
Review Article
Austin J Vet Sci & Anim Husb. 2024; 11(5): 1154.
Growing Role of Bacillus Cereus as an Emerging Potential Food Pathogen of Humans: A Review
Dereje Adugna1; Samuel Diriba2; Megersa Diriba3; Adugna Girma4*; Habtamu Mekonen5
1Meta Wolkite District Agricultural office, West shoa, Oromia, Ethiopia
2Holeta Town Administration, Oromia, Ethiopia
3Hurumu District Agricultural Office, Illu Ababor, Oromia, Ethioipa
4Yemalogi welel District Agricultural office, Kellem Wollega, Oromia, Ethioipa
5Hurumu District Agricultural Office, Illu Ababor, Oromia, Ethioipa
*Corresponding author: Adugna Girma, DVM, MSc Yemalogi Welel District Agricultural Office, Kellem Wollega, Oromia, Ethioipa. Tel: +251 911925885 Email: abdiadugna4@gmail.com
Received: August 28, 2024 Accepted: September 19, 2024 Published: September 26, 2024
Abstract
The genus Bacillus includes gram-positive and gram variable rod-shaped bacteria that sporulate under aerobic conditions. B. cereus (Bacillus cereus) is a Gram-positive spore-forming bacterium commonly found in the environment. B. cereus causes two types of food poisoning, the emetic and diarrheal syndromes, and a variety of local and systemic infections. B. cereus is widespread in nature and frequently contaminates a wide variety of food products. The incidence of both the diarrheal and emetic syndromes caused by B. cereus probably has been underestimated because the illnesses are usually self-limiting with mild symptoms. Despite the recognition of B. cereus as a foodborne pathogen over 50 years ago, its virulence mechanisms are still not fully elucidated. Cereulide has been identified as the causative agent in the emetic syndrome, and HBL is associated with diarrheal food poisoning. Nhe, a homolog of HBL, probably possesses biological activities similar to those of HBL and could be a factor in the diarrheal syndrome; however, this hypothesis has not been tested. In addition to causing food poisoning, HBL can play a role in non-gastrointestinal infections caused by B. cereus. The in vivo roles of many of the putative and potential virulence factors produced by B. cereus, such as hemolysins, phospholipases, and proteases, have not been defined. These variables are probably involved in infections and diseases caused by B. cereus based on their biological activity. The genus and several species of B. cereus are included in this review, but the strains and toxins that cause foodborne illness are the main focus. Many putative virulence factors are produced by B. cereus, however most of these factors’ involvement in particular infections are yet unknown. Thus far, only two substances have been specifi cally identifi ed as emetic and diarrhoeal toxins, respectively: cereulide and the tripartite haemolysin BL. Another homolog of haemolysin BL that has been linked to the diarrhoeal illness is nonhemolytic enterotoxin. Apart from food poisoning, B. cereus has been linked to a number of infectious diseases in the past and present, such as periodontal diseases, ocular infections (such as endophtalmitis, panophtalmitis, and keratitis), skin infections, post-operative and post-traumatic wound infections (with or without bone involvement), necrotising fasciitis, salpingitis, meningitis, and endocarditis.
Keywords: Bacterium; Bacillus; B. ceres; Hemolysin; Pathogen
Abbrevation: ATR: Acid Tolerance Response; GIT: Gastro-Intestinal Tract; MYP: Mannitol-Egg Yolk-Phenol Red-Polymyxin-Agar; PEMBA: Polymyxin Pyruvate-Egg Yolk-Mannitol-Bromothymol Blue-Agar
Introduction
Bacillus cereus is a Gram-positive spore-forming bacterium commonly found in the environments. This bacterium is also a major contaminant of raw or processed foods of plant or animal origin [58]. B. cereus exists as a soil saprophyte that can adapt and proliferate in the lower sections of the Gastro-Intestinal Tract (GIT) [90]. It is also an opportunistic pathogen responsible for local and systemic infections [88].
Bacillus cereus causes two types of food poisoning (the emetic and diarrheal syndromes) and a variety of local and systemic infections such as endophthalmitis, endocarditis, meningitis, periodontitis, osteomyelitis, wound infections, and septicemia [24]. The pathogenesis of B. cereus is still largely undefined. The organism produces a large number of potential virulence factors, including multiple hemolysins, phospholipases, and proteases [11]. However, the roles of these factors in specific infections have not been established. The emetic toxin has been identified as cereulide [4] and the tripartite Hemolysin BL (HBL) has been established as a diarrheal enterotoxin [12]. A homolog of HBL, nonhemolytic enterotoxin (Nhe), also has been associated with the diarrheal syndrome [56]. While certain B. cereus strains have been used as probiotics [48], others may cause food poisoning in humans [25]. The pathogenicity of B. cereus is attributed to the species' production of extracellular factors such as phospholipase cereulide (emetic toxin), enterotoxin Hbl, non-haemolytic toxin (Nhe), haemolysin IV, which has a strong disruptive effect on cellular membranes, and associated with the induction of necrotic enterocolitis cytotoxin (CytK) [41].
Its name derived from the cell shape (bacillus, rod) and colony appearance (cereus, wax). The facultative anaerobic B. cereus has been isolated from almost all categories of foodstuff, as it is able to grow in very diverse habitats like soil and sediments [88]. B. cereus spores can reach concentrations of up to 103-105 cells per gram soil [99]. Moreover, B. cereus has been isolated from the insect gut of several arthropod species in high frequency and a commensal lifestyle has been proposed for this bacterium [79].
A factor that plays a role in acid resistance is the mechanism of cross-protection between the different stresses microorganisms are exposed. For example, exposure of the microorganism to the various stresses they encounter in the course of food production, e.g., heat processing, dehydration, or acidifi cation, can elicit a higher tolerance to stresses encountered passing through the stomach [66]. Indeed, a sub lethal acidic environment can trigger an adaptive response that protects the bacterium during subsequent incubations at lethal acidic pH. This mechanism is known as Acid Tolerance Response (ATR) and plays an important role in the adaptation of intestinal pathogens to the pH of the stomach [21]. B. cereus vegetative cells are also able to induce ATR [79]. The ATR of B. Cereus may involve (i) F0F1 ATPase and/or glutamate decarboxylase (implicated in pHi homeostasis), (ii) modifi cations of metabolism and (iii) synthesis of proteins which act as protect and/or repair factors [79]. The ability to generate protecting biofi lms and to form endospores, which are metabolic inactive and resistant to harsh conditions such as heat (>100°C), many chemicals, radiation as well as desiccation, allows B. cereus to survive [1].
The toxicological profi le of B. cereus strains ranges from non-pathogenic strains used as probiotics in animal feed [53]. B. cereus is mainly known to evoke two types of gastrointestinal food borne poisonings. The emetic type indicated by nausea and forceful vomiting shortly after ingestion is caused by the small dodecadepsipeptide cereulide [3], which is produced by a subgroup of B. cereus [28]. The diarrheal syndrome is caused by several heat labile enterotoxins produced during growth of B. Cereus in the intestine. The Hemolysin BL (HbL) and the pore forming non-hemolytic toxin (Nhe) belong to the class of three-component enterotoxins, whereas cytotoxic K (CytK) represents a Β-barrel channel forming one-component enterotoxin [57]. In general, 6 to 12 hours after ingestion of about 105 to 107 cells, abdominal cramps and diarrhea occur, but the course of the disease is normally relatively mild and symptoms disappear within 24 hours [88]. The extent and duration of the disease depend on the infection dose and the number of produced enterotoxins, which seem to differ strongly among different B. cereus strains [26]. Besides the known toxins, B.Cereus also produces several enzymes like sphingomyelinase, phosphatidylinositol and phosphatidylcholine-specifi c phospholipases and several proteases that are so far not directly associated with gastrointestinal diseases, but may play an important role in non-gastrointestinal infections such as wound and eye infections, bacteremia, pneumonia, meningitis, periodontitis, and endocarditis [74]. Most notably, the high hydrophobic character of the spores seems to increase their adherence to the surface of food processing machines and equipment, pipelines as well as tanks leading to contamination of food products by direct contact with these different sources [30]. Consequently, once spores have entered the food, pasteurization or normal sanitation processes will not contribute to their elimination [27].
B. cereus is one of six members of the Bacillus cereus group within the genus Bacillus. The other members of this genetically closely related group are Bacillus anthracis, B. thuringiensis, B. weihenstephanensis, B. mycoides and B. pseudomycoides [22]. Although a clear separation of these species by phenol-typing or classical DNA hybridization studies failed, these bacilli differ signifi cantly in their ecological features such as the synthesis of virulence factors, specialized morphology and cold adaption [8]. These special features are mainly encoded by genes located on mega plasmids like e.g. pXO1 and pXO2 of B. anthracis. The causative agent of the fatal mammalian disease anthrax, B. anthracis, arrested attention in 2001 for its use as bioterrorism agent and biological weapon developed by several countries [46]. The insect pathogen B. thuringiensis produces toxic crystals (d-endotoxins), which are encoded on a plasmid and lyse midgut epithelial cells [14]. B. thuringiensis is routinely used as agent to control agricultural insect pests [18].
The prevalence of B. cereus induced food-borne illnesses is difficult to determine, because the symptoms associated with B. cereus infections or intoxication are mild, so it is conceivable that many B. cereus infections are not reported and that the prevalence of these infections is largely under estimated [39]. B. cereus illness recognized that there may be signifi cant under reporting due to the generally mild, short duration and self-limiting symptoms, in addition to its being infrequently tested for in routine laboratory analyses of stool samples [41] and due to lack of effective surveillance, B. cereus associated food poisoning may be largely under reported, and probably confused with Staphylococcus aureus and Clostridium perfringens food poisoning due to similar symptoms [88].
Objectives
To review on Bacillus cereus nature, morphology source of contamination, Pathogenic virulence, mode of transmission and its public health importance.
Organisms and Growth Condition
The Organism: Characteristics and Identifications
B. cereus was originally described as a mesophilic organism, growing between 10 and 500C and with an optimum temperature of 35 and 400C (Claus & Berkeley, 1986). The Latin term cereus indicates wax-like, whereas the word bacillus denotes little rod. The name refers to B. cereus's easily identifi able shape under a microscope or on blood agar plates. B. cereus is a big rod-shaped (1.0–1.2 mm by 3.0–5.0 mm) Gram-positive bacterium that grows to enormous colonies (3–8 mm diameter) on ordinary agar medium. Its appearance is fairly fawny, greyish, and reminiscent of "ground glass," with frequently uneven borders.
On blood agar, the colonies are surrounded by zones of beta hemolysis [52], the size of which is often large, but can vary depending on culturing conditions. On widely used agar media, the majority of strains will develop endospores in a few days. B. cereus spores are ellipsoidal, positioned centrally or par centrally, and do not spread the cell [36]. Employing phase contrast microscopy or spore staining techniques, the placement and morphology of the spores are much used criteria to distinguish the species of the genus Bacillus [34].
Other commonly used features for identification are motility, haemolysis, carbohydrate fermentation (B. cereus does not ferment mannitol) and the very active lecithinase (phospholipase) production [51]. Various plating media are used for the isolation, detection and enumeration of B. cereus from foods, including MYP (mannitol-egg yolk-phenol red-polymyxin-agar) and PEMBA (polymyxin pyruvate-egg yolk-mannitol-bromothymol blue-agar) [47]. These media make use of the bacterium's lecithinase synthesis (the egg-yolk reaction that results in precipitate zones) and absence of mannitol fermentation in addition to specifi c chemicals like polymyxin.
A thorough description of these media is found in Kramer & Gilbert [52]. More recently, chromogenic media have been developed for several food pathogens, including B. cereus (for instance Cereus–Ident-Agar from heipha Dr Muller GmbH, and chromogenic B. cereus Agar from Oxoid Ltd). These new media have been evaluated together with standard plating media by Fricker et al. [32].
Colony Morphology
Colony Morphology When grown under aerobic conditions on 5% sheep blood agar at 37°C, B. cereus colonies are dull gray and opaque with a rough matted surface (Figure 1). Colony perimeters are irregular and represent the configuration of swarming from the site of initial inoculation, perhaps due to B. cereus swarming motility [35]. Zones of beta-hemolysis surround and conform to the colony morphology [95].
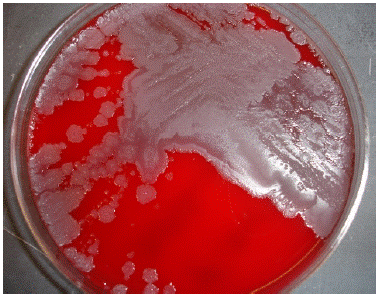
Figure 1: Gray, opaque, granular, spreading colonies with irregular perimeters growing on 5% sheep blood agar. Note the smaller smooth colonies admixed among spreading growth Wong et al., [12].
Reservoirs and Lifestyles
B. cereus is described as being of ubiquitous presence in nature and can be found in many types of soils, sediments, dust and plants (Schoeni & Wong, 2005). Spores may be passively spread and thus found also outside natural habitats. It is thought that B. cereus sensulato is present in soil as spores, which when in touch with organic matter, an insect or animal host, or both, germinate and thrive. A study revealing that B. cereus could germinate, develop, and sporulate in soil, indicating a saprophytic life cycle, was motivated by interest in the ecology of this bacteria [98].
Additionally, a fi lamentous mode of growth in a multicellular phenotype was seen and proposed as a route of translocation via soil [98]. In the intestines of insects, a multicellular fi lamentous style of growth has also been seen. When spore-forming bacteria that were later identifi ed as B. cereus were recovered from the guts of various soil-dwelling arthropod species, it was proposed that the bacteria lived in the intestines of insects. These bacteria appear to live in symbiosis with their invertebrate host [61].
The existence of different morphological modes used by B. cereus, such as the filamentous mode, may be adaptations to different life cycles like the ‘normal’ cycle of life as a symbiotic or the more infrequent pathogenic life cycle with rapid growth. Bacillus cereus has been reported to be present in stools of healthy humans at varying levels [49]. B. cereus would have a transitory existence in the mammalian gut due to its widespread low-level prevalence in surroundings, feed, and foods [52].
The suggested probiotic effect of B. cereus may stem from its potential adaption to the conditions of the animal gut. Since all strains of B. cereus are capable of producing at least one toxin linked to diarrhoeal illness, such use cannot be categorically deemed safe for humans [48]. However, the European Food Safety Authority (EFSA) has allowed the use of probiotics including particular strains that produce insignifi cant quantities of toxicity at 37 0C. Since B. cereus is widespread in so many different settings, it seems to reason that it would also be present in water. Nevertheless, there are few data on B. cereus's occurrence in water sources, and there are no established techniques for detecting the bacteria in water. Norwegian surface waters were investigated for presence of B. cereus spores, and cytotoxic strains were isolated from several rivers. This suggests the possibility that the water supply may be a means by which B. cereus enters the food processing chain microbial communities as a natural niche for part of the B. cereus life cycle is further [72].
Two distinct foodborne disease types, emetic and diarrheal, are associated with B. cereus. Both are generally mild and self-limiting, although more serious and even lethal cases have occurred [23]. Bacillus cereus was established as an organism of foodborne disease in the 1950s, with the first described outbreaks of the diarrheal type of disease in hospitals in Norway in 1947–1949 [43]. Earlier descriptions of disease which could probably be attributed to B. cereus lack the nomenclature and epidemiological framework that would allow this attribution, however there is little doubt that B. cereus has been implicated in foodborne disease historically [52]. The emetic syndrome was first identified after several outbreaks caused by eating cooked rice in the United Kingdom in the early 1970s [64]. This disease is an intoxication caused by the B. cereus emetic toxin, named cereulide, produced in foods before ingestion. The course of the disease is characteristic, with nausea and emesis occurring only a few hours after the meal. [52]. The most important differential diagnosis is intoxication with Staphylococcus aureus enterotoxins, which causes similar symptoms; however, in this disease emesis is commonly accompied by diarrhea [85]. Several severe and even lethal cases of emetic foodborne B. cereus disease have been reported [33]. The diarrheal syndrome is thought to be a toxic infection caused by vegetative cells, ingested as viable cells or spores, producing protein enterotoxins in the small intestine [20]. It is easily confused with the foodborne disease caused by another spore forming bacterium, Clostridium perfringens (Granum, 1990), and typically presents with abdominal pain, watery diarrhea and occasionally nausea and emesis. The incubation time is over 6h, normally in the range of 8–16h, and on average 12h, but in rare cases longer incubation times have been observed [52].
Non gastrointestinal Illness of B. Cereus
B. cereus has been associated with illness other than food poisoning, although these infections are not common. The bacterium has been found contaminating post-surgical or traumatic wounds and burns and causes a variety of opportunistic infections, especially in immunocompromised patients, including bacteremia, septicemia, endocarditis, meningitis, pneumonia, pleuritis, osteomyelitis, and endophthalmitis [24]. These infections can be highly fulminant and sometimes fatal. B. cereus can also play a role in sepsis caused by use of contaminated needles by intravenous drug abusers or by following penetrating wounds [11].
Involvement of B. cereus in pneumonia is rare and is usually associated with other risk factors such as leukemia. However, two unusual cases were reported that involved two previously healthy middle-aged individuals who experienced fulminant bacteremia and pneumonia caused by B. cereus, with symptoms similar to those of B. anthracis pulmonary infection [63]. Both individuals experienced chills for three to five days, fever, cough, and bloody expectoration before hospitalization. Their conditions deteriorated in the hospital, and both patients died. B. cereus is a common cause of eye infections, often causing irreversible tissue damage in a short time (within 24 h). It is one of the most common causes of post traumatic endophthalmitis, where the organism is introduced into the eye by foreign bodies as a consequence of traumatic injury [45]. It also causes metastatic endophthalmitis from hematogenous spread of the organism to the eye from other sites. Metastatic endophthalmitis is often highly fulminant and sometimes fatal. The rapid progression and fulminance of B. cereus endophthalmitis is attributed to multiple toxins elaborated by the organism, including the diarrheal enterotoxin HBL [13].
Pathogenic Strain and Pathogenicity of Bacillus cereus
The pathogenicity of B. cereus is known to be intimately associated with production of tissue-destructive/reactive exoenzymes; among these secreted toxins are three distinct phospholipases, an emesis-inducing toxin (which is responsible for the vomiting syndrome), and three pore-forming enterotoxins: the cytotoxin K, the Hemolysin BL (HBL), and a Non-Hemolytic Enterotoxin (NHE) [17].
The toxicological profi le of B. cereus strains ranges from non-pathogenic strains used as probiotics in animal feed [53] to highly pathogenic isolates, which are responsible for major outbreaks such as 173 cases of intoxication after a banquette [84] or severe individual cases leading to hospitalization and even death. B. cereus is mainly known to evoke two types of gastrointestinal food borne poisonings. The emetic type indicated by nausea and forceful vomiting shortly after ingestion is caused by the small dodecade psipeptide cereulide [3], which is produced by a subgroup of B. cereus [28]. The diarrheal syndrome is caused by several heat labile enterotoxins produced during growth of B. cereus in the intestine. The hemolysin BL (HbL) and the pore forming non-hemolytic toxin (Nhe) belong to the class of three-component enterotoxins, whereas cytotoxin K (CytK) represents a β-barrel channel forming one-component enterotoxin [57].
In general, 6 to 12 hours after ingestion of about 105 to 107 cells, abdominal cramps and diarrhoea occur, but the course of the disease is normally relatively mild and symptoms disappear within 24 hours (Stenfors Arnesen et al., 2008). The extent and duration of the disease depend on the infection dose and the amount of produced enterotoxins, which seem to differ strongly among different B. cereus strains Besides the known toxins, B. cereus also produces several enzymes like sphingomyelinase, phosphatidylinositol and phosphatidylcholine-specifi c phospholipases and several proteases that are so far not directly associated with gastrointestinal diseases, but may play an important role in non-gastrointestinal infections such as wound and eye infections, bacteremia, pneumonia, meningitis, periodontitis, and endocarditis [74].
In recent years, the number of food borne outbreaks caused by B. cereus has increased in industrial countries [88]. The ability to generate protecting biofi lms and to form endospores, which are metabolic inactive and resistant to harsh conditions such as heat (>100°C), many chemicals, radiation as well as desiccation, allows B. cereus to survive e.g. treatments of the food industry like conservation, chemical disinfection and preservation [1]. Most notably, the high hydrophobic character of the spores seems to increase their adherence to the surface of food processing machines and equipment, pipelines as well as tanks leading to contamination of food products by direct contact with these different sources [30]. Consequently, once spores have entered the food, pasteurization or normal sanitation processes will not contribute to their elimination [27].
Isolation and Identification of B. Cereus
B. cereus can be isolated and identifi ed from foods and from clinical samples taken from cases of food poisoning. Bennett and Belay (2001) and Kramer and Gilbert [52] have provided detailed descriptions of these methods. Mannitol-egg yolk-polymyxin agar and the same agar supplemented with pyruvate and bromothymol blue are two often used differential media.
These formulations take advantage of the fact that unlike many Bacillus species, B. cereus does not ferment mannitol but does produce phosphatidylcholine-preferring phospholipase C (lecithinase). Polymyxin B is used as the primary selective agent for the B. cereus group. Classical identification schemes for members of the Bacillus genus have been described extensively [94].
Generally, B. cereus colonies on solid media are 5 to 6 mm in diameter and have a ground glass or matte appearance, with edges that range from circular and entire to irregular and fimbriate. Colonies often appear greenish on blood agar. The spores are ellipsoidal or cylindrical and do not swell the sporangia. Confirmation of B. cereus involves a battery of biochemical tests. These tests can be performed using conventional methods (Bennett and Belay, 2001) or using miniaturized commercial systems that combine biochemical profiles with information in large databases. Serotyping has been a useful tool in epidemiological studies. Forty-two serotypes have been identified based on serological classification of spore, somatic, and flagellar antigens. Twenty-three of these 42 serotypes are associated with B. cereus–related disease [24]. The flagellar (H) serotypes most commonly associated with diarrheal food poisoning are 1, 2, 6, 8, 10, 12, and 19. The H serotypes 1, 5, 8, 12, and 19 are commonly associated with the emetic food poisoning syndrome. Some B. cereus strains may cause both forms of food poisoning [40]. Phage typing as a means of B. cereus identification was explored because of the specificity of Bacillus-associated bacteriophages for their host strain [97].
Biochemical Test
Bacillus Cereus colony similar with Bacillus anthrax and other bacillus groups
differentiation of B,cereus and B.anthrax on sheep blood agar
B. anthracis on sheep blood agar illustrating nonhemolytic, fl at,`ground-glass‘, dry (dull) colonies with irregular edges on let P.J.Qunnin et al., [78].
B. anthracis (left) and B. cereus (right) on demonstrating the susceptibility of B. anthracis to penicillin (10 unit disc) compared to the resistance of B. Cereus
Susceptibility to penicillin
Strong lecithinase activity by B. cereus (top) on egg yolk agar after 24 hrs incubation
B. anthracis (left) gives a weak opaque zone after 48 hrs
B. licheniformis (right) is unreactive in this medium
lecithinase activity on egg yolk agar ( B.cereus, B. anthracis and B.licheniformis)
Source: P.J.Qunnin et al., [78]
Epidemiology
B. Cereus is a ubiquitous environmental rod; specifi cally, its natural reservoir is represented by decomposing organic matter and vegetables, fresh and marine waters, and the invertebrate intestinal tract, which can contaminate soil and food and cause temporary colonisation of the human gut) [17]. When bacilli come into contact with organic matter or within an insect or animal host, they may bacilli lose their fl agella, attach to the arthropod enteric epithelium, and sporulate [17]. B. cereus also has a saprophytic life cycle where spores germinate in the soil, with the production of vegetative bacilli, that could then sporulate, keeping the cycle; following host defecation or death, cells and spores are released into the soil, where vegetative organisms may sporulate again, then surviving until the uptake by another host [17]. Survival of B. cereus in the environment is then strictly related to the spore; the latter is in fact resistant to extreme conditions including heat, radiation, drying, freezing, and may be considered to be the infective agent for this organism. In the food industry, such spores are particularly troublesome since they can be refractory to pasteurization and gamma radiation; moreover, their hydrophobic nature permits them to adhere to surfaces, and this feature enables the bacterium to spread to all kinds of food. Given the ubiquitous distribution of B. cereus in food products, therefore, the organism is ingested in small amounts and becomes part of the transitory human enteric fl ora; it is unclear, however, if the recovery of B. cereus from man stools is a function of germinating spores or the growth of vegetative cells [17].
Year
No. of cases
(fatalities)Food
Syndrome type
Country
Comments
References
2008
1 (1)
Spaghetti with tomato sauce
Emetic
Belgium
Food stored at room temperature for 5 days after preparation.
B. cereus and cereulide isolated from pastaNaranjo et al.
[68]2007
2 (1)
Asparagus sauce
Emetic
Australia
Prior to serving, the sauce was stored for 2 hours in a hot kitchen (up to 37°C), permitting B. cereus growth
NSW Food
Authority
20132003
4 (1)
Pasta salad
Emetic
Belgium
Food stored for
3 days in fridge at 14°C, permitting B. cereus growth.
B. cereus isolated from foodDierick et al.
[23]2000
173
Cake
Diarrhoeal
Italy
B. cereus isolated from food and rolling board. Rolling board likely source of contamination
Ghelardi et al.
[35]1998
44 (3)
Vegetable puree
Diarrhoeal
France
Cytotoxin K produced by B. cereus involved
Jenson and
Moir [49]1991
139
Barbequed pork
Diarrhoeal
US
B. cereus spores from dried foods, slaughtered animals or worker hands likely source of contamination. Unrefrigerated storage of cooked pork for >18 hours permitted
B. cereus growthLuby et al.
[55]1989
55
Cornish game hens
Diarrhoeal
US
Inadequate thawing and cooking, cross- contamination from basting brush used before and after cooking,
inadequate
refrigerationSlaten et al.
[87]Source: FDA [31].
Table 1: Selected major outbreaks associated with B. cereus.
Tests
B. anthracis
B. cereus
B. mycoides
B.thuringiensis
Motility
-
+
-
+
Hemolysis
-
+
Weak
+
Penicillin susceptibility (10 unit disc)
S
R
R
R
Gelatin stab culture
Inverted fir tree type of growth
Rapid liquefaction
Rapid liquefaction
Rapid liquefaction
Lecithinase activity (egg-yolk agar)
+ weak
+
+
+
Nutrient agar with 0.7% Na-bicarbonate under 10% CO2
Mucoid colonies
Unchanged
Unchanged
Unchanged
Susceptibility to cherry gamma phage
+ (lysis)
-
(+) lysis may occur
-
Pathogenicity for mice or guinea-pigs (subcut. or i.v.)
+ (death in
24-48 hrs)+ large dose (non-invasive)
-
-
Source: Qunnin et al., [78].
Table 2: Summary for differentiation of the Bacillus cereus from another common Bacillus cereus group.
Outbreaks of Bacillus Cereus Foodborne Disease
The incidence of foodborne B. cereus disease is signifi cantly underreported due to a number of causes. It is a result of the disease's often brief and mild course, which deters patients from seeking medical care and makes it difficult to distinguish between illnesses brought on by food poisoning and S. aureus intoxication (Kotiranta et al., 2000). The percentage of food poisoning outbreaks associated with B. cereus varies from country to country and is dependent on the reporting system. In The Netherlands, from 1991 to 1994 B. cereus was identified as the most common cause (19%) of food poisoning outbreaks [86]. In Taiwan, from 1986 to 1995 B. cereus outbreaks ranked third, behind those caused by Vibrio para haemolyticus and Staphylococcus aureus (Horng et al., 1997). Mead et al. [62] estimated that more than 27,000 foodborne illnesses annually in the United States are caused by B. cereus, which from 1993 to 1997 ranked seventh among the etiological agents causing reported bacterial foodborne outbreaks (14 of 655; 0.5%) and sixth as the causative agent for reported cases (691 of 43,821; 0.8%) [71]. IN Ethiopia Bacillus cereus in raw milk samples was 38.8% and 15.4% reported by Ashabir et al., and Kassa et al., respectively [2,9].
Aside from food poisoning, several infectious processes have been attributed to B. cereus in the past and recent years, including periodontal diseases, ocular infections (endophtalmitis, panophtalmitis and keratitis, that develop after the microorganism introduction into the eye due to the occurrence of a traumatic event), skin as well as post-operative and posttraumatic wound infections (with or without bone involvement), osteomyelitis, necrotizing fasciitis, salpingitis, meningitis, endocarditis, bacteremia [82].
Mode of Transmission
B. cereus food poisoning can be caused by either ingesting large numbers of bacterial cells and/or spores in contaminated food (diarrheal type) or by ingesting food contaminated with pre-formed toxin (emetic type). Food contamination, incorrect food handling and storage, and inadequate chilling of cooked food items are the main causes of this disease's spread [83]. Most remarkably, the spores' extreme hydrophobicity appears to boost their adherence to the surfaces of tanks, pipelines, and machinery used in food processing, contaminating food products when they come into direct touch with these various sources [30]. Consequently, once spores have entered the food, pasteurization or normal sanitation processes will not contribute to their elimination [26]. The diarrhoeal syndrome is thought to be a toxic infection caused by vegetative cells, ingested as viable cells or spores, producing protein enterotoxins in the small intestine [20].
Symptoms of Disease
Due to the typically mild, transient, and self-limiting symptoms of B. cereus infection, as well as the fact that it is rarely screened for in standard laboratory analysis of stool samples, there may be a large underreporting of cases [41]. B. cereus is linked to two different foodborne illness types: diarrhoeal and emetic. Both are generally mild and self-limiting, although more serious and even lethal cases have occurred [23]. This disease is an intoxication caused by the B. cereus emetic toxin, named cereulide, produced in foods before ingestion. The course of the disease is characteristic, with nausea and emesis occurring only a few hours after the meal [52].
Other than food poisoning the B. Cereus has been found contaminating post-surgical or traumatic wounds and burns causes a variety of opportunistic infections, especially in immunocompromised patients, including bacteremia, septicemia, endocarditis, meningitis, pneumonia, pleuritis, osteomyelitis, and endophthalmitis [24]. B. cereus is a common cause of eye infections, often causing irreversible tissue damage in a short time (within 24 h). It is one of the most common causes of post traumatic endophthalmitis, where the organism is introduced into the eye by foreign bodies as a consequence of traumatic injury (Boldt et al., 1989).
Diagnosis and Culturing
B. cereus and its spores have very diverse contamination sources like soil, bedding, feed, dust, air, feces, dirty teats and milking equipment [88]. After the sample collected by peptone water from clinical diseased or food sample, then spread on to Bacillus cereus selective medium plate (Oxoid Ltd, London, UK) in duplicates. After that, the inoculation plates were monitored for colony growth for 18 to 24 hours while being incubated aerobically at 30 0C. The incubation period was increased by another 24 hours and the growth of the colonies was re-examined if none appeared. B. Cereus colonies were presumed to be based on colour, morphology, and egg yolk precipitation on selective medium: they were described as crenate colonies with a diameter of around 5 mm, a colour of turquoise blue, and an opaque zone surrounding them [69].
Confirmatory and Differential Tests
Two or three colonies were selected from positive plates and moved to nutrient agar slants. These were aerobically incubated for 24 hours at 30 0C. The B. cereus group was recognized by Gramm staining as a big, rod-shaped, Gram-positive organism with short to long chains. B. cereus was differentiated from the group members as based on strong β-haemolysis on sheep blood agar (Oxoid Ltd, London, UK), diffuse growth in semisolid SIM medium (HiMedia Ltd, India) and characteristic pale green endospores without bulged sporangium and with no parasporal crystal bodies in red stained cytoplasm using rapid staining methods (warm 0.5% basic Fuchsin (BD Difco BBL Stains), Malachite green (Fluka) and Sudan Black B (Sigma-Aldrich) [2].
Antibiotics and Therapy
The antibiotic susceptibility profi le of the isolated (and accurately identifi ed) strain typically determines the therapeutic options for B. cereus illnesses; however, species-specifi c criteria for assessing and interpreting the in vitro response to medication have not yet been published. However, while waiting for the results of an antibiotic sensitivity test, empirical antimicrobial therapy may be essential in the event of a suspected B. cereus infection [17]. In general, most B. cereus isolates are resistant to penicillin and cephalosporin as a consequence of β-lactamase production; in particular, it is likely that resistance to penicillin, ampicillin and cephalosporin’s should be considered to be constant, nowadays, like that to trimethoprim [19].
A chromosomal Metallo-β-Lactamase (MBL) is widespread in B. cereus (the enzyme is called ‘BcII’) [19]. Of interest, B. cereus MBL has been the fi rst enzyme of this category that was discovered, in 1966 [80], while MBLs were later described in Stenotrophomonas maltophilia, Aeromonas spp, Bacteroides fragilis, Acinetobacter spp, Pseudomonas aeruginosa, and some fl avobacteria, and have been known to inhibit all βlactams except for the monobactam (aztreonam, to which Gram positive organisms are however intrinsically resistant) [82]. Furthermore, B. cereus usually produces Bush group 2a penicillinases I and III that typically hydrolyze penicillin and are inhibited by clavulanic acid [82].
Prevention and control
Canning is the only method that will remove B. cereus spores from food products because it is a common occurrence. Before being stored, spores can be found in practically every type of food, though usually in amounts too small to result in foodborne illness. A very signifi cant initial food contamination risk exists, although temperature abuse-induced B. cereus multiplication is typically the cause of human health risks. Cleaning is a crucial step in preventing Bacillus cereus from growing on machinery and other equipment used to transport food inside the processing facility.
Whenever possible the use of hypochlorite (pH< 8) is recommendable at least in pipelines. This will eliminate or dramatically reduce the number of spores. The use of weak acids at 30-40 °C for 20-30 min can be an alternative to chemicals that can harm the pasteurizer or other equipment’s (Anderson et al., 1995). Spores of Bacillus cereus have a pronounced ability to adhere to the surface of stainless-steel material commonly used to build processing equipment for the food industry, which may become a reservoir of spores. Therefore, the attachment of Bacillus cereus to online processing equipment may present a major problem for the food industry [73]. It is a primary factor in both its existence and its challenging control. Controlling microorganisms that are present in food products and processing equipment is crucial to delivering wholesome and safe products to consumers. This could mean doing more frequent and thorough cleanings [75].
Conclusion
B.cereus are well-known opportunistic human pathogens, which can cause two different types of foodborne illnesses, emesis and diarrhea. Although the three main diarrhea-associated toxins, Nhe, HBL, and CytK, as well as the emetic toxin cereulide, are known over a decade and considerable progress has been made on the understanding of toxin gene regulation, the exact mechanisms of toxin syntheses and toxin actions are far from understanding. However, there is still a signifi cant knowledge vacuum and no known causative genotypes for other disorders, such as food poisoning diarrhoea and (opportunistic) infections caused by pathogenic B. cereus strains. In conclusion, B. cereus is a complex microbe that exhibits a wide range of pathogenicity and ecological lifestyle variations. The virulence and food poisoning potential of these mysterious bacteria must thus be investigated using a multidisciplinary approach that integrates studies in epidemiology, molecular biology, taxonomy, metabolism, microbiology, ecology, and host-interaction.
Apart from food poisoning, B. cereus has been linked to a number of infectious diseases in the past and present, such as periodontal diseases, ocular infections (such as endophtalmitis, panophtalmitis, and keratitis, which arise from the introduction of microorganisms into the eye as a result of a traumatic event), skin infections, wound infections following surgery and trauma, osteomyelitis, necrotising fasciitis, salpingitis, meningitis, and endocarditis.
References
- Abee T, Groot MN, Tempelaars M, Zwietering M, Moezelaar R, van der Voort M. Germination and outgrowth of spores of Bacillus cereus group members: diversity and role of germinant receptors. Food microbiology. 2011; 28: 199-208.
- Alemneh Kassa, Zewude G, Sisay Tessema T. Investigation on Bacillus cereus and associated risk factors in bovine raw milk in Bishoftu town, Ethiopia. 2017; 168: 213-218.
- Agata N, Mori M, Ohta M, Suwan S, Ohtani I, Isobe M. A novel dodecadepsi peptide, cereulide, isolated from Bacillus cereus causes vacuole formation in HEp-2 cells. FEMS Microbiol. Lett. 1994; 121: 31–34.
- Agata N, Ohta M, Mori M. Production of an emetic toxin, cereulide, is associated with a specific class of Bacillus cereus. Curr Microbiol. 1996; 33: 67–69.
- Andersson A, Granum PE, Ronner U. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int J Food Microbiol. 1998a; 39: 93–99.
- Andersson A, Ronner U, Granum PE. What problems does the food industry have with the spore-forming pathogens Bacillus cereus and Clostridium perfringens? International Journal of Food Microbiology. 1995; 28: 145-55.
- Andersson MA, Mikkola R, Helin J, Andersson MC, Salkinoja-Salonen M. A novel sensitive bioassay for detection of Bacillus cereus emetic toxin and related depsipeptide ionophores. Appl Environ Microbiol. 1998; 64: 1338–1343.
- Ash C, Farrow JA, Dorsch M, Stackebrandt E, Collins MD. Comparative analysis of Bacillus anthracis, Bacillus cereus, and related species on the basis of reverse transcriptase sequencing of 16S rRNA. International journal of systematic bacteriology. 1991; 41: 343-346.
- Ashebr A, Teshome B, Sisay A, Yimer M. Bacillus Cereus isolation and load from raw cow milk sold in Markets of Haramaya District, eastern Ethiopia. International Journal of Food Contamination. 2017; 4: 15.
- Ballard CE, Yu H, Wang B. Recent developments in depsipeptide research. Current medicinal chemistry. 2002; 9: 471-498.
- Beecher DJ. The Bacillus cereus group, p. 1161–1190. In P. C. B. Turnbull (ed.), Gastro-intestinal infections: toxin-associated diseases. Academic Press, London. 2001.
- Beecher DJ, Schoeni JL, Wong AC. Enterotoxin activity of hemolysin BL from Bacillus cereus. Infect Immun. 1995; 63: 4423-4428.
- Beecher DJ, Olsen TW, Somers EB, Wong AC. Evidence for contribution of tripartite hemolysin BL, phosphatidylcholine-preferring phospholipase C, and collagenase to virulence of Bacillus cereus endophthalmitis. Infect Immun. 2000; 68: 5269– 5276.
- Berry C, O'Neil S, Ben-Dov E, Jones AF, Murphy L, Quail MA, et al. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Applied and environmental microbiology. 2002; 68: 5082-5095.
- Blume HP, Horn R, Kandeler E, Kögel-Knabner I, Kretschmar R, Stahr K, et al. Lehrbuchder Bodenkunde. Spektrum Akademischer Verlag Berlin Heidelberg. 2010.
- Bortner CD, Cidlowski JA. Caspase independent/dependent regulation of K (+), cell shrinkage, and mitochondrial membrane potential during lymphocyte apoptosis. The Journal of biological chemistry. 1999; 274: 21953-21962.
- Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010; 23: 382-398.
- Bravo A, Likitvivatanavong S, Gill SS, Soberon M. Bacillus thuringiensis: A story of a successful bio insecticide. Insect biochemistry and molecular biology. 2011; 41: 423-431.
- Brown CS, Chand MA, Hoffman P, Woodford N, Livermore DM, Brailsford S, et al. United Kingdom incident response team. Possible contamination of organ preservation fl uid with Bacillus cereus: the United Kingdom response. Euro Surveille. 2012; 17: 20165.
- Clavel T, Carlin F, Lairon D, Nguyen-The C, Schmitt P. Survival of Bacillus cereus spores and vegetative cells in acid media simulating human stomach. J Appl Microbiol. 2004; 97: 214–219.
- Merrel DS, Camilli A. Acid tolerance of gastrointestinal pathogens, Current Opin Microbiol. 2002; 5: 51-5.
- Didelot X, Barker M, Falush D, Priest FG. Evolution of pathogenicity in the Bacillus cereus group. Systematic and applied microbiology. 2009; 32: 81-90.
- Dierick K, Van Coillie E, Swiecicka I, Meyfroidt G, Devlieger H, Meulemans A, et al. Fatal family outbreak of Bacillus cereus-associated food poisoning. Journal of clinical microbiology. 2005; 43: 4277-4279.
- Drobniewski FA. Bacillus cereus and related species. Clinical. Microbial. Rev. 1993; 6: 324–338.
- EFSA. Opinion of the scientifi c panel on biological hazards on Bacillus cereus and other Bacillus spp. in foodstuffs, EFSA J. 2005; 1: e 48.
- Ehling-Schulz M, Knutsson R, Scherer S. Bacillus cereus. Genome of Foodborne and Waterborne Pathogens, ASM Press. 2011a; 149-164.
- Ehling-Schulz M, Messelhäusser U, Granum PE. Bacillus cereus in milk and dairy products. In J Hoorfar (ed), Rapid Detection, Identifi cation, and Quantifi cation of Foodborne Pathogens, ASM Press. 2011b: 275-289.
- Ehling-Schulz M, Svensson B, Guinebretiere MH, Lindback T, Andersson M, Schulz A, et al. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology. 2005a; 151: 183-197.
- Fagerlund A, Ween O, Lund T, Hardy P, Granum E. Genetic and functional analysis of the cytK family of genes in Bacillus cereus. Microbiology. 2004; 150: 2689-2697.
- Faille C, Tauveron G, Le Gentil-Lelievre C, Slomianny C. Occurrence of Bacillus cereus spores with a damaged exosporium: consequences on the spore adhesion on surfaces of food processing lines. Journal of food protection. 2007; 70: 2346-2353.
- FDA. Bad bug book: Foodborne pathogenic microorganisms and natural toxins handbook, 2nd ed, US Food and Drug Administration, Silver Spring. 2012: 93–96.
- Fricker M, Reissbrodt R, Ehling-Schulz M. Evaluation of standard and new chromogenic selective plating media for isolation and identifi cation of Bacillus cereus. Int J food Micro. 2008; 121: 27–34.
- Fricker M, Messelhausser U, Busch U, Scherer S, Ehling-Schulz M. Diagnostic real-time PCR assays for the detection of emetic Bacillus cereus strains in foods and recent food-borne outbreaks. Applied and environmental microbiology. 2007; 73: 1892-1898.
- Fritze D. Bacillus identification - Traditional approaches. Applications and Systematics of Bacillus and Relatives (Berkeley R, Heyndrickx M, Logan N & De Vos P, eds). Blackwell Science Ltd, Oxford. 2002; 121: 27–34.
- Ghelardi E, Celandroni F, Salvetti S, Barsotti C, Baggiani A, Senesi S. Identifi cation and characterization of toxigenic Bacillus cereus isolates responsible for two food-poisoning outbreaks. FEMS microbiology letters. 2002; 208: 129-134.
- Gilbert RJ, Kramer JM. Bacillus cereus food poisoning. Progress in Food Safety (Proceedings of Symposium) (Cliver DC & Cochrane BA, eds). Food Research Institute, University of Wisconsin-Madison, Madison, WI. 1986: 85-93.
- Gilbert RJ. Bacillus cereus gastroenteritis. In H. Reimann and F. L. Bryan (ed.), Food-borne infections and intoxication, 2nd ed. Academic Press, New York. 1979: 495-518.
- Gordon RE, Haynes WC, Pang CHN. The genus Bacillus. U.S. Department of Agriculture agricultural handbook no. 427. U.S. Government Printing Office, Washington. 1973.
- Granum PE. Bacillus cereus. Food microbiology: fundamentals and Frontiers. In: Beuchat LR, editor. Doyle MP. Washington, DC: ASM Press. 2007: 445–55.
- Granum PE. Bacillus cereus. Food Microbiology Fundamentals and Frontiers, 2nd ed (eds M Doyle, L Beuchat, and T Montville,) ASM press, Washington DC. 2001: 373–382.
- Hall G, Kirk MD, Becker N, Gregory JE, Unicomb L, Millard G, et al. Estimating foodborne gastroenteritis, Australia. Emerg Infect Dis. 2005; 11: 1257–64.
- Harmon SM, Kautter DA, Golden DA, Rhodehamel EJ. Bacillus cereus. In U.S. Food and Drug Administration bacteriological analytical manual, 7th ed. AOAC. 1992: 191-198.
- Hauge S. Food poisoning caused by Bacillus cereus (in Norwegian, English abstract). Nord Hyg Tidsskr. 1955; 31: 189–206.
- Hauge S. Food poisoning caused by aerobic spore forming bacilli. J Appl Bacteriol. 1955; 18: 591–595.
- Hermady R, Zaltas M, Paton B, Foster CS, Baker AS. Bacillus-induced endophthalmitis: a new series of 10 cases and review of the literature. Br J Ophthalmol. 1990; 74: 26–29.
- Hoff master AR, Fitzgerald CC, Ribot E, Mayer LW, Popovic T. Molecular subtyping of Bacillus anthracis and the 2001 bioterrorism-associated anthrax outbreak, United States. Emerging infectious diseases. 2002; 8: 1111-1116.
- Holbrook R, Anderson JM. An improved selective and diagnostic medium for the isolation and enumeration of Bacillus cereus in foods. Can J Microbial. 1980; 26: 753–759.
- Hong HA, Ducle H, Cutting SM. The use of bacterial spore formers as probiotics. FEMS Microbial Rev. 2005; 29: 813–835.
- Jensen GB, Hansen BM, Eilenberg J, Mahillon J. The hidden lifestyles of Bacillus cereus and relatives. Environ Microbial. 2003; 5: 631–640.
- Jenson I, Moir CJ. Bacillus cereus and other Bacillus species. Ch 14 In: Hocking AD (ed) Foodborne microorganisms of public health signifi cance. 6th ed, Australian Institute of Food Science and Technology (NSW Branch), Sydney. 2003: 445–478.
- Johnson KM. Bacillus cereus food-borne illness. An update. J Food Prot. 1984; 47: 145–153.
- Kramer JM, Gilbert RJ. Bacillus cereus and other Bacillus species. Foodborne Bacterial Pathogens (Doyle MP, ed). Marcel Dekker, New York. 1989: 21-70.
- Lodemann U, Lorenz BM, Weyrauch KD, Martens H. Effects of Bacillus cereus var. toyoi as probiotic feed supplement on intestinal transport and barrier function in piglets. Archives of animal nutrition. 2008; 62: 87-106.
- Logan NA, Berkeley RCW. Identifi cation of Bacillus strains using the API system. J Gen Microbiol. 1984; 130: 1871-1882.
- Luby S, Jones J, Dowda H, Kramer J, Horan J. A large outbreak of gastroenteritis caused by diarrheal toxin-producing Bacillus cereus. Journal of Infectious Diseases. 1993; 167: 1452–1455.
- Lund T, Granum PE. Characterization of a non-hemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbial Lett. 1996; 141: 151–156.
- Lund T, De Buyser ML, Granum PE. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Molecular microbiology. 2000; 38: 254-261.
- Torre Del M, Della Corte M, Stecchini ML. Prevalence and behavior of Bacillus cereus in a REPFED of Italian origin. Int J Food Microbial. 2001; 63: 199-e207.
- Mahler H, Pasi A, Kramer JM, Schulte P, Scoging AC, Bar W, et al. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N Engl J Med. 1997; 336: 1142–1148.
- Marahiel MA, Stachelhaus T, Mootz HD. Modular Peptide Synthetases Involved in Nonribosomal Peptide Synthesis. Chem Rev. 1997; 97: 2651-2674.
- Margulis L, Jorgensen JZ, Dolan S, Kolchinsky R, Rainey FA, Lo SC. The Arthromitus stage of Bacillus cereus: intestinal symbionts of animals. Proc Natl Acad Sci USA. 1998; 95: 1236–1241.
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999; 5: 607–625.
- Miller JM, Hair JG, Herbert M, Herbert L, Roberts FJ. Fulminating bacteremia and pneumonia due to Bacillus cereus. J Clin Microbial. 1997; 35: 504–507.
- Mortimer PR, McCann G. Food-poisoning episodes associated with Bacillus cereus in fried rice. Lancet. 1974; 1: 1043–1045.
- Mossel DA, Koopman MJ, Jongerius E. Enumeration of Bacillus cereus in foods. Appl Microbial. 1967; 15: 650–653.
- Browne N, Dowds BC. Acid stress in the food pathogen Bacillus cereus. J Appl Microbial. 2002; 92: 404-414.
- Nakamura LK. Bacillus pseudomycoides spnov. International journal of systematic bacteriology. 1998; 48: 1031-1035.
- Naranjo M, Denayer S, Botteldoorn N, Delbrassinne L, Veys J, Waegenaere J, et al. Sudden death of a young adult associated with Bacillus cereus food poisoning. Journal of Clinical Microbiology. 2011; 49: 4379–4381.
- Nicoletta N, Royston G. Rapid and quantitative detection of the microbial spoilage in milk using Fourier transform infrared spectroscopy and chemo-metrics. Analyst. 2008; 133: 1424–1431.
- Okstad OA, Gominet M, Purnelle B, Rose M, Lereclus D, Kolsto AB. Sequence analysis of three Bacillus cereus loci carrying PIcR-regulated genes encoding degradative enzymes and enterotoxin. Microbiology. 1999; 145: 3129-3138.
- Olson SJ, MacKinon LC, Goulding JC, Bean NH, Slutsker L. Surveillance for foodborne-disease outbreaks-United States, 1993–1997. Morb Mortal Wkly Rep. 2000; 49: 1–62.
- Østensvik Ø, From C, Heidenreich B, O’Sullivan K, Granum PE. Cytotoxic Bacillus spp. belonging to the B. cereus and B. subtilis groups in Norwegian surface waters. J Appl Microbial. 2004: 96: 987-93.
- Peng JS, Tsai WC, Chou CC. Surface characteristics of Bacillus cereus and its adhesion to stainless steel. International Journal of Food Microbiology. 2001; 65: 105- 111.
- Pillai A, Thomas S, Arora J. Bacillus cereus: the forgotten pathogen. Surgical infections. 2006; 7: 305-308.
- Pontefract RD. Bacterial adherence: its consequences in food processing. Canadian Institute Science and Technology Journal. 1991; 24: 113-117.
- Priest FG, Barker M, Baillie LW, Holmes EC, Maiden MC. Population structure and evolution of the Bacillus cereus group. Journal of bacteriology. 2004; 186: 7959-7970.
- Priest FG, Good fellow M, Todd C. A numerical classifi cation of the genus Bacillus. Journal of general microbiology. 1988; 134: 1847-1882.
- Quinn J, Carter E, Markey B, Carter R. Clinical veterinary microbiology. Philadelphia: Elsevier Limited. 1999: 178–83.
- Thomassin S, Jobin MP, Schmitt P. The acid tolerance response of Bacillus cereus ATCC14579 is dependent on culture pH, growth rate and intracellular pH, Arch. Microbiol. 2006; 186: 229-e239.
- Sabath LD, Abraham EP. Zinc as a cofactor for cephalosporinase from Bacillus cereus. Biochem J. 1996; 98: 11C-3C.
- Sandra M Tallent, Ann Knolhoff E, Jeffery Rhodehamel, Stanley M, Harmon, Reginald W, Bennett. Bacteriological Analytical Manual, 8th Edition, Revision A, 1998. Chapter 14: Section H.8. Addition of link to protocol for quantitative analysis for cereulide in food products. 2019.
- Savini V, Favaro M, Fontana C, Catavitello C, Balbinot A, Talia M, et al. Bacillus cereus heteroresistance to carbapenems in a cancer patient. J Hosp Infect. 2009; 71: 288-290.
- Schneider KR, Parish ME, Goodrich RM, Cookingham T. Preventing Foodborne Illness: Bacillus cereus and Bacillus anthracis. 2004.
- Senesi S, Celandroni F, Scher S, Wong ACL, Ghelardi E. Swarming motility in Bacillus cereus and characterization of a fliY mutant impaired in swarm cell differentiation. Microbiology. 2002; 148: 1785–1794.
- Seo KS, Bohach GA. Staphylococcus aureus. Food Microbiology: Fundamentals and Frontiers (Doyle MP and Beuchat LR, eds). ASM Press, Washington, DC. 2007: 493-518.
- Simone E, Goosen M, Notermans SHW, Borgdorff MW. Investigations of foodborne diseases by food inspection services in The Netherlands, 1991–1994. J Food Prot. 1997; 60: 442–446.
- Slaten DD, Oropeza R, Werner SB. An outbreak of Bacillus cereus food poisoning; are caterers being supervised sufficiently? Public Health Reports. 1992; 107: 477–480.
- Stenfors Arnesen LP, Fagerlund A, Granum PE. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS microbiology reviews. 2008; 32: 579-606.
- Strauss R, Mueller A, Wehler M, Neureiter D, Fischer E, Gramatzki M, et al. Pseudomembranous tracheobronchitis due to Bacillus cereus. Clin Infect Dis. 2001; 33: E39-41.
- Barbosa T, Serra C, La Ragione R, Woodward M, Henriques A. Screening for Bacillus isolates in the broiler gastrointestinal tract, Appl. Environ. Microbial. 2005; 71: 968-978.
- Lund T, De Buyser M, Granum P. A new cytotoxic from Bacillus cereus that may cause necrotic enteritis. Mol Microbial. 2000; 38: 254-e261.
- Taylor AJ, Gilbert RJ. Bacillus cereus food poisoning: a provisional serotyping scheme. J. Med. Microbial. 1975; 8: 543–550.
- Thompson NE, Ketterhagen MJ, Bergdoll MS, Schantz EJ. Isolation and some properties of an enterotoxin produced by Bacillus cereus. Infect Immun. 1984; 43: 887–894.
- Turnbull PCB, Kramer JM. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. 1995: 349-356.
- Turnbull PCB, Kramer J, Melling J. In W. W. C. Topley and G. S. Wilson (ed.), Topley and Wilson’s principles of bacteriology, virology and immunity, 8th ed., Edward Arnold, London, United Kingdom. 1990; 2: 188-210.
- Turnbull PC, Kramer JM, Jorgensen K, Gilbert RJ, Melling J. Properties and production characteristics of vomiting, diarrheal, and necrotizing toxins of Bacillus cereus. The American journal of clinical nutrition. 1979; 32: 219-228.
- Vaisanen OM, Mwaisumo NJ, Salkinoja-Salonen MS. Differentiation of dairy strains of the Bacillus cereus group by phage typing, minimum growth temperature and fatty acid analysis. J Appl Bacterial. 1991; 70: 315–324.
- Vilain S, Luo Y, Hildreth MB, Brozel VS. Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil. Appl. Environ Microbial 72: 4970–4977.
- Von Stetten F, Mayr R, Scherer S. Climatic infl uence on mesophilic Bacillus cereus and psychrotolerant Bacillus weihenstephanensis populations in tropical, temperate and alpine soil. Environmental microbiology. 1999; 1: 503-515.
- Yang IC, Shih DY, Huang TP, Huang YP, Wang JY, Pan TM. Establishment of a novel multiplex PCR assay and detection of toxigenic strains of the species in the Bacillus cereus group. 2005; 68: 2123-30.