
Review Article
Austin J Vet Sci & Anim Husb. 2024; 11(3): 1145.
Seroprevalence of Small Ruminant Brucellosis in Ethiopia: Systematic Review and Meta-Analysis
Desalegn Zemene¹*; Tsegaw Fentie²; Tsegaye Aseredie³; Adem Beyan¹
¹Livestock Resources Development Office, Lay Armachiho Woreda, Ethiopia
²Department of Veterinary Epidemiology and Public Health, College of Veterinary Medicine and Animal Sciences, Gondar, Ethiopia
³Agricultural Research Institutes, Gondar & Livestock Resources Development Office Lay Armachiho, Ethiopia
*Corresponding author: Desalegn Zemene Livestock Resources Development Office, Lay Armachiho Woreda, Ethiopia. Email: desalegnzemene2008@gmail.com
Received: March 13, 2024 Accepted: May 01, 2024 Published: May 08, 2024
Abstract
The World Health Organization (WHO) has classified brucellosis as a neglected zoonotic bacterial disease and determined that it has the largest public health burden among all community segments. The purpose of this research is to perform a Meta analysis and systematic review on seroprevalence of small ruminant brucellosis in Ethiopia.The data searching journal like PubMed, Science Direct, Scopus, Embase and Google Scholar was used to search the articles. All articles are screened, which was reported seroprevalence of small ruminant brucellosis in Ethiopia to be included in the study. This systematic review and meta-analysis included 19 papers. Meta-analysis using random-effects models were made to calculate the pooled seroprevalence of brucellosis. The study determined that the estimated pooled seroprevalence of small ruminant brucellosis was 3.0% (95% CI: 0.02, 0.03). According to the subgroup analysis, a statistically significant difference was found between the disease and the study region, publication year, laboratory technique used and studies years. Subgroup analysis by study regions the highest prevalence was observed in the Oromia region, with a prevalence of 3%, whereas the lowest prevalence was reported in the Amhara region, with a prevalence of 1%. Additionally, there was some indication of publication bias in papers reporting the prevalence of small ruminant brucellosis in Ethiopia (Egger’s test, p = 0.001). This analysis demonstrates the high seroprevalence of small ruminant brucellosis in Ethiopia and the necessity of suitable intervention strategies, such as increased public awareness creations and vaccination campaigns, as well as ongoing surveillance to manage and prevent brucellosis in cattle husbandry methods.
Keywords: Brucellosis; Ethiopia; Meta-analysis; Seroprevalence; Small ruminant
Introduction
The World Health Organization (WHO) has classified brucellosis as a neglected zoonotic bacterial disease and determined that it has the largest public health burden among all community segments [18]. This is due to lack of effective control and proper disease surveillance [33,21]. Animal and public health issues related to brucellosis persist in many poor nations, such as Ethiopia, where the disease is still endemic [6].
The currently recognized species includes Brucella abortus, B. Melitensis, B. Suis, B. Ovis, B. Canis, B. Ceti, B. Pinnipedialis, B. Neotomae, B. Microti and B. Inopinata [27]. The two major Brucella species known to infect sheep and goats are B. melitensis and B. ovis; however, B. abortus has also been observed to periodically increase in sheep and goat populations [29,5]. In human, Brucellosis is always caused by B. melitensis (cause Undulant or Malta fever) followed by B. suis, B. abortus and B. canis [15].
The disease is transmitted to humans primarily from eating raw or undercooked animal products or through direct contact with infected animals. It causes a systemic infection with clinical manifestations such as fever, sweats, fatigue and joint pain [22]. The prevalence of brucellosis is affected by several risk factors such as production system, host and environmental factors [29]. In sheep and goats that are sexually mature, brucellosis is limited to the reproductive tract and typically causes placentitis and abortion in pregnant. Brucella melitensis and B. abortus are zoonotic pathogens that cause disease in humans [28,29].
Brucellosis causes significant financial losses, such as a trade barrier for animals and animal products and restricts to free animal movements [37]. In addition, it causes in losses from fetus abortion, breeding failure (culling), and decreased milk production in the affected animal population.
The disease is often prevalent in traditional pastoral communities both in animals and humans but, due to lack of awareness the disease is not diagnosed and treated [2].
Generally, poor hygiene, prevalence of the disease in animals that expose humans from infected animals or their products influence the occurrence of the disease in humans. Cattle producers, veterinarians, animal health professionals, workers in abattoirs, laboratory personnel, and members of the general public who consume animal products are among the occupational categories most at risk of infection [3]. The conventional way of living, prevailing attitudes and inadequate understanding of the illness provide conducive circumstances for the dissemination and exchange of Brucellosis. The dearth of readily available alternatives and straightforward, reasonably priced remedies makes it challenging to control the hazards connected to these behaviors. Cost-effectiveness in brucellosis control is likely to exist. To illustrate the advantages of intervention, accurate quantitative data on brucellosis in humans and livestock is crucial [35].
The prevention and control of brucellosis in small ruminants will contribute to reduce human brucellosis incidence, especially in the endemic regions of Ethiopia. Therefore, adequate knowledge of the epidemiology of Brucellosis is of great public health and economic importance, particularly amongst cattle owners, animal and animal product consumers, as this will greatly assist in controlling its infections. This study aimed to determine the pooled seroprevalence of small ruminant brucellosis by a systematic review and meta-analysis in Ethiopia.
Methods
The systematic review and Meta-analysis were performed according to the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) flow chart guideline [24]. The STROBE checklist was used to ensure the inclusion of relevant information from the selected articles in the analysis. The outcome of interest was the proportion for small ruminant brucellosis.
Literature Search Strategy and Eligibility Criteria
The purpose of this meta-analysis and comprehensive review was to calculate the weighted seroprevalence of small ruminant brucellosis. Literature was searched in Pub Med, Science Direct, Scopus, African Journal Online and Google Scholar databases until July 18, 2022 to September 27, 2022. During an online article search, a Boolean operator and/or was utilized by combination of keywords related to the issue.
The following were the main search terms: "brucellosis" or "Brucella"; "seroprevalence" or "prevalence" or "Seroepidemiology"; "risk factors" or "potential factors"; and "sheep and goat" or "ovine and Caprine" or “Ethiopia" AND "Small Ruminants".
We find studies with any of the keywords in their titles, abstracts, and complete texts using the Boolean operators "OR" and "AND." Moreover, unpublished thesis manuscripts were also accessed from University of Gondar library and College of Veterinary Medicine and Animal Sciences.
Inclusion and Exclusion Criteria
To ensure that the papers found during the search were eligible, we employed the subsequent inclusion criteria: 1) unique, peer-reviewed research papers and theses from Ethiopia; 2) cross-sectional studies that provided the seroprevalence of brucellosis in small ruminants; 3) articles with full-text studies; 4) research that included small ruminants in any type of management system as part of the targeted study population (intense or extensive). In this case, extensively managed small ruminants are kept on the grazing pasture and obtain their feed by grazing without supplementation; intensively managed small ruminants are those that are kept indoors the entire day or leave the house for leisure only a few hours each day; Studies reported the overall sample size and the outcome of interest (number of positive samples); 5) studies were conducted utilizing serological diagnostic tests such as, RBPT for screening and CFT or ELISA for confirmation; 6) studies provided the total sample size and the outcome of interest (number of positive samples); 7) studies published only in English language; and 8) studies published online between 2011 up to 2022. Papers which did not meet the above-mentioned criteria were excluded. Besides, the references of the selected papers will be check manually to find relevant papers that were not retrieved in the database search [36].
Study selection and Data Extraction Procedure
Records identified from various electronic databases, indexing services and directories would be exported to Endnote software version X7. We found, noted, and deleted duplicate records. Two independent researchers were extract full text data and evaluate the eligibility of them for final inclusion. In each case, the rest authors play a critical role in solving discrepancies arose between two authors to come up to consensus.
Similarly, data extraction format was prepared based on first author, publication year, study year, geographical location (region), study design, sampling method, sample size, diagnostic test, setting and number of positive samples among the study groups. Seroprevalence of small ruminant brucellosis would calculate by dividing the number of positive cases by the total number of individuals used for the study in a given population at a given period. The study effect size and their corresponding confidence intervals would be calculated from the extracted data. Microsoft Excel datasheet was used to code and manage all extracted information from all relevant studies.
Study Quality Assessment
Two independent researchers were evaluated the quality of the included papers using a quality assessment checklist (standard strengthening the Reporting of Observational Studies in Epidemiology checklist (STROBE) which includes 22 items to make up the quality assessment checklist, which covers the title, abstract, introduction, methodology, results, and discussion of the articles.
The checklist comprised items evaluating the objectives, includes various material and methods (e.g., sample size, study population, bias, statistical methods), outcomes and constraints of the research.
The checklist included items assessing objectives, different components of the methods (eg, study design, sample size, study population, bias, statistical methods), results, limitations, and funding of the studies. The article quality scores were ranged from 0 to 44. Following the checklist (STROBE), searched papers were classified into 3 groups: low quality score (<15.50), moderate quality score (15.50-29.50) and high-quality score (30.0-44.0) [17].
Meta-Analysis
Data on the seroprevalence and corresponding 95% Confidence Intervals (CIs) of the disease were calculated for each study. The pooled prevalence estimates would be compute using the formula given by [9]. Forest plot diagram was employed to present the heterogeneity among studies, outcomes of meta-analysis that display estimates of the seroprevalence, and their corresponding CIs of all included studies together with the pooled effect size. Similarly, subgroup analyses for the primary outcome (seroprevalence of brucellosis) would be done by study region, publication year, laboratory technique employed (CFT or ELISA) and sample size category.
Cochran’s Q-statistics and inverse variance index (I2) would be computed to determine the heterogeneity and inconsistency (true variation) among studies, respectively. Similarly, we considered the I2 values of 25%, 50% and 75% as low, medium and high heterogeneity respectively [19]. The tau statistics (t2) was used to assess the variance of the effect size estimates across the population of the study.
Based on the heterogeneity assessment result, we used DerSimonian and Laird’s random-effects method (if the p-value of the Q test is 5%) or Mantel-Haenszel’s fixed-effects method to pool the estimations [39]. Small study effects and publication bias presence were then visualized using funnel plot diagrams and, Egger’s and Begg’s asymmetry tests [11]. A funnel plot was computed using effect size and its corresponding standard error of the effect size. STATA software version 17 is used to do the meta-analysis.
Results
Descriptive Literature Search Results
A total of 187 potentially relevant studies were identified from several sources including PubMed, Science Direct, Scopus and Google scholar. From these, 27 duplicated articles were removed with the help of Endnote 7. The remaining 160 records were screened using their titles and abstracts and 134 of them were excluded. Full texts of 26 records were then evaluated for eligibility. From these, 7 articles were excluded due to the outcome of interest was found missing, insufficient and/or ambiguous.
A total of 19 articles were eligible for the final systematic review and Meta-analysis from all screened studies. All of the eligible studies have been used RPBT and ELISA or CFT for antibody detection. These selected eligible articles were conducted namely; Oromia, Tigray, Amhara, Somali and Oromia and Somali. From 19 published articles a total of 10,067 samples of small ruminant (both sheep and goats) were subjected to disease detection. The sample size of shoat ranges from 226 to 985 in each study area of Ethiopia. The seroprevalence of the disease in the 19 articles was ranges from 1.40% to 9.1%. The mean sample size from overall report was 528.94. Finally, a total of 19 articles fulfilled the eligibility criteria and quality assessment and thus included for systematic review and meta-analysis.
Descriptive Study Characteristics
The final 19 eligible studies which were considered for determining the seroprevalence of brucellosis in small ruminants are summarized for systematic review and Meta-analysis. The studies were published in the year between 2011 and 2022. All the selected studies were cross-sectional study design in nature.
Meta-Analysis
Pooled prevalence estimate: Due to the expected variation between studies, random-effects meta-analyses were employed using the total sample size and number of positives (effect size and standard error of the effect size). An overall pooled prevalence of the disease was estimated to be 3% (0.02 to 0.03 of 95% CI).
Summary of Meta-analysis: Random-effects meta-analyses were employed using the prevalence and standard error of prevalence for effect size and standard error of the effect size and using author and publication year for the study label of the Meta-analysis.
Forest Plot
Due to the expected variation between studies, random effects meta-analyses were carried out using the prevalence and standard error of prevalence (effect size and standard error of the effect size). (2 = 0.00; I2 = 85.65%, DF = 18, H2 =6.96, Q - test = 82.72 and P - value 0.00). Individual study prevalence estimates ranged from 1.40% to 9.1% with the overall random pooled prevalence of 3% (95% CI: 0.02, 0.03). Studies weighted approximately equal with weights on individual studies ranging from 2.94% to 6.19% due to high heterogeneity between studies.Due to the expected variation between studies, random effects meta-analyses were carried out using the prevalence and standard error of prevalence (effect size and standard error of the effect size). (2 = 0.00; I2 = 85.65%, DF = 18, H2 =6.96, Q - test = 82.72 and P - value 0.00). Individual study prevalence estimates ranged from 1.40% to 9.1% with the overall random pooled prevalence of 3% (95% CI: 0.02, 0.03). Studies weighted approximately equal with weights on individual studies ranging from 2.94% to 6.19% due to high heterogeneity between studies.
Subgroup Meta-Analysis
Subgroup Analysis by study Regions: Subgroup analyses were done for study Regions (Oromia, Somali Oromia and Somali, Amhara and Tigray regions of Ethiopia). Thus, high seroprevalence was observed in Oromia region 3% (95% CI: 0.02 - 0.05), whereas the same prevalence was observed in both Somali and Tigray region 2% (95% CI: 0.01– 0.02) and the lowest prevalence was observed in Amhara region 1% (95% CI: 0.00– 0.01).
Subgroup Analysis by publication year category: Subgroup analyses were done by articles publication year category. Thus, the same seroprevalence was observed the publication year category from 2011– 2014 and 2015 – 2018 with the prevalence 3 % (95% CI: 0.00 - 0.06 and 0.01 - 0.04) and the publication year category from 2019 - 2022 with prevalence of 2% (95% CI: 0.01 - 0.03) respectively.
Subgroup Analysis by Laboratory techniques: Subgroup analyses were done for laboratory techniques (RBPT, CFT and ELISA). Thus, high seroprevalence was observed in ELISA6% (95% CI: 0.03 - 0.09) followed by CFT and RBPT with both the same seroprevalence of 2% (95% CI: 0.00 - 0.02 and 0.00 – 0.03) respectively.
Subgroup analysis by sample size: Subgroup analyses were done for sample size which has been categorized into three parts like <300, 300 - 600 and >600. Thus, high seroprevalence was observed in both sample size category of <300 and 300 - 600 with the seroprevalence of 3% (95% CI: 0.00 - 0.06 and 0.01 - 0.05), whereas the least prevalence was observed in sample size category of >600 with 2% (95% CI: 0.01– 0.03) respectively.
Publication Bias
Funnel plot for visualizing publication bias: We assessed publication bias and small study effects by funnel plot observation and Egger’s test for small study effects. The funnel plot that visually observed there were asymmetry in which the result of effect estimates against its standard error showed that there was some evidence of publication bias and small study effect on studies reporting the seroprevalence of brucellosis in small ruminant in Ethiopia.
Egger test detecting publication bias: From Egger’s test statistics result there was publication bias and small study effect since the estimated bias coefficient 4.729 with standard error 0.666 and p - value 0.001.
Meta-Regression
Meta-regression analysis was done for each variable included in the study separately. The variables coded as categorical variables and those variables included were study regions, publication year, study year, laboratory techniques and sample size were employed. Those variables with p-values <0.05 were used in the multivariable Meta regression analysis. Only laboratory techniques had significant value and retained in the final multivariable Meta regression analysis.
Discussion
Brucellosis induces considerable human suffering and huge economic losses in animals [8,34]. It has a significant public health implication for a pastoral community in consequence of lifestyles, feeding habits, close contact with animals, low awareness, and poor hygienic conditions which favors infection [7]. Also, it can generally cause significant loss of productivity through abortion, prolonged calving, kidding, or lambing interval, low herd fertility, and comparatively low milk production in farm animals.
Item No
Recommendation
1
(a) Indicate the study’s design with a commonly used term in the title or the abstract
(b) Provide in the abstract an informative and balanced summary of what was done and what was found
2
Explain the scientific background and rationale for the investigation being reported
Objectives
3
State specific objectives, including any pre specified hypotheses
Study design
4
Present key elements of study design early in the paper
Setting
5
Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection
Participants
6
(a) Give the eligibility criteria, and the sources and methods of selection of participants
Variables
7
Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable
For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group
Bias
9
Describe any efforts to address potential sources of bias
Study size
10
Explain how the study size was arrived at
11
Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why
12
(a) Describe all statistical methods, including those used to control for confounding
(b) Describe any methods used to examine subgroups and interactions
(c) Explain how missing data were addressed
(d) If applicable, describe analytical methods taking account of sampling strategy
(e) Describe any sensitivity analyses
(a) Report numbers of individuals at each stage of study eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed
(b) Give reasons for non-participation at each stage
(a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders
(b) Indicate number of participants with missing data for each variable of interest
Outcome data
Report numbers of outcome events or summary measures
Main results
16
(a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included
(b) Report category boundaries when continuous variables were categorized
(c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period
17
Report other analyses done eg analyses of subgroups and interactions, and sensitivity analyses
Key results
18
Summarize key results with reference to study objectives
Limitations
19
Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias
Interpretation
20
Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence
Generalizability
21
Discuss the generalizability (external validity) of the study results
Funding
22
Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based
Table 1: STROBE Checklist for quality assessment of included studies STROBE Statement Checklist of items that should be included in reports of cross-sectional studies.
Author
Publication Year
Study area
Laboratory Techniques
Total Sample
Diseased
Prevalence
Quality Score
[35]
2018
Oromia
CFT
424
11
0.026
34
[16]
2012
Oromia
CFT
384
35
0.091
38
[41]
2018
Oromia
ELISA
283
23
0.081
37
[25]
2021
Oromia
CFT
470
14
0.030
32
[1]
2015
Oromia
ELISA
840
39
0.046
36
[20]
2014
Oromia
RBPT
384
6
0.016
28.5
[26]
2017
Tigray
CFT
558
10
0.018
29
[39]
2014
Oromia and Somali
CFT
420
15
0.036
30.5
[12]
2013
Oromia
CFT
384
9
0.023
31
[27]
2019
Oromia
CFT
762
11
0.014
27.5
[10]
2011
Somali
CFT
730
11
0.015
28
[22]
2017
Somali
CFT
291
4
0.014
27.5
[36]
2015
Amhara
CFT
714
5
0.007
27
[4]
2021
Somali
CFT
226
4
0.018
30
[32]
2013
Tigray
CFT
985
15
0.015
29
[31]
2015
Somali
CFT
285
6
0.021
30
[38]
2015
Oromia
CFT
853
15
0.018
29.5
[42]
2022
Oromia
CFT
384
6
0.016
31
[14]
2022
Oromia
CFT
690
23
0.033
35
Table 2: Characteristics of selected studies describing the seroprevalence of small ruminant brucellosis in Ethiopia.
Author with Publication year
Effect size
(95% conf. interval)
% Weight
[35]
0.026
0.011 - 0.041
5.17
[16]
0.091
0.062 – 0.120
3.27
[41]
0.081
0.049 - 0.113
2.94
[25]
0.030
0.014 - 0.045
5.13
[1]
0.046
0.032 – 0.061
5.30
[20]
0.016
0.003 - 0.028
5.57
[26]
0.018
0.007 - 0.029
5.77
[39]
0.036
0.018 - 0.053
4.77
[12]
0.023
0.008 - 0.039
5.17
[27]
0.014
0.006 - 0.023
6.09
[10]
0.015
0.006 - 0.024
6.05
[22]
0.014
0.000- 0.027
5.43
[36]
0.007
0.001 - 0.013
6.34
[4]
0.018
0.001 - 0.035
4.85
[32]
0.015
0.008 - 0.023
6.19
[31]
0.021
0.004 - 0.038
4.93
[38]
0.018
0.009 - 0.026
6.05
[42]
0.016
0.003 – 0.028
5.57
[14]
0.033
0.020 – 0.047
5.43
Theta
0.025
0.018- 0.032
Table 3: Summary of selected studies with its Author and Publication year.
Std.Eff
Coefficient
Std. err
p-value
95% conf. interval
Slope
-0.0072
0. 004
0.072
-0.015 - 0.0006
Bias
4.729
0.666
0.000
3.423 - 6.035
Table 4: Egger test that assesses publication bias.
Variables
Coefficient
Std. errs.
P- value
95%Conf. interval
Laboratory techniques
Ref.
2
0.041
0.016
0.009
0.010 - 0 .072
3
0.006
0.012
0.635
-0.018 - 0.030
Table 5: Summary of final multivariable Meta-regression analysis.
The disease impairs socio-economic development for livestock owners, which represents a vulnerable sector in rural populations in general and pastoral communities in particular. Even though, most reports have made either limited geographic coverage or are relatively confined to a single agro ecology, these stated evidences strongly suggest that brucellosis might be a widespread problem in Ethiopia [33].
But the seroprevalence of the disease is affected by different factors like, environmental factors, the number of samples, type of strains, stage of infection and type of diagnostic techniques used. The approaches of Meta-analysis allow identifying the role of such factors, by combining results of different reports, with different designs, agro ecology and locations. Good meta-analysis outputs are relevant for the management and control of an infectious disease like Brucellosis that could not be identified by individual studies alone [13]. This is the first quantitative meta- analysis on the sero-prevalence of small ruminant brucellosis in Ethiopia to the best of our knowledge for evidence-based decision.
Figure 1: PRISMA guide line flow chart format describing the article selection procedure.
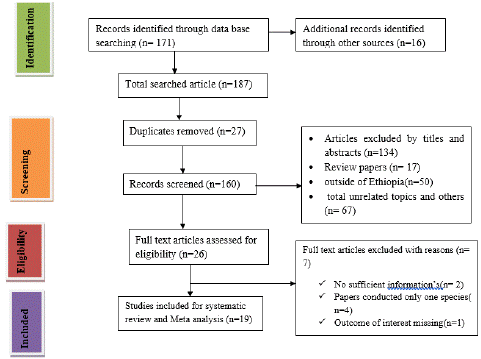
Figure 2: PRISMA guide line flow chart describing the article selection process.
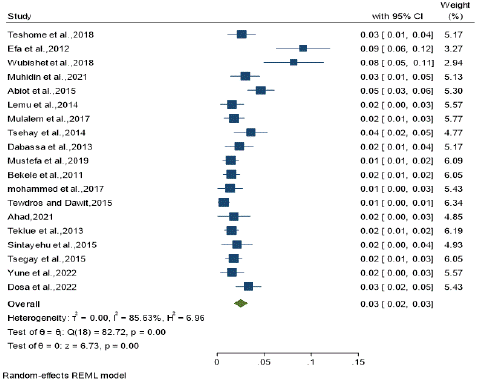
Figure 3: Forest Plot depicting the seroprevalence of small ruminant brucellosis in Ethiopia.
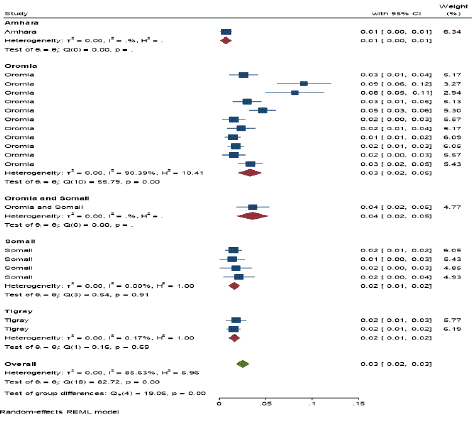
Figure 4: Subgroup analysis by study regions.
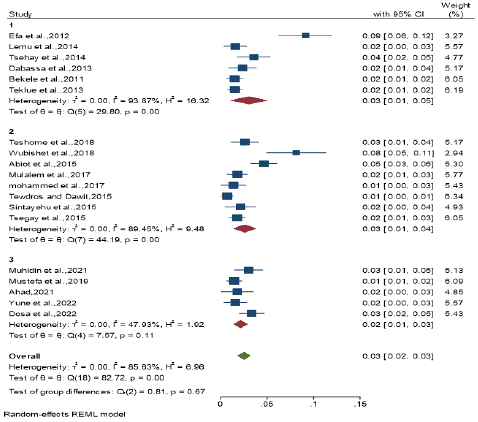
Figure 5: Subgroup analysis by publication year category
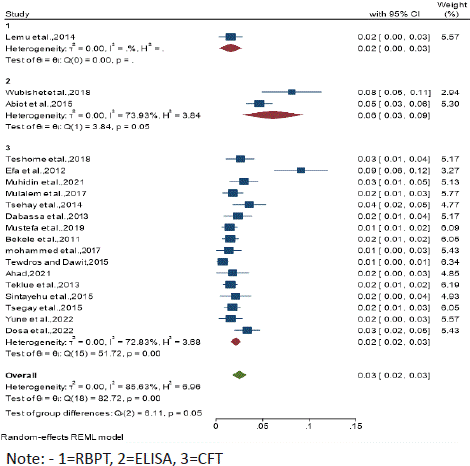
Figure 6: Subgroup analysis by Laboratory techniques.
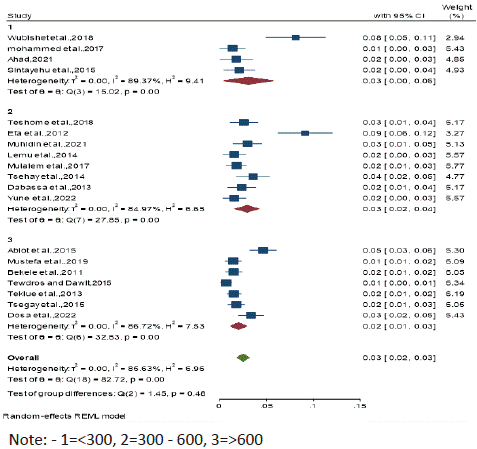
Figure 7: Subgroup analysis by Sample size.
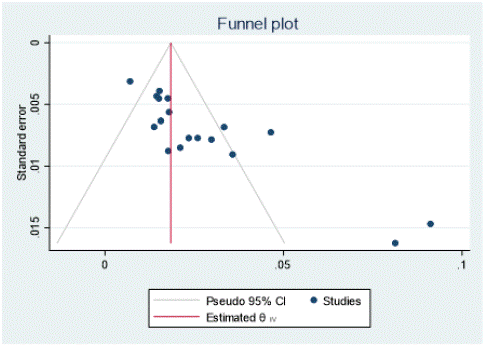
Figure 8: Funnel plot that assesses publication bias.
We have used 19 cross sectional studies with 10067 serum samples that have been undertaken between years from 2011 to 2022 in Ethiopia were included in this study; the pooled seroprevalence of small ruminant brucellosis was 3.0%. This result is higher than the meta-analysis report of [30] from sheep and goat in China where the pooled prevalence was 2%. Similarly, the current finding was higher than the reports of [38] who reported prevalence of 1.80% from small ruminant selected in different area in Oromia region in Ethiopia. The current finding is in line with the report of [14,25] from small ruminant brucellosis in Oromia region in Ethiopia where the pooled prevalence was 2.97 and 3.30% respectively.
Meanwhile, the current finding was lower than the reports of [1,16,41] from small ruminant brucellosis in different districts of Oromia region in Ethiopia where the pooled seroprevalence was 4.64%.8.13% and 9.1% respectively. The difference in the seroprevalence of small ruminant brucellosis in the different studies could be due to differences in the geographical location and animal husbandry practice between the different study areas. Therefore, information on the actual seroprevalence of the small ruminant brucellosis in the country helps the policymakers to develop appropriate strategies regarding prevention and control protocols. In the present study, the subgroup analysis showed that there was a statistically significant association between the disease and study regions, publication year, laboratory technique employed and study years. Also, there was evidence of publication bias and small study effects (Egger’s test, p = 0.001) on studies reporting the seroprevalence of small ruminant brucellosis in Ethiopia.
Limitations
The potential limitation of this systematic review and meta-analysis was the heterogeneity. The heterogeneity of the included studies could be due to various factors such as differences in study design, diagnostic criteria, and study populations, which can affect the generalizability of the findings. All studies included in this systematic review and meta-analysis had a cross-sectional design. The cross-sectional design of these studies may limit their ability to establish causal relationships or capture seasonal variations in the incidence of small ruminant brucellosis. Another potential limitation is the language restriction of the English-language articles, which may have excluded relevant studies published in other languages.
Conclusion and Recommendations
We conduct a systematic review and meta-analysis to assess the seroprevalence of brucellosis in small ruminant in Ethiopia. The seroprevalence of brucellosis in small ruminant is different in different parts of Ethiopia. There is a limited knowledge and studies about the systematic review and Meta-analysis in many regions of the country and the findings are heterogeneous. The result of this meta-analysis shows that the pooled prevalence estimate of the disease in the country is 3.0%. Therefore, the pooled seroprevalence of small ruminant brucellosis is used for evidence-based disease control in Ethiopia.
Based on the above conclusions the following recommendations are forwarded;
• The overall data demands intervention measures, including vaccination and enhanced public awareness, and further surveillance for the control and prevention of brucellosis in livestock husbandry practices.
• Further studies were needed to understand the epidemiology of brucellosis in Ethiopia, including the risk factors, transmission dynamics, and genetic diversity of the causative agent. This should provide valuable information for the development of effective prevention and control strategies.
• To consider the significance of small ruminant brucellosis in the national economy, strategies to reduce the prevalence and burden of brucellosis the government and other stakeholders prioritized and offered adequate funding to carry out necessary activities, such as screening, diagnosis, treatment, and surveillance.
Author Statements
Data Sharing Statement
All data generated during this study are included.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interest.
References
- Abiot D, Tesfaye S, Getachew T. Seroprevalence and risk factors of small ruminant brucellosis in selected districts of Arsi and East Shoa zones, Oromia region, Ethiopia. African Journal of Microbiology Research. 2015; 9: 1338-1344.
- Addis M. Small ruminant brucellosis: serological survey in Yabello District, Ethiopia. Asian Journal of Animal Sciences. 2013; 7: 14-21.
- Adesokan HK, Alabi PI, Cadmus SI, Stack JA. Knowledge and practices related to bovine brucellosis transmission amongst livestock workers in Yewa, south-western Nigeria. Journal of the South African Veterinary Association. 2013; 84: 1-5.
- Ahad A. Seroprevalence and public health perception of small ruminant brucellosis in South eastern Somali Region, Ethiopia. 2021.
- Akhvlediani T, Clark DV, Chubabria G, Zenaishvili O, Hepburn MJ. The changing pattern of human brucellosis: clinical manifestations, epidemiology, and treatment outcomes over three decades in Georgia. BMC infectious diseases. 2010; 10: 346.
- Asmare K, Megersa B, Denbarga Y, Abebe G, Taye A, Bekele J, et al. A study on seroprevalence of caprine brucellosis under three livestock production systems in southern and central Ethiopia. Tropical animal health and production. 2013a; 45: 555-560.
- Asmare K, Sibhat B, Molla W, Ayelet G, Shiferaw J, Martin A, et al. The status of bovine brucellosis in Ethiopia with special emphasis on exotic and cross bred cattle in dairy and breeding farms. Acta tropica. 2013b; 126: 186-192.
- Lopes LB, Nicolino R, Pa Haddad J. Brucellosis-risk factors and prevalence: a review. The Open Veterinary Science Journal. 2010; 4: 72-84.
- Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013; 67: 974-978.
- Bekele M, Mohammed H, Tolosa T. Small ruminant brucellosis and community perception in Jijiga district, Somali Reginal State, Eastern Ethiopia. Tropical Animal Health and Production. 2011; 43: 983-898.
- Borenstein M. Effect sizes for continuous data. The handbook of research synthesis and meta-analysis. 2009; 2: 221-235.
- Dabassa G, Tefera M. Addis M. Small ruminant brucelosis: Serological survey in Ybello District, Ethiopia. Asian J Anim Sci. 2013; 7: 14-21.
- Dohoo I, Martin S, Stryhn H. Veterinary epidemiologic research. VER. Inc., Charlottetown, PE, Canada. 2009.
- Dosa D, Mohammed N, Mathewos M. Study on small ruminant brucellosis and owners awareness in two selected districts of southern region, Ethiopia. Veterinary Medicine and Science. 2023; 9: 907-916.
- Dungan R. Board-invited review: Fate and transport of bioaerosols associated with livestock operations and manures. Journal of Animal Science. 2010; 88: 3693-3706.
- Efa N, Shihun S, Desta B. Seroprevalence of small ruminant brucellosis and its public health awareness in selected sites of Dire Dawa region, Eastern Ethiopia. Journal of Veterinary Medicine and Animal Health. 2012; 4: 61-66.
- Erik Von Elm M, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007; 147: 573e7.
- Hegazy YM, Moawad A, Osman S, Ridler A, Guitian J. Ruminant brucellosis in the Kafr El Sheikh Governorate of the Nile Delta, Egypt: prevalence of a neglected zoonosis. PLoS neglected tropical diseases. 2011; 5: e944.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta- analysis. Statistics in medicine. 2002; 21: 1539-1558.
- Lemu A, Mamo D, Madera P. A study on seroprevalence of brucellosis in goats and sheep in East Shewa, Ethiopia. Ethiop. Int J Multidis Res. 2014; 1: 14-18.
- Mcdermott J, Grace D, Zinsstag J. Economics of brucellosis impact and control in low-income countries. Revue scientifique et technique (International Office of Epizootics). 2013; 32: 249-261.
- Mohammed M, Mindaye S, Hailemariam Z, Tamerat N, Muktar Y. Sero-Prevalence of Small Ruminant Brucellosis in Three Selected Districts of Somali Region, Eastern Ethiopia. J Vet Sci Anim Hus. 2017; 5: 105.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery (London, England). 2010; 8: 336-341.
- Muhidin M, Degafu H, Abdurahaman M. Seroprevalence of brucellosis in small ruminants, its risk factors, knowledge, attitude and practice of owners in Berbere district of Bale Zone southeast Ethiopia. Ethiopian Journal of Applied Science and Technology. 2021; 12: 10-23.
- Mulalem ZK, Getachew G, Yohannes H, Habtamu T. Sero-prevalence and associated risk factors for Brucella sero-positivity among small ruminants in Tselemti districts, Northern Ethiopia. Journal of Veterinary Medicine and Animal Health. 2017; 9: 320-326.
- Mustefa M, Andargie B, Zerihun T. Sero-Prevalence and Associated Risk Factors Of Brucellosis In Small Ruminants Slaughtered At Selected Abattoirs In Eastern Ethiopia. Haramaya University. 2019.
- Pappas G, Panagopoulou P, Christou L, Akritidis N. Biological weapons. Cellular and molecular life sciences CMLS. 2006; 63: 2229-2236.
- Radostits O, Gay C, Blood D, Hinchliff K, Constable P. Vet. Med: A Text Book of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. WB Saunders Co. Ltd., London. 2007.
- Ran X, Chen X, Wang M, Cheng J, Ni H, Zhang XX, et al. Brucellosis seroprevalence in ovine and caprine flocks in China during 2000–2018: A systematic review and meta-analysis. BMC veterinary research. 2018; 14: 393.
- Sintayehu G, Tamerat N, Hussein D. Epidemiological survey of brucellosis in sheep and goat in selected pastoral and agro-pastoral lowlands of Ethiopia. Revue Scientifique et Tecnoique de I’OIE. 2015; 34: 881-893.
- Teklue T, Tolosa T, Hailu B. Sero-prevalence and risk factors study of brucellosis in small ruminant in Southern Zone of Tigray Region, Northern Ethiopia. Tropical Animal Health and Production. 2013; 45: 1809-1815.
- Terefe Y, Girma S, Mekonnen N, Asrade B. Brucellosis and associated risk factors in dairy cattle of eastern Ethiopia. Tropical Animal Health and Production. 2017; 49: 599-606.
- Tesfaye A, Dejene H, Admassu B, Kassegn TA, Asfaw D, Dagnaw GG, et al. Seroprevalence of bovine brucellosis in Ethiopia: systematic review and meta-analysis. Veterinary Medicine: Research and Reports. 2021; 12: 1-6.
- Teshome A, Geremew H, Lijalem N. Asero-Prevalence of Small Ruminant Brucellosis in Selected Settlements of Dire Dawa Administrative Council Area, Eastern Ethiopia. ARC J Immunol Vaccin. 2018; 3: 7-14.
- Tewodros A, Dawit A. Sero-Prevalence of Small Ruminant Brucellosis in and around Kombolcha, Amhara Regional State, North-Eastern Ethiopia. J Vet Sci Med Diagn. 2015; 4.
- Thrusfield M. Comparative epidemiology. Veterinary Epidemiology, 3rd edition. London: Blackwell Science. 2008; 231-232.
- Tsegay A, Tuli G, Kassa T, Kebede N. Seroprevalence and risk factors of Brucellosis in small ruminants slaughtered at Debre Ziet and Modjo export abattoirs, Ethiopia. The Journal of Infection in Developing Countries. 2015; 9: 373-380.
- Tsehay M, Getachew G, Morka A, Tadesse B, Eyob H. Seroprevalence of brucellosis in small ruminants in pastoral areas of Oromia and Somali regional states, Ethiopia. J Vet Medi and Anim Health. 2014; 6: 289-294.
- Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Systematic reviews of effectiveness. Joanna Briggs Institute reviewer’s manual. The Joanna Briggs Institute Adelaide, Australia. 2017.
- Wubishet Z, Sadik K, Abdala B, Mokonin B, Getachew T, Getachew K. Small ruminant brucellosis and awareness of pastoralists community about zoonotic importance of the disease in Yabello districts of Borena Zone Oromia Regional State, Southern Ethiopia. Current Trends in Biomedical Engineering & Biosciences. 2018; 12: 5-10.
- Yune N, Ibarahim N, Worku S, Hailu T, Edao A. Seroprevalence of brucellosis in small ruminants and associated risk factors in ziway Dugda woreda, Arsi Zone southeast Ethiopia. J Vet Sci Research & reviews. 2022.