
Review Article
Austin J Vet Sci & Anim Husb. 2024; 11(5): 1155.
Study on the Prevalence of Ixodid Tick Infestation and its Associated Risk Factors in Cattle in and Around Lay Armachiho Districts of Amhara Region, Northwest Ethiopia
Desalegn Zemene*
Livestock Resources and Development Office Lay Armachiho, Ethiopia
*Corresponding author: Desalegn Zemene Livestock Resources and Development Office Lay Armachiho, Gondar, Ethiopia. Email: desalegnzemene2008@gmail.com
Received: September 04, 2024 Accepted: September 24, 2024 Published: October 01, 2024
Abstract
Ticks are harmful blood sucking external parasites and cause economic loss towards livestock production and have a negative impact on food security, animal product and by products. A cross-sectional study was conducted from January 2023 to March 2024 to estimate the prevalence of Ixodid Tick and its Associated Risk Factors in cattle in Lay Armachiho District, Amhara region, Norhwest Ethiopia. Simple random sampling procedure was used for selecting study animals. Descriptive statics and mixed effect logistic regression was used to assess the associations between ticks and its potential risk factors. A total of 384 Ixodid tick samples were collected from selected study cattle to idenitfy ticks at genus and species level. The overall prevalence of tick-borne hemoparasite was 37.76% (95% CI: 0.33-0.43) in the study area. From identified ticks, the genus level prevalence was 14.32%, 11.98%, 7.55% and 3.91% for Amblyomma, Boophilus, Rhipicephalus and Hyalomma, respectively. Groin and scrotum/udder is the most favorable tick attachment sites followed by dewlap, belly, neck, under tail and legs/hoof.
Based on mixed effect logistic regression analysis, communal grazing land, agroecology, study kebeles, body condition score, season and communal watering point were identified as potential risk factors. Tick infestation plays an important role for the reduction of production and productivity in livestock industries. Therefore, strategic tick control measures should be carried out in order to minimize losses attributed to ticks in Ethiopia.
Keywords: Cattle; Lay Armachiho; Prevalence; Tick; Risk factors
Abbreviations: ASL: Above Sea Level; BCS: Body Condition Score; CSA: Central Statistical Agency; LALRDO: Lay Armachiho Livestock Resources Development Office; NMA: National Metrological Agency; OIE: World Animal Health Organization; QGIS: Quantum Geographic Information System; TBD: Tick Borne Disease; WHO: World Health Organization
Introduction
Background
Ethiopia has an extremely diverse topography, a variety climatic features and agro ecological zones that are expedient to host a very large animal population [3]. It has the largest livestock population in Africa with an estimate of 78 million cattle, 42.9 million sheep, 52.5 million goats, 2.11 million horses, 0.38 million mules, 8.98 million donkeys, 8.1 million camels and 57 million of poultry [13]. Livestock sub sector plays vital roles in national economy like in generating income to farmers, creating job opportunities, ensuring food security, providing services, contributing to asset, social, cultural and environmental values, and sustain livelihoods but its development is hampered by different constraints (Hussein et al., 2018). The most important constraints to cattle productions are widespread endemic diseases including parasitic infestation, poor veterinary service and lack of attention from government [16]. Ectoparasite is widespread and the most important prevalent constraints to the livestock sector that affect the production and productivity of cattle [33,44]. Ticks are very significant and harmful blood sucking external parasites of mammals, birds and reptiles throughout the world [43]. It has a considerable impact on animals either by inflecting direct damage or by transmission of tick-borne pathogens [40]. Tick and tick born disease affect 90% of the world’s cattle population and are widely distributed throughout the world [62]. The country's environmental condition and vegetation are highly conducive for ticks and tick-borne disease perpetuation [1]. Ticks are more prevalent in the warmer climates, especially in tropical and sub-tropical areas [30].
Tick distribution and their population in the country vary according to their adaptability to ecology, eco-climate, microhabitats, ambient temperature, rainfall and relative humidity which is critical factors affecting life cycle of ticks [40]. Ticks comprise various types of genera, including Amblyomma, Rhipicephalus, Haemaphysalis, Hyalomma and Rhipicephalus (Boophilus). The genus Amblyomma and Rhipicephalus (Boophilus) are predominating in many parts of country [20]. The life of ticks depends on the host animal which results in retardation to animal growth, loss of milk and meat production.
Generally, ticks could be affecting the market price and decreasing the annual income of humans [31]. In Ethiopia, ticks in cattle population cause serious economic loss to smallholder farmers, the tanning industry and the country as a whole through the mortality of infected animals, decreased production, down grading and rejection of hide [24]. The effects of ticks estimated annual loss of US$ 500,000 from hide and skin downgrading and approximately 65.5% of major defects of hides in Eastern Ethiopia [17].
Various Tick prevalence studies have been conducted in different localities of Ethiopia. The prevalence of ticks in different studies has shown a range of 23-85%. In the study area, information is scarce as there have not been any epidemiological studies conducted on ticks in cattle. Therefore, this study was initiated to generate baseline information on the epidemiology of tick in cattle for developing disease control and prevention programs. It also expected to guide community-based awareness programs about ticks in the study area to improve effective tick prevention and control measures in cattle.
Statement of the Problem
Tick infestations in cattle are economically important ectoparasite and cause a major impediment to the health and productive performance of cattle in tropical and subtropical area [17]. This disease causes economic loss towards livestock production and has a negative impact on food security, animal product and by products [14]. In Ethiopia, ticks and tick-borne diseases in cattle population cause serious economic loss to smallholder farmers, the tanning industry and the country as a whole through the mortality of infected animals, decreased production, down grading and rejection of hide [24]. In addition, the influence of livestock farming practices on the spread and maintenance of ticks in cattle is poorly understood [6].
Despite the widespread distribution of various tick species across the study area little is known about the spread of harmful hemoparasite carried by ticks. Thus, there is paucity of information on tick in Lay Armachiho. In order to address the above problems, a study is required to fill the gaps in knowledge about the disease and its vectors in order to create baseline information that can be used to develop efficient disease control and prevention program. So, because of these problems the following research questions are formulated.
Research Questions
This research work was attempted to answer the following research questions.
¾What are the prevalence Ixodid ticks in cattle in the study district?
¾What are the associated risk factors of Ixodid tick species in cattle in the study district?
Objectives
General objective: The aim of this study was to quantify the epidemiology of Ixodid tick infestation in cattle in Lay Armachiho districts of Amhara region, Northwest Ethiopia.
Specific objectives: The specific objectives of this study are:
To determine the prevalence Ixodid ticks infestation in cattle in the study district.
To identify the associated risk factors of Ixodid tick species in cattle in the study district.
Significance of the Study
This study would be used to quantify the epidemiology of Ixodid tick infestation in cattle in the study area. To update the required bodies about the important risk factors responsible for the occurrence of ticks in cattle. The study would be promoted to future researchers to use the gap for further investigating the occurrence of this ectoparasite in cattle. It also investigating the occurrence of tick-borne diseases in cattle in the study area and bordering districts. Therefore, this study would facilitate zonal and regional animal health sectors used to designing and implementing effective control and prevention strategies of Ixodid ticks in cattle.
Literature Review
Biology and Classification of Ixodid Ticks of Cattle
Ticks are among the most significant blood-sucking arthropods and distributed worldwide. They transmit various pathogens that can cause disease and death in cattle. Ticks have several morphologic features and physiologic mechanisms that facilitate host selection, ingestion of vertebrate blood, mating, survival and reproduction [40].
Ticks are within a member called the phylum (Arthropoda), class (Arachnida), sub class (Acari) and Order (Parasitiformes) [59]. Within the Parasitiformes, ticks belong to the suborder Ixodida, which contains a single super family, the Ixodoidea, which is divided into two major families, Argasidae (soft ticks) and Ixodidae (hard ticks), and the rare family Nuttalliellidae, with a single African species [54].
The family Ixodidae, or hard ticks, contains some 683 species [32]. As adults, Ixodids exhibit prominent sexual dimorphism: the scutum covers the entire dorsum in males, but in females (and immatures) the scutum is reduced to a small podonotal shield behind the capitulum, thereby permitting great distention of the idiosomal integument during feeding [38].
Ixodidae ticks are relatively large and comprise thirteen genera. Seven of these genera contain species of veterinary and medical importance: Amblyomma, sub genus Rhipicephalus (Boophilus), Rhipicephalus, Haemaphysalis, Hyalomma, Dermacentor and Ixodes [54]. The family Argasidae, or soft ticks, consists of about 185 species worldwide and have one important genus that infests cattle, Ornithodoros [37]. Adult argasids lack a dorsal sclerotized plate or scutum, their integument is leathery and wrinkled, their mouthparts are not visible from above, and they show no obvious sexual dimorphism. Argasidae are wandering ticks, which only remain on their host while feeding [7].
Life Cycle of Ticks
There are four major stages in the life cycle of ticks: egg, larva, nymph, and adult. Following their engorgement on the host, female ticks drop off the host and seek protected places, such as in cracks and crevices or under leaves and branches, to lay their eggs [29]. During the passage through these stages, Ixodidae ticks take a number of large blood meals, interspersed by lengthy free-living periods. The time spent on the host may occupy as little as 10% of the ticks. They are relatively long-lived and each female may produce several thousand eggs [48].
The lifecycle of ticks (both Ixodids and Argasids) undergo four stages in their development (Figure 1); eggs, 6-legged larva, 8-legged nymph and adult [63]. According to the numbers of hosts, Ixodids ticks are classified as one host ticks, two-host ticks, three-host ticks and Argasids classified as multi-host ticks.
In one-host ticks, all the parasitic stages (larva, nymph and adult) are on the same hosts; in two- host ticks, larva attach to one host, feed and molt to nymph stage and engorged, after which they detach and molt on the ground to adult; and in three-host ticks, the larva, nymph and adult attach to different hosts and all detach from the host after engorging, and molt on the ground. In multi-host ticks (Argasids), a large number of hosts are involved and it is common to have five molts, each completed after engorging and detaching from the hosts [40].
Epidemiology of ticks
Geographical Distribution of Ixodid ticks in Ethiopia: Ticks are more prevalent in the warmer climates, especially in tropical and sub-tropical areas [30]. Ticks are considered to be most important to the health of domestic animal in Ethiopia. The distribution and abundance of tick species infesting domestic ruminants in Ethiopia vary greatly from one area to another area [17]. In Ethiopia, study on ticks begun early in the 19th century. Different researchers from Ethiopia and other worlds determine the pattern of ticks and ticks are common in all agro-ecological zones [47,52].
The main tick genera found in domestic animals of Ethiopia are Amblyomma, Hyalomma, Rhipicephalus, Haemaphysalis and Rhipicephalus [17]. Among the genera Rhipicephalus, Rhipicephalus lunulatus species were observed in Central Ethiopia [41], and Rhipicephalus muhasmae in Borena [53], in wetter western areas of the country [15,52]. Among the genera Rhipicephalus, Rhipicephalus lunulatus species were observed in Central Ethiopia in wetter western areas of the country [8]. Rhipicephalus evertsi evertsi is the most widespread species of Rhipicephalus [23,56]. Rhipicephalus pulchellus is distributed widely in the north eastern and southern parts of the country [23,55]. Of the genus Amblyomma four species that commonly infest cattle includes Amblyomma variegatum, A. gemma, A. lepidum and A. cohaerens and are known to exist in Ethiopia [42]. The study conducted by Mekonnen et al. (2001) in Borena zone showed that A. variegatum, A. gemma and A. lepidum distributed in wider area of southern Ethiopia. Amblyomma variegatum and A. coherence are the two most prevalent Amblyomma species in Awassa areas in decreasing order [11]. It is clearly associated with dry types of vegetation or semi-arid rangelands [52].
Two species of Rhipicephalus (Boophilus) sub genus are known to exist in Ethiopia, which include Rhipicephalus (Boophilus) decoloratus and Rhipicephalus (Boophilus) annulatus [41,55]. Rhipicephalus (Boophilus) annulatus is known to present in Gambella region and recorded by [52].
In Ethiopia, about eight species of Hyalomma that affect cattle are identified, which includes Hyalomma marginatum rufipes, Hyalomma Dromedarii, Hyalomma Tuncatum, Hyalomma Marginatum, Hyalomma Impelatum, Hyalomma Anatolicum excavatum, Hyalomma Anatolicum anatolicum and Hyalomma Albiparmatum [8].
Risk Factors
Host factors: Heavy infestations can kill calves and even adult cattle. Previously unexposed cattle become heavily infested until they build up a degree of resistance. Bos Indicus (tropical breeds of cattle) and their crosses, develop a greater degree of resistance than Bos Taurus (British and European breeds of cattle [1]. Cattle ticks transmit the organisms that cause tick fever, which is a serious blood parasite disease of cattle [37].
The genus Rhipicephalus, Haemaphysalis and Ixodes larvae, nymphs and adults will quest on vegetation [40]. The tick grabs onto the host using their front legs and crawl over the skin to find a suitable place to attach and feed. Adult tick of genera Amblyomma and Hyalomma are active hunters, they run across the ground after nearby hosts [1].
Pathogen factors: They are considered second only to mosquitoes as the most medically important group of arthropods [60]. The pathogenic effect of ticks on host species can be divided into cutaneous and systemic effects [42]. In cutaneous infection the sites of tick bite local dermal necrosis and hemorrhage occur, followed by an inflammatory response, dermal necrosis is sufficient to damage the hide. Tick bites wounds can become infected with staphylococcus bacteria causing local cutaneous abscess or pyaemia.
Heavy tick infestation can result in significant blood loss, reduced productivity, reduced weight gain, and can cause restlessness (NMA, 2008). In the systemic effect blood-feeding habit of ticks are important as vectors of animal disease-transmitting a wide range of pathogenic viruses, Rickettsial, bacteria, and protozoa (Urquhart et al., 1996).
Environmental factors: Ticks are more prevalent in the warmer climates, especially in tropical and sub-tropical areas [30]. The country's environmental condition and vegetation are highly conducive for ticks and tick-borne disease perpetuation [1]. The disease occurs when there is much tick activity, mainly during summer but a single tick can cause fatal infection (Pieszko, 2015). The heaviest losses occur in marginal areas where the tick population is highly variable depending on the environmental conditions [29].
There is a seasonal variation in the prevalence of clinical babesiosis, the greatest incidence occurring soon after the peak of the tick population, climatic factors and air temperature is the most important because of their effect on tick activity, higher temperatures increase its occurrence [3]. In temperate regions seasonal occurrence of the disease is associated with the occurrence of the vectors and the prevalence of Anaplasmosis is found higher in hot and humid weather associated with the abundance of ticks (Sajid et al., 2017).
The presence of ticks on animals are an important risk factors for the spread of Theileriosis reported as there is higher prevalence of T. annulata in hot dry summer (Niaz et al., 2021). The pattern of seasonal occurrence of R. appendiculatus (Vector of T. parva) is determined by climate. Rhipicephalus appendiculatus is most active following onset of rain, outbreak of ECF may be seasonal or, where rainfall is relatively constant, may occur at any time [46].
Clinical Findings of Ticks
Tick infestations in cattle are economically important ectoparasite and cause a major impediment to the health and productive performance of cattle in tropical and subtropical area (Desalegn et al., 2024). They can cause fever, anemia, jaundice, anorexia, weight loss, swelling of lymph nodes, dyspnoea, diarrhea, nervous disorders, and even death by affecting the blood and/ or lymphatic system of the animals [6].
It also causes economic loss towards livestock production and has a negative impact on food security, animal product and by products [14]. In Ethiopia, ticks and tick-borne diseases in cattle population cause serious economic loss to smallholder farmers, the tanning industry and the country as a whole through the mortality of infected animals, decreased production, down grading and rejection of hide [24].
Prevention and Control Methods
Vector Control: It is done by repeated treatment of cattle with acaricides in areas of high challenge, such treatment may require to be carried out twice weekly in order to kill the tick before the infective sporozoite develops in the salivary gland [29]. Significant factors currently affecting the control of babesiosis include increased resistance to acaricides by ticks and the numerous draw backs of the current live vaccines (Suarez et al., 2019). Tick-borne pathogen controls include tick control, vaccines against ticks, and parasites and drugs against ticks and parasites [29].
Biological tick control methods: Ticks have numerous natural enemies, but only a few species have been evaluated as tick biocontrol agents. Some laboratory results suggest that several bacteria are pathogenic to ticks, but their mode of action and their potential value as bio-control agents remain to be determined (Uilenberg, G.1994).
Natural enemies of ticks include insectivorous birds, parasitoid wasps, nematodes, Bacillus thuringiensis bacteria, and deuteromycete fungi (largely Metarhizium anisopliae and Beauvaria bassiana). The potential of each of these taxa as bio-control agents will be discussed in turn. Mammals and birds typically consume ticks during self-grooming [40].
Tick vaccine: Tick infestations affect animal health and production worldwide, with an impact of ecto-parasites. Acaricides are a major component of integrated tick control strategies, but their application had limited efficacy in reducing tick infestations and often accompanied by serious drawbacks, it includes the selection of acaricide-resistant ticks, environmental contamination and contamination of milk and meat products with drug residues (Uilenberg, G.1994). All of these issues re-inforce the need for alternative approaches to control tick infestations and pathogen transmission that is the use of vaccines (a vaccine which is prepared from infected ticks) with tick antigens (Suarez et al., 2019).
Breed selection for tick resistance: Indigenous Sanga and Zebu cattle which are predominantly reared by communal farmers have a high degree of tick and tick-borne disease resistance and require minimal tick control methods. This tick control method is suitable and cost effective (minimize) for usages, even farmers (Kaur et al., 2015). Tick resistance has been shown to be heritable reported a heritability estimate of 34% for tick resistance, indicating that genetic improvement through selection should be effective. Resistance of cattle to tick infestation was reported to consist of innate and acquired components [1].
Application of chemicals methods: The use of acaricides in the control of ticks has improved the viability of cattle farming in the tick infested areas. Ticks can be killed by dipping or spraying cattle with an appropriate chemical (acaricides). Ticks can develop resistance to acaricides [29]. Dipping animals are immersed in a dipping tub containing solution of chemicals. Infested cattle should be dipped in the organophosphate acaricide coumaphos (0.3% active ingredient. In general dipping vats provide a highly effective method of treating animals with acaricides for tick control [40].
Spray the application of fluid acaricides to an animal by means of a spray has many advantages and has been successfully practiced for controlling ticks on most of the animals (Solomon and Molla, 2020). Spraying equipment is highly portable, and only small amounts of acaricides need to be mixed for a single application. However, spraying is generally less efficient in controlling ticks than immersion in a dipping vat because of problems associated with applying the acaricides thoroughly on all parts of the animal body (Oundo et al., 2022).
The application of insecticides with aerosols and in oils, smears, and dusts by hand to limited body areas is time-consuming and laborious, but in certain instances it may be more effective and economical (in terms of cost) of acaricides than treating the entire animal [29].
Economic Importance of Tick infestations
In Ethiopia, ticks occupy the first place among the external parasites through mortality of animals, decreased production, downgrading and general rejection of skins and hides [14]. The impacts of ticks on animals were either by inflecting direct damage or by transmission of tick-borne pathogens. They are responsible for severe economic losses both through the direct effects associated with their blood sucking behavior [36] and also indirectly act as reservoirs and vectors for a wide range of human and animal pathogens [32].
Ticks and tick-borne diseases affect 90% of the world’s cattle population and are widely distributed throughout the world [19]. In Ethiopia, ticks and tick-borne diseases in cattle population cause serious economic loss to smallholder farmers, the tanning industry and the country as a whole through the mortality of infected animals, decreased production, down grading and rejection of hide [24]. Generally, ticks could be affecting the market price and decreasing the annual income of humans [31]. The effects of ticks estimated annual loss of US$ 500,000 from hide and skin downgrading and approximately 65.5% of major defects of hides in Eastern Ethiopia [17].
Materials and Methods
Study Area
The study was conducted in Lay Armachiho district in Amhara region, Northwest Ethiopia from February 2023 to April 2024. The administrative zone was selected purposively based on their livestock population agro ecology representation and accessibility.
Lay Armachiho district is found in Central Gondar zone with an area of 1,059.33 square kilometers. It is located in Central Gondar zone, Amhara regional state, Northwest Ethiopia, located at latitude of 13° north and longitude of 37.2° east at an altitude range from 1,500-2,700 meters above sea level. The administrative center of the district is Tikel Dingay, with 29 rural and two town administrative units/kebeles. The agro climatic zone of the area is characterized as highland (7%), midland, (61%) and lowland (32%) with the temperature and rainfall ranges between 18-30°C and 800-1,500 mm, respectively.
It is located 749 kilo-meters away from Addis Ababa, the capital city of the country, and 207 kilo-meters away from Bahir Dar, the capital city of the region (Demeke et al., 2020). The livestock populations of the district were 456,522 (cattle 145,733, sheep 40,917, goats 72,247, horses 330, mules 295, donkeys 20,219 and poultry 197,000) (LALRDO, 2023). A total human population of a district estimated to be 157,836, of whom 79,538 were males and 78,298 females.
Study Population
The study was conducted on local and cross breed of cattle with different age, sex and Body Condition Scores (BCS). Cattle are kept under extensive and semi-intensive management system in which it depends on grazing for their feed sources.
Study Design
A cross-sectional study was conducted from February 2023 to April 2024. to estimate the prevalence of ixodid ticks and identify its associated risk factors in cattle in Lay Armachiho district. Body condition scores of each cattle was evaluated during sample collection and the cattle was classified as emaciated (poor), moderate (medium) and good based on anatomical parts and the flesh and fat cover at different body parts (Nicholson and Butterworth, 1986) (Annex 1). Animals were conveniently classified as young (<3 years) and Adult (>3 years) age categories as described by De-lahunta and Habel (1986).
Sampling Method and Sample Size Determination
The study districts were selected purposively based on their livestock population, agro ecology representation and accessibility. Simple random sampling techniques were used to select study kebeles, villages and animals. Sample size was calculated according to the formula given by Thrusfield (2007) with 95% confidence level and 5% absolute precision.
![]()
Where, n = required sample size
Pexp = expected prevalence
d = desired absolute precision
Thus, a total of 384 both local and cross breed cattle were used for this study.
Data Collection
Tick sample collection and preservation: The entire body surface of the animal was examined thoroughly for the presence or absence of ticks from each body part of animals. Ticks were collected after cattle restrained properly. Then, ticks were collected carefully and gently in a horizontal pull to the body surface of cattle by using forceps and care was taken to avoid decapitulation [62]. The collected ticks were preserved in clean universal bottle containing 70% ethyl alcohol and labeled it. During examination the selected animal’s age, sex, breed, body condition score, tick infestation, date of collection, kebeles and its tick predication sites were recorded on a data recording format designed for this purpose.
Tick identifications: Ticks were identified to the genus and species level according to their morphological key structures such as shape of scutum, leg colour, scutum ornamentation, body grooves, punctuations, basis capitulum, coaxes and ventral plates. During tick identification in the laboratory, the sample was put on Petri dish and adult ticks were identified under a stereomicroscope using the standard identification keys [28,62].
Data Analysis
The collected data was entered into Microsoft Excel, coded and summarized using descriptive statistics. The prevalence was calculated for all data by dividing positive samples over the total number of examined samples and multiplying by hundred. All statistical analyses were done using Stata 17 statistical software. A mixed effect logistic regression model (household was taken as random effect) was used to assess the association of risk factors with the occurrence of hemioparaite infections in cattle. Districts, breed, sex, age category, health status, season, body condition score, tick infestation, communal grazing land and watering points were the predictor variables in which associations examined. Factors with p-value less than 0.25 in the univariable analysis were incorporated into the multivariable mixed effect logistic regression model. In the multivariable mixed effect logistic regression, P-value < 0.05 was considered as statistically significant and Odds Ratio (OR) and 95% CI were also calculated. Correlation, confounding and interaction tests were checked.
Results
Prevalence of Bovine Ixodid tick infestation at Kebele Level
A total of 384 cattle were examined for tick infestation. Of which, 145 cattle were found positive for tick infestation with overall prevalence of 37.76 % was recorded at 95% confidence interval in the study areas. Examined animals were considered to be positive for a given tick infestation when at least one tick was collected from them. Out of the total animals exposed to Ixodid tick infestation 32 (42.1%), 30 (40.5%), 27 (39.7%), 23 (36.5%), 18 (32.14%) and 15 (31.91%) were from Atsemider, Janikaw, Jiha, Shumara lomeye, Kerker and Chira kebeles respectively (Figure 1).
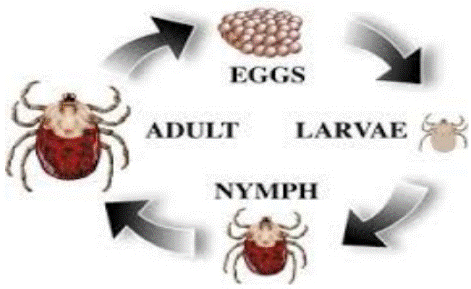
Figure 1: Life cycle of ticks (Walker et al., 2003).
Figure 2: Maps of the study area.
Sources: QGIS software version 3.22
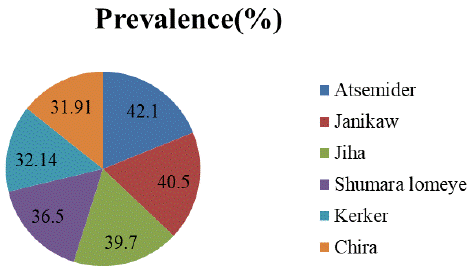
Figure 3: Prevalence of Ixodid tick infestation in cattle at kebele level.
Prevalence of Bovine Ixodid tick infestation at Genus Level
Out of 384 cattle examined, 37.6% were found to be infested with four different genera of ticks. Amblyomma (14.32%) takes the largest infesting ticks in cattle in the study area followed by Boophilus (11.98%), Rhipicephalus (7.55%) and Hyalomma (3.91%) (Table 1).
Districts
Kebeles (Villages)
Cattle population (S)
Sample size (n)
Lay Armachiho
Atsemider
6930
76
Janikaw
6774
74
Jiha
6150
68
Shumara
5705
63
Kerker balegezihaber
5032
56
Chira
4235
47
Total
34,826
384
Sources: (Lay Armachiho district livestock resources and development office, 2024); S = Cattle population, n = sample size.
Table 1: Proportional sampling allocations from the selected kebeles in the study district.
Prevalence of Bovine Ixodid tick infestation at Species Level
Out of 145 infested cattle, 1470 tick species were observed during the study period. Amblyomma variegatum and Boophilus decoloratus were the most abundant tick species with a prevalence of 39.18% and 23.95% in the study area respectively (Table 2).
Tick Genera
Tick counts
Proportion (%)
Amblyomma
55
14.32
Boophilus
46
11.98
Rhipicephalus
29
7.55
Hyalomma
15
3.91
Total
145
37.6
Table 2: Prevalence of Bovine Ixodid tick infestation at Genus Level.
Tick Species
Tick counts
Proportion (%)
Amblyomma variegatum
576
39.18
Amblyomma cohorense
201
13.67
Amblyomma lepidum
56
3.81
Boophilus decoratus
352
23.95
Boophilus evertsi evertsi
251
17.07
Hyalomma marginatum
34
2.31
Total
1470
100
Table 3: Prevalence of Bovine Ixodid tick infestation at species Level.
Tick Species
Tick predilection sites
S/U
Groin
Dewlap
Belly
Neck
Under tail
Legs/hoof
Over all
Amblyomma variegatum
270
164
60
82
-
-
-
576
Amblyomma cohorense
90
65
-
-
-
30
16
201
Amblyomma lepidum
21
16
12
-
-
7
-
56
Boophilus decoratus
42
145
130
-
35
-
-
352
Boophilus evertsi evertsi
27
101
75
48
-
-
-
251
Hyalomma marginatum
8
15
11
-
-
-
-
34
Total
458
506
288
130
35
37
16
1470
S/U =Scrotum or Udder.
Table 4: Distribution of tick species at their predilection sites.
Distribution of Tick Species at Predilection Sites
The distribution of ticks species were widely distributed in different parts of cattle bodies such as scrotum/udder, groin, dewlap, under-tail, belly, legs/hoof and neck. Groin and scrotum/udder is the most favorable tick attachment sites followed by dewlap, belly, neck, under tail and legs/hoof. The most favorable predilection sites for Amblyomma species were the udder/scrotum and groin. Moreover, Boophilus decoloratus was preferred groin, dewlap, and neck. Rhipicephalus evertsi was preferred groin and belly. Other tick species attached other body parts of cattle like under tail and legs/hoofs.
Risk Factors for Ixodid Tick Infestation in cattle
In univariable logistic regression, the risk factors such as study kebeles, breed, sex, age, body condition score; season, agro ecology, communal grazing land and communal watering points were analyzed. Among these factors study kebele, age, body condition score, season, agro ecology, communal grazing land and communal watering points were found to be statistically significant association with Ixodid tick infestation (P<0.25) effects on the occurrence of these infections in cattle. However, the factors considered in the initial univariable logistic regression analysis only breed and sex were removed for multivariable logistic analysis in which p-value greater than 0.25.
For final mixed effect logistic regression model, factors with p-value <0.05 in the multivariable logistic regression analysis were included to fit the model. In the final multivariable logistic regression analysis; communal grazing land, study kebele, agro ecology, body condition score, season and communal watering points were found to be the potential risk factors for the occurrence of Ixodid tick infestation in the study area.
Animals having communal grazing land access had higher prevalence of Ixodid tick infestation (41.04%) compared to absence of communal grazing land access (31.6%). The odds of animals having access of grazing on communal land for the occurrence of tick were 3.26 times more likely of infestation higher than in absence of grazing communal land access (Table 6).
The prevalence of tick infestation was significant (P=0.031) based on the agro ecology of the study animals. The highest prevalence was recorded in animals with a low land agro ecology (54.7%) followed by mid land agro ecology were (44.53%) and the lowest in animals with high land agro ecology were (14.06%).
As indicated in table 5, the prevalence of Ixodid tick infestation in Atsemider, Janikaw and Jiha kebele have nearly similar prevalence such as 42.5%, 40.5% and 39.5% as compared to other study kebeles with statistically significantly (OR= 3.61; 95% CI=0.96-13.55; p=0.05) (Table 6).
Variables
Categories
Number examined
Number positive (%)
OR (95% CI)
P-value
Kebeles
Chira
47
15 (31.91)
Ref.
-
Kerker
56
18 (32.14)
1.09 (0.37 - 3.20)
0.868
Shumara lomeye
63
23 (36.5)
3.29 (1.27 - 8.52)
0.014
Jiha
68
27 (39.7)
3.12 (1.12 - 8.02)
0.018
Janikaw
74
30 (40.5)
9.06 (3.58 - 22.92)
0.001
Atsemider
76
32 (42.1)
5.87 (2.33 -14.75)
0.001
Breed
Cross
109
34 (31.2)
Ref.
-
Local
275
111 (40.36)
1.26 (0.57 - 2.78)
0.562
Sex
Male
174
55 (31.61)
Ref.
-
Female
210
90 (42.9)
0.86 (0.57 -1.30)
0.475
Age
Young
82
24 (29.3)
Ref.
-
Adult
302
121 (40.07)
0.61 (0.38 - 0.99)
0.045
Season
Dry
192
35 (18.2)
Ref.
Wet
192
110 (57.3)
1.68 (1.11-2.54)
0.015
BCS
Good
70
20 (28.6)
Ref.
Medium
215
73 (33.95)
1.92 (1.18 - 3.10)
0.008
Poor
99
42 (42.4)
1.19 (0.65 - 2.17)
0.573
Agro ecology
High land
128
18 (14.06)
Ref.
Mid land
128
57 (44.53)
2.26 (1.28 - 3.99)
0.005
Low land
128
70 (54.7)
6.10 (3.48 - 10.70)
0.001
Communal grazing land
Absent
133
42 (31.6)
Ref.
Present
251
103 (41.04)
2.52 (1.58 - 4.02)
0.001
Communal watering points
Absent
150
50 (33.3)
Ref.
Present
234
95 (40.6)
1.61 (1.04 – 2.51)
0.034
BCS= body condition score, OR= odd ratio, CI= confidence interval, Ref. = Reference
Table 5: Univariable logistic regression analysis of risk factors associated with Ixodid tick infestation in cattle.
The prevalence of Ixodid tick infestation was significant (P=0.012) based on the body condition score of the study animals. The highest prevalence was recorded in animals with a poor body condition (42.4%) followed by medium body condition animals were (33.95%) and the lowest in animals with good body condition scores were (28.6%). According to multivariable logistic regression analysis, the odd of Ixodid tick occurrence in poor and medium body condition score of animals were 1.16 and 1.83 times more likely than good body condition score of animals positive for tick infestation respectively (Table 6).
Variables
Categories
Number examined
Number positive (%)
OR (95%CI)
P-value
Communal grazing land
Absent
133
42 (31.6)
Ref.
-
Present
251
103 (41.04)
3.26 (1.91 - 5.58)
0.001
Agro ecology
High land
128
18 (14.06)
Ref.
-
Mid land
128
57 (44.53)
1.05 (0.09 - 11.90)
0.968
Low land
128
70 (54.7)
2.95 (1.10 - 7.90)
0.031
Kebeles
Chira
47
15 (31.91)
Ref.
-
Kerker
56
18 (32.14)
1.22 (0.40 - 3.73)
0.730
Shumara lomeye
63
23 (36.5)
3.00 (0.21 - 41.71)
0.414
Jiha
68
27 (39.7)
3.78 (0.31 - 46.75)
0.299
Janikaw
74
30 (40.5)
4.34 (1.21 - 15.60)
0.024
Atsemider
76
32 (42.1)
3.61 (0.96 - 13.55)
0.05
BCS
Good
70
20 (28.6)
Ref.
-
Medium
215
73 (33.95)
1.83 (1.06 - 3.16)
0.030
Poor
99
42 (42.4)
1.16 (0.59 - 2.28)
0.672
Season
Dry
192
35 (18.2)
Ref.
-
Wet
192
110 (57.3)
2.23 (1.36-3.65)
0.001
Communal watering points
Absent
150
50 (33.3)
Ref.
-
Present
234
95 (40.6)
1.74 (1.04 - 2.90)
0.034
BCS= body condition score, OR= odd ratio, CI= confidence interval, Ref. = Reference
Table 6: Final multivariable logistic regression model of factors associated with Ixodid tick infestation in cattle.
The season of study animals, the prevalence of Ixodid ticks in cattle was estimated to be higher in wet season (57.3%) compared to dry season (18.2%). The odd of occurrence of tick infestation in wet season were 2.23 times more likely than in dry season and there was statistically significant difference between the two groups wet and dry season (OR=2.23; CI=1.36 – 3.65; P =0.001) (Table 6).
Regarding the prevalence of tick infestation in animals having access of communal water and non-access of communal water in cattle, the higher prevalence was found in sharing access of communal water (40.6%) cattle than non-sharing communal water access (33.3%) cattle. This difference was statistically significant (OR=1.74; 95% CI=1.04- 2.90; P=0.034) (Table 6).
In the present study, the most favorable predilection site for tick species were Groin, scrotum or udder and Dewlap with a proportion of 34.42%, 31.16% and 19.6% respectively as compared to other tick attachment sites. This is due to ticks need smooth body parts and short hairs to attached the host body.
Discussion
In the present study, a total of 1470 ticks were collected from 145 cattle with the overall prevalence of Ixodid tick infestation was found to be 37.76% in cattle in the study districts. The present result coincides with the previous report of Fesseha and Mathewos (2022), Yalew et al. (2017) and Kindalem (2015) in Hadiya zone, Kombolcha and Haramaya town respectively. However, the present finding disagrees with the studies made in Ethiopia by Tiki and Addis (2011), Mekonnen (2017), Misgana (2017) and Meaza et al. (2014) with a prevalence of 25.64%, 56%, 91.5% and 91.7%, respectively. The variation in the prevalence of ticks could be due to the climatic factors which directly influence the vector distribution, agroecology, study design, animal husbandry practices and time of study.
The hard tick prevalence distribution in the present study was varied among genus and species of the hemoparasites. In the current study, Amblyomma, Rhipicephalus, Subgenus Rhipicephalus (Boophilus) and Hyalomma were the four tick genera identified. Amblyomma (14.32%) was the most abundant hard tick genus whereas Hyalomma (3.91%) was the least abundant genus among the identified genera in the study areas. This finding is in agreement with the report of the study done by Amante et al. [4] in Diga, Western Ethiopia. Hyalomma was the least abundant tick species in the study areas with overall prevalence of 4.1%. This report coincides with the previous study with 3% prevalence by Hailu et al. [27] in and around Bishoftu town, Oromia, Ethiopia. However, the current study is disagreed with the study done by Fesseha and Mathewos [22] in Hosanna district, Hadiya zone, Ethiopia stated that Hyalomma was the most abundant tick genus with the prevalence of 11.9%. This variation could be due to the difference of the season of tick collection and agro ecological systems in study areas.
The finding of the present study indicated that Amblyomma variegatum, Amblyomma coherence, Amblyomma lepidum Boophilus decoloratus, Rhipicephalus evertsi evertsi and Hyalomma marginatum are the most abundant tick species in the study districts. On the other hand, Amblyomma lepidum and Hyalomma marginatum were collected in a small occurrence the study area. In this study, Amblyomma variegatum (39.18%) was the most prevalent tick species. This report coincides with a study reported by Bimrew et al. (2015), Bossena and Abdu, (2012) and Nigus and Basaznew (2016), who reported a prevalence of 37.5% in Dangila District, 43.6% in and around Assosa and 51% in Jabitehnan district Northwest Ethiopia. Similarly, the finding of the present study disagrees with the finding of Fanos et al. (2012), Ammanueal & Abdu (2014) and Bekere (2022), who reported 18.1%, 54.9% and 61.8% prevalence in Southern Ethiopia and Awi zone Northwest Ethiopia respectively. Boophilus decoloratus was the second most observable tick species in the study areas with the prevalence of 23.95%. This finding was in line with the previous study reported by Mekonnen (2017), Mohamed et al. (2014) and Bekere (2022), with the prevalence of 18%, 26.3% and 34.6% in North Gondar zone, Haramaya town and Mesela District, respectively. This finding is inconsistent with the previous studies reported by Gurmessa et al. (2015), Tsegaye et al. (2014) and Solomon and Tamirat (2022) with a prevalence of 10.7%, 15.5% and 15.7% in and around Sebeta Town, in and around Haramaya town and Awi zone, respectively. Rhipicephalus evertsi evertsi was the third most abundant tick species in the study areas with a prevalence of 17.07%. This is in accordance with the previous studies who reported by Bossena and Abdu, 2012; Mohamed et al. (2014) with a prevalence range from 15-16%. However, our report is inconsistent with the studies reported by Adugna and Tamirat (2022), Mekonnen (2017), Belay and Eneyew (2016 and Gurmessa et al. (2015), with a prevalence of 7.4%, 11%, 28.6% and 53.4% in Awi zone, North Gondar zone, Sude district and in and around Sebeta town respectively.
Amblyomma cohorense was the fourth most abundant tick species in the study areas with a prevalence of 13.7%. This finding is consistent with the previous study conducted by Adugna and Tamirat (2022), Mekonnen (2017) and Amante et al. (2014) in Awi zone, Diga and North Gondar zone, North West Ethiopia, respectively. The finding of the present study was in strongly disagrees with the previous studies reported by Getachew et al. (2014), Gurmessa et al. (2015) and Nibret et al. (2012) with the prevalence of 1.95%, 2.4% and 5.21% Dembia, Chilga district and in and around Sebeta Town, Ethiopia, respectively
Hyalomma marginatum was the least occurrence of tick species during the study area with 2.31% of prevalence. This finding is agreeing with the previous study reported by Mekonnen (2017), Bossena and Abdu (2012) and Temesgen et al., (2016) in North Gondar zone, Assosa and in and Around Bishoftu Town, Ethiopia respectively. The finding of the present study was strongly disagreeing with the previous studies reported by Gurmessa et al. (2015), Nigus and Basaznew (2016) and Meaza et al. (2014) with a prevalence of 0.8%, 23.5% and 33.1% in Sebeta and Jabitehnan district, Ethiopia respectively. Generally, the difference for the occurrence of tick could be due to the climatic factors which directly influence the vector distribution, agroecology, study design, animal husbandry practices and time of study [2].
Animals having access of grazing on communal land was significantly affected and higher occurrence of tick infestation than animals having no access of grazing on communal and. This could be due to the reason that cattle having access of grazing on communal land have high chance of contact with tick infected animals and tick vectors getting their preferable host to attach the different body parts of animals.
In the present study, the prevalence and abundance of hard tick infestation of cattle was highest in lowland and midland than highland. This finding is coinciding with previous results in Ethiopia reported by Mekonnen et al. (2007). It was due to the fact that lowland and midland agro ecological systems with high temperature and humidity are more suitable for tick proliferation and survival as compared to highland area previously reported by Kumsa et al. (2012). In the present finding, the occurrence of ticks in Atsemider, Janikaw and Jiha kebeles were significantly affected and higher occurrence of tick than other study kebeles. This variation could be due to the difference in cattle management system, burden of tick and agro-climatic condition of the study areas.
In the present study, poor body condition cattle higher number of tick infestation was observed (33.95%), it was agreed with Mekonnen (2017) and Belay and Eneyew (2016), they reported prevalence of 80.7% and 100% in North Gondar zone and Sude district, Arsi Zone, Ethiopia. Because poor body conditioned animals had reduced resistance to tick infestation and they exposed to tick victors easily during grazing on the field than medium and good body conditioned animals.
Ticks are more prevalent in the warmer climates, especially in tropical and sub-tropical areas [6]. In the present study, the occurrence of tick was higher in wet season than dry season. This report was in consistent with the report of Kumsa et al. (2012) that high humidity and temperature are crucial factors that influence the seasonal variation of ticks. However, this study is in disagreement with the study done by Kemal et al. (2016) who reported that there was no considerable difference in the prevalence of ticks within the wet and dry season. Additionally, this finding is in agreement with the study done by Mekonnen et al. (2007) that ticks were found on cattle throughout the study period, although higher tick counts were observed during the rainy than dry season. This might be attributed that Dry environmental conditions are a serious danger to ticks, particularly to the questing larvae, which are very susceptible to drying out fatally [16].
In the present study, higher prevalence was recorded in animals having access of communal watering points (40.6%) than animals having no access of communal watering points (33.3%). This variation could be due to the reason that cattle having access of communal watering points having high chance of contact with tick infected animals and tick vectors that transmit from tick infested to tick non-infested animals.
In the present study, Groin, scrotum or udder and Dewlap were the most favorable predilection site for tick species with a proportion of 34.42%, 31.16% and 19.6% respectively as compared to other tick attachment sites. This is due to ticks need smooth body parts and short hairs to attach the host body (Table 4).
Conclusion and Recommendations
An overall prevalence of Ixodid tick infestation from the present study was 37.76% and this study revealed that tick infestation (Amblyomma, Boophilus, Rhipicephalus and Hyalomma) are important cattle health problems in the study districts. The main risk factors found to be significantly associated with the occurrence of tick infestation were communal grazing land, agroecology, study kebele, body condition season and communal watering points. In this study, a total of four genera and six tick species were identified. Of them Amblyomma variegatum and Boophilus decoloratus were the predominant species encountered. In agro ecological study, tick infestation is prevalent in the lowland and midland as compared to highland areas. Therefore, in conclusion ticks play a major role in reducing production and productivity and cause health problems of cattle.
Based on the above conclusions the following recommendations are forwarded;
¾Strategic tick control measures should be carried out mainly during season of high tick occurrence in order to minimize losses attributed to ticks in the area.
¾Awareness creation should be made about cattle owners for harmful effect of ticks through training.
¾Tick resistance breed is identified to minimize tick infestation problems in high tick burden area.
¾The economic impact and effects of ticks on cattle productivity should be further studied at zonal and regional level.
Author Statements
Acknowledgements
I express my sincere gratitude to my colleagues, for their considerable guidance, appropriate supervision, valuable suggestion and constant encouragement, intellectual advice and devotion of time starting from the title selection to his overall support and precious comments in every step of the paper write-up.
I thank livestock owners for accepting our request and give permission for sampling their cattle and to give response of questionnaires. I would like to thank district animal health personnel and kebele animal health practitioners for their cooperation and technical supports during field work.
I would like to express greatest thanks to Lay Armachiho Woreda Livestock Resources and development Office for their motivation for my research work. I would like to express my gratitude to Lay Armachiho Woreda veterinary clinic in providing laboratory medical equipments and reagents for my research work.
References
- Abdela N, Bekele T. Epidemiology and Control of Bovine Theileriosis in Ethiopia. Review. Journal of Medicine, Physiology and Biophysics. An International Peer-reviewed Journal ticks in North Gondar. Global Veterinaria. 2016; 23: 186-190.
- Abunna F, Kasasa D, Shelima B, Megersa B, Regassa A, Amenu K. Survey of tick infestation in small ruminants of Miesso district, West Hararghe, Oromia Region, Ethiopia. Trop Anim Health Prod. 2009; 41: 969-972.
- Adugna H, Tamrat H. Epidemiological study on Ixodid tick infestation and tick borne haemopathogen on cattle in Awi Zone, northwest Ethiopia. Veterinary Medicine and Science. 2022; 8: 2194-2205.
- Amante M, Alelegn Z, Hirba E. Prevalence of Ixodid ticks on cattle in and around Diga Town, West Ethiopia. European Journal of Biological Sciences. 2014; 6: 25–32.
- Ammanuel W, Abdu M. Prevalence of Ixodid Ticks on Bovine in Soddo Zuria Districts, Wolayita Zone, Ethiopia. Acta Parasitological Globalis. 2014; 5: 188-197.
- Ayana M, Feyisa O. Review on epidemiology of bovine hemoparasites in Ethiopia. 2022.
- Barker S, Murrell A. Systematic and Evaluation of Ticks with a List of Valid Genus and Species Names. Journal of Parasitology. 2004; 129: 15 36.
- Bedasso M, Abebe B, Degife H. Species Composition, Prevalence and Seasonal Variations of Ixodidae Cattle Ticks in and around Haramaya Town, Ethiopia. Full Length Research Paper. 2014; 6: 131-137.
- Bekere H. Identification of Ixodid Tick Species on Bovine in and Around Mesela (Shanan Dhugo) District, Eastern Ethiopia. J Vet Med Health. 2022; 6: 136.
- Belay W, Eneyew M. Identification and Prevalence of hard tick in and around Sude Woreda, Arsi Zone, Ethiopia. 2016: 28.
- Berhane M. Distribution of Livestock Tick Species in Awassa Area. DVM Thesis, AAU, FVM, Debre-Zeit. 2004: 1-16.
- Bossena F, Abdu M. Survey on the distribution of tick species in and around Assosa Town, Ethiopia. Research Journal of Veterinary Science. 2012; 5: 32-41.
- CSA. Agricultural sample survey volume II: report on livestock and livestock characteristics (private peasant holding) in Addis Ababa, Ethiopia. Central statics Agency (CSA). Statistical bulletin. 2021: 589.
- Dabassa G, Shanko T, Zewdie W, Jilo K, Gurmessa G, Abdela N. Prevalence of small ruminant gastrointestinal parasites infections and associated risk factors in selected districts of Bale zone, south eastern Ethiopia. Journal of Parasitology and Vector Biology. 2017; 9: 81–88.
- de Castro JJ. A Survey of the Tick Species in Western Ethiopia Including the Previous Findings and Recommendation, for Further Tick Survey in Ethiopia. FAO, Rome. 1994: 1-83.
- Adugna H, Tamrat H. Epidemiological study on Ixodid tick infestation and tick borne haemopathogen on cattle in Awi Zone, northwest Ethiopia. Veterinary Medicine and Science. 2022; 8: 2194-2205.
- Desalegn T, Fikru A, Kasaye S. Survey of Tick Infestation in Domestic Ruminants of Haramaya District, Eastern Hararghe, and Ethiopia. Journal of Bacteriology & Parasitology. 2015; 6: 246.
- Desalegn T, Fikru A, Kasaye S. Survey of tick infestation in domestic ruminants of Haramaya District, Eastern Hararghe, and Ethiopia. Journal of Bacteriology & Parasitology. 2015: 6.
- Estrada-Peña A. Ticks as vectors: Taxonomy, biology and ecology. Revue Scientifique et Technique. 2014; 34: 53–65.
- Eyob. A Review on the Diagnostic and Control Challenges of Major Tick-Borne Hemoparasite Diseases of Cattle. Journal of Biology, Agriculture and Healthcare. 2015; 5: 1.
- Fanos T, Gezali A, Sisay Girma B, Tariku J. Identification of tick species and their preferred site on cattle’s body in and around Mizan Teferi, Southwestern Ethiopia. Journal of Veterinary Medicine and Animal Health. 2012; 4: 1-5.
- Fesseha H, Mathewos M. Prevalence and identification of bovine Ixodid tick with their associated risk factors in Hosanna District, Hadiya Zone Southern. Acta Scientific Pharmaceutical Sciences. 2018; 4: 20–25.
- Gebre S, Mekonnen S, Kaaya Godwin P, Tekle T, Jobre Y. Prevalence of Ixodid Ticks and Trypanosomiasis in Camels in Southern Rangelands of Ethiopia. Ethiopian Veterinary Journal. 2004; 8: 23-24.
- Getachew A, Mersha C, Desalegn M, Basaznew B. Prevalence of Ixodid Ticks on Cattle in North West Ethiopia. Acta Parasitological Globalis. 2014; 5: 139-145.
- Gurmessa H, Mukarim A, Solomon G, Benti D. Identification of bovine tick species and their prevalence in and around Sebeta Town, Ethiopia. Journal of parasitology and vector biology. 2015; 7: 1-8.
- Fesseha H, Mathewos M. Prevalence and Identification of Bovine Ixodid Tick with their Associated Risk Factors in Hosanna District, Hadiya Zone Southern Ethiopia. Acta Scientific Pharmaceutical Sciences. 2020; 6: 20-25.
- Hailu Y, Yosef D, Nuraddis I. Prevalence and species composition of ticks infesting cattle in and around Bishoftu Town, Oromia Region, Ethiopia. Global Veterinaria. 2016; 16: 238–246.
- Houseman RM. Guide to ticks and tick-borne diseases. University of Missouri Extension, IPM1032. 2013.
- Hussein A. Prevalence of Bovine Babesiosis Associated Risk Factors and Tick Species Identification in Galka-Ayo District, Somalia. Haramaya University. 2022.
- Ikpeze O, Eneanya C, Onyido A. Burden, seasonality, sex ratio and preferred sites of ticks of public health importance on cattle found at amansea. Anambra State Nigeria. 2015; 3: 61-71.
- Jelalu K, Nateneal T, Temesgen T. Infestation and Identification of Ixodid Tick in Cattle: The Case of Arbegona District, Southern Ethiopia. Journal of Veterinary Medicine. 2016: 1-8
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004; 29: S13–S14.
- Kemal J, Nateneal T, Temesgen T. Infestation and identification of Ixodid tick in cattle: The case of Arbegona District, Southern Ethiopia. Journal of Veterinary Medicine. 2016; 2016: 9618291.
- Kindalem B. Prevalence of bovine tick infestation in and around Kombolcha town. DVM thesis, University of Gondar Faculty of Veterinary Medicine. 2015.
- Kumsa B, Socolovschi C, Parola P, Rolain J, Raoult D. Molecular detection of Acinetobacter species in lice and kids of domestic animals in Oromia Regional State, Ethiopia. PLoS One. 2012; 7: e52377.
- Kumsa B, Socolovschi C, Raoult D, Parola P. New Borrelia species detected in Ixodid ticks in Oromia, Ethiopia. Ticks and Tick-borne Diseases. 2015a; 6: 401–407.
- Latif A, Walker A. An Introduction to the Biology and Control of Ticks in Africa. ICTTD-2 Project. 2004: 1-29.
- Lora RB. The Practical Veterinaria, Arthropods. Butterworth-Heinemann, a Member of the Reed Elsevier Group. Library of Congress Cataloging United State of America. 2001: 16-21.
- Gedilu M, Mohamed A, Kechero Y. Determination of the prevalence of ixodid ticks of cattle breeds, their predilection sites of variation and tick burden between different risk factors in Bahir Dar, Ethiopia. Global Veterinaria. 2014; 13: 520-529.
- Mekonnen B. Epidemiology and species identification of Ixodida ticks on cattle in three selected districts of north Gondar zone, northwest Ethiopia. 2017.
- Mekonnen S, Hussein I, Bedane B. The Distribution of Ixodid Ticks (Acari: Ixodidae) in Central Ethiopia. The Onderstepoort Journal of Veterinary Research. 2001; 68: 243-251.
- Mekonnen S, Pegram L, Solomon G, Abebe M, Yilma J, Silesh Z. A synthesis review of Ixodid (Acari: Ixodidae) and Argasids (Acari: Argasidae) ticks in Ethiopia & their possible roles in disease transmission. Ethiopian Veterinary Journal. 2007; 11: 1–24.
- Mesele A, Tirazu M, Rahmeto A, Kassaye A, Jemere B. Survey of Ixodid ticks in domestic ruminants in Bedelle district, Southwestern Ethiopia. Trop Anim Health Prod. 2010; 42: 1677–1683.
- Misgana N. Species diversity and seasonal pattern of ticks and tick-borne pathogens in cattle of Ada’a and Boset districts, central Oromia, Ethiopia. MSc Thesis, Addis Ababa University. 2017.
- Mohamed B, Belay A, Hailu D. Species composition, prevalence and Seasonal variation of Ixodid cattle ticks in and around Haramaya town, Ethiopia. 2014.
- Mohammed H, Moa M, Tilahun B. Study on Prevalence and Identification of Bovine Tick Species in Hetosa District of East Arsi Zone, Eastern Ethiopia. Int J Adv Res Biol Sci. 2018; 5: 105-114.
- Morel P. Manual Tropical Veterinary Parasitological. CAB International, United Kingdom. 1989: 299-460.
- Nejash A. Review of Important Cattle Tick and Its Control in Ethiopia. Open Access Library Journal. 2016; 3: e2456.
- Rahmeto A, Thedrous F, Mesele A, Jemere B. Survey of Ticks Infesting Cattle in Two Districts of Somali Regional State, Ethiopia. Veterinary World. 2010; 3: 539-543.
- Nigus B, Basaznew B. Prevalence of Ixodid Ticks on Cattle in and around Jabitehnan Woreda, North Western Ethiopia. Acta Parasitological Globalis. 2016; 7: 22-26.
- NMA (National Metrological Agency). SNNP Metrological office. 2008.
- Pegram R, Hoogstraal H, Wassef H. Ticks (Acari: Ixodidae) of Ethiopia. Distribution, Ecology and Host Relationship of Tick Species Infecting Livestock. Bulletin of Entomology Research. 1981; 71: 339 359.
- Regassa A. Tick Infestation of Borena Cattle in the Borena Province of Ethiopia. Onderstepoort Journal of Veterinary Research. 2001; 68: 41-45.
- Rodríguez-Vivas R, Mata M, Pérez G, Wagner W. The Effect of Management Factors on the Seroprevalence of Anaplasma marginale in Bos indicus Cattle in the Mexican Tropics. Tropical Animal Health Production. 2004; 36: 135-143.
- Seyoum Z. Study of Ticks and Tick-Borne Diseases on Cattle at Girana Valley in the North Wollo Zone. Proceeding of the Ethiopian Veterinary Association. 2001: 15.
- Sinshaw S. Distribution of Ticks and Tick-Borne Diseases at Metekel Ranch. Ethiopian Veterinary Journal. 2000; 1: 40-59.
- Temesgen T, Yosef D, Nuraddis I. Prevalence and species composition of ticks infesting cattle in and around Bishoftu Town, Oromia Region, and Ethiopia Global Veterinaria. 2016; 16: 238-246.
- Tiki B, Addis M. Distribution of Ixodid ticks on cattle in and around Holeta town, Ethiopia. Global Veterinaria. 2011; 7: 527-531.
- Torr S, Eisler M, Coleman P, Morton J, Machila N. Integrated Control of Ticks and Tsetse. A Report for the DFID Advisory and Support Service Contract, Project ZV. NR International Ltd. 2003: 1-135.
- Torres DF. The Brown Dog Tick Rhipicephalus sanguinus (Acari: Ixodidae): From Taxonomy to Control. Veterinary Parasitology. 2008; 152: 173 185.
- Tsegaye A, Yacob H, Bersissa K. Ixodid ticks infesting cattle in three agro ecological zones in central Oromia: species composition, seasonal variation and control practices. Comp Clin Pathol. 2014; 23: 1103–1110.
- Walker A, Bouattour A, Camicas J, Estrada-Pena A, Horak I, Latif A, et al. Ticks of domestic animals in Africa: A guide to identification of species, Edinburgh, UK. Bioscience Report. 2014: 1–221.
- Walker A, Bouattour J, Camicas A, Estrada Pena I, Horak A, Latif Pegram R, et al. Ticks of domestic animals in Africa: A guide to identification of species. Bioscience Report. 2003: 1-221.
- Yalew A, Adugna S, Keffale M. Identification of major Ixodid ticks on cattle in and around Haramaya Town, Eastern Hararghe, and Ethiopia. Acta Parasitological. 2017; 8: 09–16.