to avoid IBV infection are problematic. However, biosecurity and management measures such as strict isolation, repopulation of single-day chickens after cleaning and disinfecting the room, distinct age groups of birds reared separately, and a vaccination program can be used to prevent IBV from spreading. After each chicken flock, restocking must take place at least 10-14 days apart [34,35]. Vaccines are administered through drinking water, via eye drops, nasal illustration, or aerosol sprays. Despite commercially available live and inactivated vaccinations, monitoring infections is difficult due to considerable variation. When the vaccine is available, the best way to treat IBV is to utilize vaccine strains that are similar to those found in a particular farm or field. Broilers, as well as breeders and layer pullets, frequently receive activated or live immunizations inactivated oil emulsion vaccines preceded by a live virus vaccine produce persistent antibody response. Strict biosecurity measures and good husbandry practices will minimize the spread of IBV. During outbreaks, the virus should be serotyped and vaccine should be altered. In areas where there is no IB, use of live vaccine should be minimized [33].
Conclusion and Recommendations
Avian infectious bronchitis continues to be a major problem for the global chicken industry. Since the virus is constantly changing, many localized and worldwide variations have been found. However, the previous analysis of the existing data does not suggest additional vaccinations at every turn. Thankfully, a few live vaccines offer wide protection against the majority of genotypes known to cause nephrotropic and respiratory forms of the disease. Including selected strains in inactivated vaccines is a viable substitute. Aside from this, it seems that enhanced management, ongoing active surveillance, and farm biosecurity are all crucial to reducing losses brought on by IBV infections.
Author Statements
Authors’ Contributions
Conceptualization, writing-original draft preparation, T.M and O.M, Writing-review and editing, T.M
Competing Interests
The authors declare that there are no conflicts of interest.
References
- Roba YT, Bejura W, Teshome T, Tesfaye A, Mengesha N, Tadesse F. ‘Serological Detection of Antibodies Against Gamma Coronavirus Infection in Scavenging Village Chickensin Ada’aDistrict Ethiopia. 2021; 4: 26106-26110.
- Mihret T, Bitew M, Oni OO, Enbiyale G, Workneh D, Birhanu K, et al. Seroprevalence, Isolation and Molecular Detection of Infectious Bronchitis in Backyard and Commercial Chickens in Central Gondar Zone, Northern Ethiopia. Epidemol Int J. 2023; 7: 00027.
- Dolz R, Pujols J, Ord óñez G, Porta R, Maj ó, N. Molecular epidemiology and evolution of avian infectious /bronchitis virus in Spain over a fourteen -year period. Virology. 2008; 374: 50-59.
- Cavanagh and Gelb. Infectious Bronchitis Virus in: Diseases of Poultry. Iowa State University Press, Ames, Iowa, 12th edition, 2008; 117-130.
- Hodgson T, Britton P, Cavanagh D. Neither the RNA nor the Proteins of Open Reading Frames 3a and 3b of the Coronavirus Infectious Bronchitis Virus Are Essential for Replication. J Virol. 2006; 80: 296–305.
- Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of corona virus cell entry mediated by the viral spike protein. Viruses. 2012; 4: 1011–1033.
- Casais R, Dove B, Cavanagh D, Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J Virol. 2003; 77: 9084 9089.
- Tegegne D, Deneke Y, Sori T, Abdurahaman M, Kebede M, Cecchinato M, et al. ‘veterinary sciences Molecular Epidemiology and Genotyping of Infectious Bronchitis Virus and Avian Metapneumovirus in Backyard and Commercial Chickens in Jimma Zone, Southwestern Ethiopia. 2020.
- Khataby K, Fellahi S, Loutfi C, Mustapha M. ‘Avian infectious bronchitis virus in Africa: review’. Veterinary Quarterly. 2016; 36: 71-75.
- Laconi A, Van Beurden SJ, Berends AJ, Krämer-Kühl A, Jansen CA, Spekreijse D, et al. Deletion of accessory genes 3a, 3b, 5a or5b from avian coronavirus infectious bronchitis virus induces an attenuated phenotype both in vitro and in vivo. J Gen Virol. 2018; 99: 1381–1390.
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus, Avian Pathol. 2003; 32: 567–582.
- Jackwood MW, Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, et al. In Diseases of Poultry, 13th ed.; Iowa State University Press: Ames, IA, USA. 2013; 13: 139–159.
- Cavanagh D. Coronavirus, avian infectious bronchitis virus. Vet Res. 2007; 38: 281-297.
- Cook JA, Jackwood M, Jones RC. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012; 41: 239–250.
- Jackwood MW. Review of infectious bronchitis virus around the world. Avian Dis. 2012; 56: 634- 641.
- Liu HJ, Lee LH, Shih WL, Lin MY, Liao MH. Detection of infectious bronchitis virus by multiplex polymerase chain reaction and sequence analysis. J Virol Methods. 2003; 109: 31 -37.
- Lee HJ, Youn HN, Kwon JS, Lee YJ, Kim JH, Lee JB, et al. Characterization of a novel live attenuated infectious bronchitis virus vaccine candidate derived from a Korean nephropathogenic strain. Vaccine. 2010; 28: 2887-2894.
- Jones RC, Savage CE, Naylor CJ, Cook JK, El-Houadfi MA. Possible North African strains of infectious bronchitis. 2004.
- Cavanagh D, Davis PJ, Cook JK, Kant A, Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992; 21: 33-43.
- Hutton S, Bettridge J, Christley R, Habte T, Ganapathy K. Detection of infectious bronchitis virus 793B, avian metapneumovirus, Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Ethiopia. Trop Anim Health Prod. 2017; 49: 317–322.
- Birhan M, Temesgen M, Shite A, Berhane N, Bitew M, Gelaye E, et al. Seroprevalence and Associated Risk Factors of Infectious Bronchitis Virus in Chicken in Northwest Ethiopia’. Scientific World Journal. 2021.
- Shiferaw J, Dego T, Tefera M, Tamiru Y. Seroprevalence of Infectious Bronchitis Virus in Broiler and Layer Farms of Central Ethiopia. BioMed Research International. 2022.
- Tesfaye T, Kassa S, Mesfin S. Four serotypes of infectious bronchitis virus are widespread in unvaccinated backyard chicken and commercial farms in Ethiopia. World Journal of Veterinary Science. 2019; 1: 1001.
- Yonas T, Wabi B, Tsedale T, Asamnew T, Naol M, Fanos T. Serological detection of antibodies against gamma coronavirus infection in scavenging village chickens in Ada’a District, Ethiopia. Biomedical Journal of Scientific & Technical Research. 2021 33: 26106–26110.
- Mungadi HU, Mera U, Adamu Y, Musa U, Achi C. Seroprevalence of Infectious Bronchitis Virus antibodies in local chickens in live bird markets in Sokoto State, Nigeria. Scientific Journal of Animal Science. 2015; 4: 53–56.
- Duguma R, Yami A, Dana N, Hassen HH, Esatu W. Marek’s disease in local chicken strains of Ethiopia reared under confined management regime in central Ethiopia. Revue deMedecine Veterinaire. 2005; 156: 541-546.
- Oyejide A, Demangam VL, Akinyemi JO. Serological survey of antibodies to Infectious bronchitis in commercial and indigenous Nigerian chickens using ELISA. Bull. Anim Health Prod Afr. 1988; 3: 259-262.
- Roussan DA, Khawaldeh GY, Shaheen IA. Infectious bronchitis virus in Jordanian chickens: Seroprevalence and detection. Can Vet J. 2009; 50: 77-80.
- Shettima YM, El-Yuguda AD, Zanna MY. Serological evidence of infectious bronchitis virus among some poultry species in Maiduguri, Nigeria. Alexandria Journal of Veterinary Sciences. 2016; 51: 135-139.
- Ayim M, Akonor DD, Owusu Ntumy HE. Serological and molecular surveillance of infectious bronchitis virus infection in free-range chickens and Guinea fowls in the Ga-east district of Ghana. Journal of Veterinary Medicine. 2018; 2018: 4949580.
- Javed T, Siddique M, Hameed A. Persistence and morpho pathological studies on infectious bronchitis virus in chickens in Pakistan. Assiut Veterinary Medical Journal. 1991; 25: 216–228.
- Shane SM. As a Handbook on Poultry Diseases. 2005.
- Rana C, Bhattarai B, Rana M, Khil B, Panth Y. Avian infectious bronchitis and its management in Nepal: a review. Journal of Agriculture and Natural Resources. 2021; 4: 211–226.
- Welchman DDB, Bradbury JM, Cavanagh D, Aebischer NJ. Infectious agents associated with respiratory disease in pheasants. Veterinary Record. 2002; 150: 658-664.
- Dhama K, Sawant PM, Kumar D, Kumar R. Diagnostic applications of molecular tools and techniques for important viral diseases of poultry. Poultry World. 2011; 6: 32-40.