
Review Article
Austin J Vet Sci & Anim Husb. 2017; 4(1): 1030.
Bovine Mastitis and Its Therapeutic Strategy Doing Antibiotic Sensitivity Test
Hossain MK¹*, Paul S², Hossain MM³, Islam MR³ and Alam MGS4
¹Department of Surgery and Theriogenology, Sylhet Agricultural University, Bangladesh
²Department of Public Health and Epidemiology, Sylhet Agricultural University, Bangladesh
³Department of Medicine, Sylhet Agricultural University, Bangladesh
4Department of Surgery and Obstetrics, Bangladesh Agricultural University, Bangladesh
*Corresponding author: Hossain MK. Department of Surgery and Theriogenology, Faculty of Veterinary and Animal Science, Sylhet Agricultural University, Tilagor, Sylhet-3100 Bangladesh
Received: November 23, 2016; Accepted: March 02, 2017; Published: March 10, 2017
Abstract
Mastitis is one of the most costly devastating diseases in the dairy industry. Bovine mastitis can be classified as clinical or subclinical based on clinical features of the disease. Subclinical mastitis occurrence is more prominent compared to clinical form and its prevalence is more than 20 to 50% of cows in given herds. Both forms cause substantial financial losses due to reduced milk yield, degraded milk quality, drug costs and veterinary expenses, early culling of cows and increased labour costs for the farmers. The chief causal organisms of mastitis are cell-walled bacteria, even though mycoplasma, algae and yeast have also been identified to cause mastitis. Numerous influencing factors like physiological, pathological, environmental or genetic factors may contribute to the occurrence of mastitis in dairy cattle. Presently, different types of antibiotics are prescribed for the treatment of mastitis. However, therapeutic success rate of mastitis is generally poor because of pathological changes in the udder parenchyma, mastitogenic bacterial and related factors, indiscriminate use of antibiotics and poor animal husbandry practices. These evidences focus the necessity for discovering completely newer strategies for treatment of mastitis. This review will highlight the key etiological agents and factors affecting the manifestation of mastitis, and the recent advancement of therapeutic strategies including application of antibiotic-based therapies by doing antibiotic sensitivity test as a prospective approach to prevent and control mastitis in dairy herds.
Keywords: Mastitis; Causal agents; Factors; Antibiotics; Sensitivity test
Abbreviations
MIC: Minimum Inhibitory Concentration; SCC: Somatic Cell Count; CMT: California Mastitis Test; kPa: Kilo Pascal; NAGase: N-acetyl-β-D-glucosaminidase; LDH: Lactate Dehydrogenase Activity; EC: Electric Conductivity; MBC: Minimum Bactericidal Concentration, IL: Interleukins; TNF-a: Tumor Necrosis Factor alpha
Introduction
Mastitis is one of the important devastating diseases in cattle striking the greatest challenge to the dairy industry [1,2]. Mastitis remains the most economically destructive and zoonotic imminent disease for consumers irrespective of many years of research worldwide with different levels of economic losses identified by different countries [2-6]. Milk production is reduced considerably in the affected animals and estimated loss of milk yield may range from 100 to 500 kg per cow per lactation [7]. The predictable loss causing clinical mastitis in cows is nearly 700kg in first lactation and 1,200kg in the second lactation [8]. In United States, expenses related to mastitis on dairy farms are around U$ 200 per cow per year and an estimated annual loss of 2 billion dollars for dairy industry [9]. Australian dairy industry is losing more than $130 million every year because of poor udder health causing reduced milk production that is mainly associated with mastitis [10]. In India, annual economic loss suffered by dairy industry owing to udder infections is estimated to be more than $120 million [2,11]. Economic consequences of mastitis, clinical or sub-clinical, include reduced milk yield, poorer quality milk, increased culling rate, increased cost of veterinary services and medicine and increase labor cost for the farmers [12-15]. Bacterial contaminated milk from mastitis infected cows can impede with food manufacturing process or can cause food poisoning; may render unsuited for human consumption, and in some circumstances may have the possibility to transmit disease to humans [16].
Medical therapy involving antibiotics is a key tool in the scheme of mastitis treatment and control. For successful antibacterial mastitis therapy, the active medicine must achieve and conserve concentrations above the minimum inhibitory concentration (MIC) at the center of infection for long enough to breakdown the production and toxin-producing phase of the causal pathogen [17-18]. This may be prohibited by several factors that include pathological changes in the udder parenchyma, mastitogenic bacterial and related factors, poor selection and indiscriminate use of antibiotics, and is also influenced by the route of administration [16]. Enormous economic losses by mastitis are also experienced due to unmarketable milk or milk-products adulterated with antibiotic residues originating from treatment in the developing nations along with usage of antibiotics as growth promoters predominantly in dairy feedlots in the advanced world [20-21]. The long time use of antibiotics in the treatment of mastitis has directed further problem of occurrence of antibiotic resistant strains, therefore there is continual worry about treatment failure and the resistant strains entering the food chain [22-24]. Treatment failures also lead to longer periods of infectivity, which increase the numbers of infected cows moving in the farm and thus expose the whole herd to the risk of contracting a resistant strain of infection. Continual attention has given by the researchers to discover the proper antibiotic to treat and control mastitis doing antibiotic sensitivity test. Therefore, this review will summarize the key etiological agents and factors affecting the manifestation of mastitis, and the recent status of therapeutic strategy including antibioticbased therapies by doing antibiotic sensitivity test as a prospective approach to prevent and control mastitis in dairy herds.
Mastitis Pathogens
Huge quantities of microorganisms have been known to cause bovine mastitis. The major causal microorganisms are bacteria, but mycoplasma, fungi and algae have also been reported to cause mastitis problems in some herds or regions [25-28]. It is very much interesting, about 137 species and subspecies of possible pathogens can be accompanying with infection of the mammary gland [29]. The proportionate importance of the pathogens has varied between countries, regions and farms, mostly owing to differences in management and housing systems [30]. Conversely, mastitis in dairy herds is commonly of two types, these are environmental mastitis and contagious mastitis (Table 1).
Environmental pathogens
Contagious pathogens
Others
References
Escherichia coli
Staphylococcus aureus
Coagulase-negative
staphylococci (CNS)
[31], [32], [33],
[34], [35], [43], [44], [45], [46]
Streptococcus spp.
Streptococcus agalactiae
Streptococcus uberis
Streptococcus dysgalactiae
Pseudomonas spp.
Corynebacteriumspp.
Klebsiella pneumonia
Mycoplasma spp.
Serratiamarcescens
Citrobacterspp.
Enterococcus faecalis
Enterococcus faecium
Proteus spp.
Arcanobacteriumpyogenes
Yeast spp.
Table 1: Pathogens responsible for bovine mastitis in dairy herds.
The primary source of environmental mastitis pathogens is the habitat of the cow like soil, plant material, manure, bedding, and contaminated water or potential pathogens usually in the digestive tract [31,32]. Interaction to environmental pathogens ensues primarily between milking. These microorganisms usually proliferate nearly 1,000,000 or more cells per gram of bedding. This issue increases the possibility of mammary glands infection leading to clinical mastitis [33,34]. There is a progressive association among the number of coliforms existing in the bedding material and the bacterial load on the teat ends along with the incidence rates of clinical mastitis [35]. Primary environmental pathogens include two types of bacteria: coliform bacteria and species of streptococci other than Strep. Agalactiae. Coliforms bacteria chiefly Escherichia coli, Enterobacter aerogenes, Klebsiella pneumonia and Serratia marcescens, and a Streptococcus sp, Streptococcus Uberis are the chief organisms found as the of source environmental mastitis [31,36,37]. Coliform bacteria mostly cause clinical form of mastitis. Environmental mastitis has formerly founded less than 10% of total mastitis cases, but there has been an increase in the occurrence of environmental mastitis more recently [38-40] mostly related with S. Uberis infection. This pathogen is most frequently linked with chronic mastitis, which does not respond to antibiotic treatment [31].
Contagious mastitis is commenced by bacterial pathogens that grow well on the udder skin and lesions of teat. They cannot persist for long times in the environment and are usually spread from one cow to another at milking time through the hands of milkers, the milking machine, milk-contaminated fomites or the sponge [41,42]. The pathogens typically interrelated with contagious mastitis are Staphylococcus aureus and Streptococcus agalactiae [43-45]. Though Streptococcus dysgalactiaeis considered as an environmental pathogen, there is indication of its spread from cow to cow as a contagious pathogen triggering mastitis [43]. These bacteria are common causes of both clinical and sub clinical mastitis. Mycoplasma species are also another source of contagious mastitis. Mycoplasma mastitis may be accessed to the herd by newly purchased animals and can be transmit during milking. Mycoplasma bovis is the chief species occasionally leading to severe complications like abrupt onset, decrease in milk yield, quick transmission and lack of response to treatment [41,46]. Conversely, the most predictable pathogen in the majority of clinical and subclinical mastitis cases in most countries is Staphylococcus Aureus [47-51]. These bacteria are of enormous importance as they are responsible for more than 25% of intra-mammary infections and undesirably distressing the quality of milk in enormous number of clinical cases [52,53]. They are also supposed to be the most emerging pathogens leading bovine mastitis as they are commonly isolated as the topmost bacterial pathogens [51,54].
Risk Factors of Bovine Mastitis
There are plenty of predisposing factors that can influence emergence of mastitis at individual and herd level in dairy cattle. The factors may be physiological, genetic, pathological or environmental [55] which are given below:
Age of cows has significant affects in occurring mastitis. It has been shown that manifestation of mastitis in infected quarters increases with advancement of age in cows [41,56-59], the highest occurrence are being observed in cows of more than 7 years of age [60]. This may be due to more dilated teat canals in older age, permanent udder tissue damage resulting from the primary infection or due to an increased cellular response to intra mammary infection [61,62]. Another reason may be effective innate host defense mechanism that makes the younger animals less susceptible to infection [63]. Moreover, cow’s parity has significant influence of on prevalence of mastitis in farms. Cows in parity number more than 3 have considerably higher mastitis prevalence than those of parity 2-3 and primiparous one [64]. Primiparous cows have stronger defense mechanism than multiparous cows that make them less susceptible to mastitis.
Several genetic traits may also have substantial effect upon susceptibility to mastitis in bovine. These genetic traits are natural resistance of cows, comparative distance between teats, teat shape and conformation, position of udders and milk yield and fat content of milk. High yielding dairy cows with high fat contents are reported to be more vulnerable to mastitis [65-67]. Udder conformation and shape of the teat are significant genetic traits that may also affect susceptibility to mastitis. Cows with extended teats are more susceptible to mastitis infection compared to cows with reversed teat ends [68,69]. An additional important influencing factor for mastitis is super numerous teats, which may be responsible for extra reservoirs for potential pathogens leading to manifestation of mastitis.
Lactation has a highly significant consequence on prevalence of mastitis. In bovine, there is possibility of increased oxidative stress and reduced antioxidant defense mechanisms immediately after parturition, early lactation and during the dry period and the incidence of mastitis is reported to be higher during these times [70]. Somatic cell counts (SCC) mainly neutrophils which is the cow’s natural first line of defense to prepare the onset of the new lactation, seems to be increased immediately after parturition, which remains high for a few weeks regardless of the presence or absence of infection [71-73]. Recent studies have shown that higher amount SCC count in later stage of lactation in cows [74]; possibly signifying increased subclinical infection leading to a decrease in milk production.
Herd management and milk hygiene are also considered significant risk factors for mastitis [64,75]. The occurrence of bovine mastitis is remarkably higher in less clean udder in contrast to clean one. The farms that have followed pre and post teat dipping practice for milking have been less affected by mastitis infection. Several studies have found that left front quarter (LFQ) are more affected by mastitis than the other quarters and may be due to contaminations from the operators left hands without proper washing and disinfection [76,77]. In farms where milking machines are used for milking, it is essential to conserve ideal pressure like 50kPa for most machines, because extra pressure may lead to incomplete milking and tissue damage in the teat [78]. These may cause increased risk of both contagious and environmental mastitis. Therefore, it is better to use those milking machines that can achieve complete milking as well as fewer incidences of teat injuries [79].
The incidence of mastitis is obviously influenced by the weather and climatic conditions. A greater percentage of mastitis has been observed to arise mostly during summer and rainy months [80-83]. Bacterial propagation as well as the load of pathogens increases as heat and humidity increases in the environment [84]. On the contrary, another study has reported a greater occurrence of coliform mastitis during the cold months when the temperature was recorded to be less than 21°C [85,86] in the environment.
Plan of nutrition is another important factor that influences clinical manifestation of mastitis in heifers and cows [87]. Vitamin E has been described to boost the immune response of cows by enhancing the phagocytic properties of neutrophils after parturition [88,89]. Vitamin E combined with selenium acts as an anti-oxidant substance by preventing oxidative stress [90,91]. Numerous investigations have proved that neutrophils of selenium fed cows are more active at killing mastitis triggering microorganisms than those not complemented with selenium [92-94]. Beta-carotene and Vitamin A have also been recognized to be active in inhibiting the manifestation of mastitis, most possibly owing to their antioxidant and immune-enhancing properties to influence mucosal surface reliability of the mammary gland [95,96]. Dairy feed supplemented with Zinc and copper contribute in mammary gland health by promoting cellular repair, wound healing and reduction in SCC [95,97,98] assisted by rises in metallothionein synthesis with antioxidant potential.
Mastitis Diagnostic Approaches
Udder health performance observation is difficult without any reliable and reasonable diagnostic techniques. Hence, there is a continuous necessity for development of advanced diagnostic methods which are readily available, cost effective and more accurate. The key diagnostic tools that are used in detecting mastitis are somatic cell count (SCC) and bacteriological culturing of milk [33,99]. Presently, techniques such as California Mastitis Test (CMT), modified white side test, somatic cell count (SCC), trypsin inhibition test, milk pH, N-acetyl-β-D-glucosaminidase (NAGase), quantification of lactate dehydrogenase activity (LDH), electric conductivity (EC) are less frequently practiced [100]. Diagnosis of mastitis begins with visual distress of the udder and of the milk via fore stripping [101]. If there is any palpable change in quarter or any visible defect in the milk, the affected quarter is defined as clinical mastitis. It is essential not to overlook clinical signs. A rapid, easy and cheap on farm mastitis diagnostic tool is California Mastitis Test. This test can be more readily used by farmers and veterinarians to diagnose and treat the early stages of mastitis and thus devising the ability to stop the magnitude of disease in dairy herds. Increasing the body temperature is one of the significant sign associated with mastitis. Assessment of the intensities of inflammation-linked enzymes might also be used for the recognition of mastitis as these display proper association with SCCs. Additional enzymatic tests comprise the revealing of an esterase secreted by somatic cells using an enzymatic assay on a dipstick is another method of diagnosis. Another assay called bioluminescencedetermination, built on assessment of the ATP concentrations in somatic cells or the response of somatic cell DNA by fluorescent staining, may also be able for trust worthy determination of higher SCC levels and consequently the apparent occurrence of mastitis [102]. Determination of pathogens initiating mastitis is significant for control of disease and epidemiological studies. Bacterial culture can be performed at herd, along with cow and quarter level, each with its individual definite goal. Probable identification of the species is done interpreting cultural, biochemical and serological examination. The usage of molecular diagnostic tools in pathogen finding has advanced over the last years. PCR-based techniques have been defined for a number of mastitis pathogens [103]. Newly, multiplex PCR devices have been developed to detect quite a lot of pathogens at the same time [104]. Moreover, real-time PCR analyses are being advanced for identification and quantification of mastitis pathogens in milk. Twodimensional gel electrophoresis and mass spectroscopy of relevant proteomics techniques have also directed for identification of several new proteins involved in mastitis expressed by invading pathogens. This signal can be useful not only to discover new therapeutic goals but also to search new diagnostic biomarkers. The effective application of these novel biomarker devices are still remains a challenge for clinical mastitis diagnosis. Recent progresses in micro fluidics and so-called ‘Biochips’ have the capability to modernize diagnostic procedure in early diagnosis of mastitis and to discover and develop specific therapeutic strategy [105] (Figure 1).
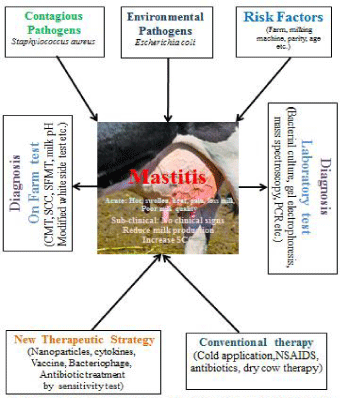
Figure 1: Graphical abstract of bovine mastitis.
Schematic presentation of causes of bovine mastitis and therapeutic
strategies to prevent and control of the disease. Environmental and contagious
pathogens and various risk factors causes mastitis occurrence in dairy herds.
Clinical cause’s pain and swelling of udder and subclinical mastitis reduce
the milk production and increase SCC. Mastitis can be diagnosed on farm
and by laboratory examination. Various conventional treatment procedure
including antibiotics, NSAIDs and new advanced therapeutic strategies like
nanoparticles, vaccines, antibiotic susceptibility testing specific antibiotics are
used to prevent and control bovine mastitis.
Principles of Bovine Mastitis Therapy
Therapeutic success of mastitis depends mainly on accurate diagnosis, severity of udder pathology, drug selection, and relevance of route of administration, supportive treatment, and elimination of predisposing factors [106]. Actually, there is no standard treatment regime yet developed for mastitis; however, it is encouraged to treat clinical forms of mastitis according to the severity of the udder inflammation. Once cows are infected with mastitis, the aim is to diminish the length of infection by treatment of infected cows or culling of cows from the farm. Effective and appropriate treatment of mastitis used all over the season called lactation therapy or dry cow therapy at the end of the season is vital [107].
Before starting antibiotic treatment it should be ensure that the gland is milked out as completely as possible. This will eliminate millions of bacteria, milk clots and debris that can delay the magnitude of antibiotic treatment [106,108]. Some bacteria can also release toxins along with mastitis in infected cows that can make them very sick. In that case, milking out will also remove these toxins. It is recommended milking cows more frequently to remove the infective bacteria and toxins as much as possible. Oxytocin influences contraction of muscle cells that can squeeze milk from the glands and into the udder. The drug helps relief the cow milk out completely if the animal is being exposed out in between regular milking [108].
Bovine mastitis is generally treated by intra-mammary infusion of drugs [109,110]. This route is drug of choice in subclinical, mild or moderately severe form of mastitis, and is practiced as an aid to parenteral administration in severe cases. Some clinicians choose only intra-mammary administration even in severe disease [111]. It is noteworthy, intra-mammary drugs should be dispensed throughout the udder and be quickly immersed into the general blood circulation for effective functions. In severe case, spreading of drugs through the udder may be decreased by inflammation or blockage of milk ducts by debris. In that case, parenteral administration may over whelm these complications, even though it is common to administer agents simultaneously by the intramammary route [112]. Acutely inflamed udders should be milked out repeatedly, with the support of oxytocin if needed. Combined systemic and intramammary treatment with well-suited antibiotics, supplemented with supportive therapy like cold application and anti-inflammatory drug is recommended in peracute or acute clinical mastitis cases with systemic clinical signs [110].
It is commonly desired to treat infectious mastitis consistent with the antimicrobial drug sensitivity outline of the pathogens and clinical experiences [111]. The fundamental rule in selecting the drug is to select as narrow a spectrum as possible against a specific pathogen and shorten side-effects. Initially, broad-spectrum antibiotics must be given on the basis of pharmacokinetic properties and formulation of the drugs [109,113,114]. Response to treatment of disease rises with persistence of the antibiotic in the udder. Therefore, the concentration of the drug must exceed the minimum inhibitory concentration (MIC) value for the pathogen, if possible also the minimum bactericidal concentration (MBC) value in the udder [106]. In case of bacteriostatic drugs, high concentrations in the target organ should be maintained.
Success rate of intravenous or intramuscular therapy in case of mastitis is governed by rate of dispense of drugs from blood to reach into foci of infection. The dispense rate is mainly regulated by degree of ionization, lipid solubility, and the amount of protein-binding of the drug with plasma [115-117] as drugs can cross the blood–milk barrier by passive diffusion passageway [118]. Conversely, a cow that is producing 20kg of milk per day, blood can circulate through her udder approximately 7–10 l/min [119]. Reasonably selected antibacterial can thus be used for systemic administration as a single treatment in acute mastitis. For parenteral mastitis therapy, an model antibiotic should have a low MIC against most of the udder pathogens, abundant bioavailability, acceptable lipid solubility, a low grade of serum protein binding, a long half-life in the body, no distribution to the gastrointestinal tract and similar clearance from organs, tissues and blood [111,114,120]. Immediately after diagnosis, acute mastitis therapy should begin using a remarkably bio available antibiotic, with required supportive drugs [107,121,122]. Treatment duration will depend on disease anamnesis, clinical signs, and microbiological diagnosis. It is not worthy that prolonged treatment means a long milk withdrawal period, which makes the treatment expensive, so treatment duration should not be so long. Chronic mastitis that cannot be cured throughout lactation, culling is the apparent solution. Dairy cows that have had clinical mastitis throughout lactation should be prescribed drying off therapy.
Specific Therapy for Various Forms of Mastitis
Per acute mastitis
Suitable parenteral antibiotic with identical or synergistic action are usually preferred for 3–5 days, depending on clinical cure and special care must be taken to prevent shock. The intravenous route must be chosen to accomplish highest parenchymal diffusion [109,110]. While the response to antibiotic and other supportive therapy is unsatisfactory, extra measures may be necessary to avoid death or to treat a severely affected quarter. When the infected quarter is severely damaged or gangrenous, intra-mammary infusion is unproductive, and if it is economically reasonable teat amputation may support drainage. In severe cases tying off the mammary veins has also been encouraged to decrease the uptake of toxins into the circulation [123].
Acute clinical mastitis
In acute mastitis, recurrent failure of intramammary antimicrobial therapy is due to poor or irregular dissemination of the drug in the acutely swollen udder parenchyma. Parenteral and local antibiotic therapy may be chosen applying either the same antibiotic, or wellsuited antibiotics with identical or synergistic action for 3–5 days, depending on clinical medication. However, intravenous route is the best to achieve extreme parenchymal diffusion [109,110]. Primarily, intramammary antibiotic preparation should be infused in double dose into the exaggerated quarters and a single infusion is suggested into each of the healthy quarters to inhibit new intramammary infection. After the preliminary infusion, intramammary antibiotic preparations should be infused 12-hourly and continued for 3–5 days as recommended by the manufacturer. For enhanced bioavailability of the drug, a great volume should be injected intramuscularly at two sites [114,123-125]. Mastitis caused by Gram-positive bacteria is potentially best treated using macrolide antibiotics and gentamicin, or polymyxin B for Gram-negative infections. Penicillin G is the drug of choice for mastitis infection caused by Clostridium perfringens and Bacillus cereus organism [124,126]. Mastitis caused by penicillinsensitive coagulase-negative staphylococci, S. agalactia and S. dysgalactiaeis usually treated by penicillin for 2–3 days [126]. The organism S. Uberis may be more challenging and treatment should be continued for 3–5 days. The intracellular bacteria Staphylococcus aureus has been identified as therapy-resistant and treatment for 5 days has made better results [126].
In case of mild clinical mastitis, bacteriocidal drugs can be used as intramammary therapy to act only against Gram-positive bacteria. Application frequency of intramammary preparations depends on the pharmacokinetics of the selected drug. Procaine penicillin that are absorbed slowly is sufficient to apply once daily; drugs for example cephalexin, erythromycin that are quickly absorbed must be given twice a day [122]. Frequent intramammary administration of slowrelease formulations is more dynamic than using fast-released osage forms [111]. There are very limited antimicrobial drugs appropriate for treating coliform mastitis. Some drugs for example tetracycline, novobiocin-penicillin, chloramphenicol and streptomycin with high concentrations have been found to suppress phagocytosis in the udder. This consequence is boosted if corticosteroids are integrated with the drugs.
Sub-acute clinical mastitis
It is most predominant form of clinical mastitis in dairy herds where the duration of treatment is based on the causal organism and clinical progress [127,128]. Intra-mammary drugs are rapidly absorbed and usually administered and thus can usually be prescribed for 3 days at 12 hours intervals supplemented by repeated hand stripping to eliminate secretion and debris [111]. After the disappearance of clinical signs [128] treatment should be continued for at least another 24 hours to eliminate the bacterial contamination. In high-yielding dairy cows, 2 syringes of intra-mammary antibiotic preparation may be infused initially into the affected quarter. The introduction of cefoperazone for intra-mammary administration with single-dose treatment is a significant advance to treat sub-acute form of mastitis [129].
Chronic mastitis
In chronic form of mastitis, parenteral and intramammary antibiotic treatment may be used for 3–5 days in combination with anti-inflammatory products, but the prospects remains disappointing. Infusion of a 5% or 10% dextrose solution in combined with antibiotics for 2-3 days at 12 hours intervals has been suggested to which the microbes are sensitive into the affected quarter [123,130] but the prognosis also remains poor. Therefore, it is usually necessary to cull the cow or to destroy the affected quarter by means of infusing concentrated ether to eliminate an important potential source of bacterial infection for healthy quarters [131,132].
Subclinical mastitis
To make a note worthy impact reducing the occurrence of mastitis, it is obligatory to treat subclinical as well as clinical cases. There is usually being 15–40 subclinical cases for every clinic alone. Treatment of infected cows at drying-off with a dry-cow antibiotic preparation is useful and economical. Subclinical mastitis treatment in lactation time is not usually recommended, unless large number of infection rate threatens the selling of milk [120]. The cost of treatment and milk rejection at the time of withdrawal period exceedingly decreases the advantage of the therapy. The microbe that can be treated mainly during lactation is Streptococcus agalactiae, with success rate of medication is usually in the range of 90–95% [128]. The therapeutic success rate for environmental streptococci and other staphylococci may be approximately 10% and will seldom exceed 40–50 %. It is best to treat these cases at drying-off. Generally, antibiotic preparations in the form of intramammary infusion are recommended for 3 days at 12-hourly interval. Remarkably, parenteral antibiotic therapy can be advised when highly infectious bacteria or very valuable dairy cows are affected [123,133]. It is only cost effective to treat subclinical mastitis when the influencing factors are very limited [134,135,136]. In chronic infections, proven by earlier clinical incidents and persistent high SCC, may not respond to dry-cow therapy and should be culled from the dairy herd [128].
Dry cow therapy
Dry cow therapy with antibiotics has been recommended as one of the choices to control intra-mammary infections and inhibit progress of mastitis [137,138]. The cow is at the maximum danger of obtaining new intra-mammary infections with both contagious and environmental pathogens during the dry period [139,140]. It has been shown that about 61% of new infections are achieved during this period [140]. Therapy with antibiotics during dry period is beneficial as there is no need to remove milk from treated quarters. Antibiotics are applied near the end of lactation [141] and may persist in the udder in high concentrations to destroy pathogenic bacteria for 20- 70 days. The applied antibiotic has enhanced penetration capacity because of long time contact and curing intramammary infections unless resistance to novel antibiotics is developed by the entering pathogen [142]. Dry cow therapy has been described to remove nearly 100% of mastitis caused by S. agalactiae [139,143]. Conversely, dry cow therapy is less effective to inhibit S. aureus mastitis compared to streptococcal mastitis [138]. The treatment success rate of dry cow therapy against S. aureus mastitis is about 50% and vaccination against this pathogen throughout the dry season may improve the antibiotic efficiency [140]. Dry cow treatment for a duration of two weeks exhibited substantial decrease in the number of clinical mastitis cases owing to infection with S. dysgalactiae and S. uberis [144].
New Therapeutic Strategies for Mastitis
Nanotechnology
Wide ranges of antibiotics have been used for past 40 years in control of bovine mastitis. However, antibiotic resistance develops mainly due to their overuse and for other possible causes and creates the cure rate very poor. Nanotechnology has endowed researchers to produce nanosized particles less than 100nm in size, apply them in a wide range of sectors, mostly in drug delivery system. The nanoparticles may be revealed prospective delivery methods in the treatment of bovine mastitis from when they are used by phagocytes. For example, silver nanoparticles exhibited inhibitory activity against S. aureus isolated from subclinical mastitis [145] and to be perfect for a wonderfully economical antimicrobial solution. Furthermore, nowadays antibiotics are incorporated in most of the nanoparticles delivery system to combat against microorganisms. Amoxicillin incorporated into nanoparticles exhibited biologically dynamic against S. aureus, E. coli, and S. agalactiae [146]. The key challenge seems to be obtaining an abundant antibiotic loading inside these delivery systems.
Mastitis vaccines
Mastitis vaccines have been in use with the expectation of decreasing the occurrence mastitis in dairy farming and upholding the profit of industry. The practice of killed whole cell vaccines to handle infective organism on-farm in dairy cattle is common like other disease vaccine. A lot of studies have been prepared to produce vaccine against mastitis, but very few have claimed acceptable results. It is important to discover a vaccine capable of protective against an extensive range of strains as multiple strains can be existent within a herd and within a single cow [147]. It is defined that only one vaccine will not prevent mastitis affected by the diverse numbers of pathogens and their different strategies of pathogenesis. Coliform vaccines can decrease the prevalence, severity and length of infections in both milking cows and heifers [148]. Vaccines against S.aureus can decline the occurrence of new intra-mammary contamination as well as increase the impulsive remedy, and should be practiced in herds where this kind of organism is a main issue [149,150]. Conversely, it should be noticed that there are lots of chance of vaccine failure if the farms are not immunized against similar type organism and it will make extra burden for diary industry.
Bacteriophage therapy for mastitis
Bacteriophage therapy is one of the alternate treatments for mastitis which practices pathogen specific bacteriophages in the cure of a bacterial infection. Bacteriophages are viruses capable to invade and destroy bacteria [151]. Phages are proved to be determinants as new antimicrobial agents for veterinary applications. Certain studies specify that phage therapy could create advantageous effect against E.coli and S.aureus influenced mastitis infection [148]. Still, further research is necessary to discover the therapeutic prospective of bacteriophages to treat mastitis associated bacterial infections.
Cytokines
In mammary gland, the roles of the various immune system mechanisms against infection are not successful. Conversely, cytokine generation and leukocyte populations carry out significant roles in host defense system and pathophysiological devices throughout the bacterial infection [148]. Numerous cytokines like complement component, interleukins (IL) and tumor necrosis factor alpha (TNF-a) have been recognized as key actors during the acute-phase reaction. They play chemotactic action liable for leukocyte enrollment and motivation and they can also advance the bactericidal action of phagocytes. Numerous experimental works point out that use of cytokines with or without antibiotics significantly improved the cure rate in S. aureus infected mastitis. Cytokines therapy appears to be a promising method; however, advanced research has to be done to accept therapeutic attention.
Antibiotics therapy doing Antibiotic sensitivity test
Antibiotics have extensively reduced the hazard simulated by mastitis pathogens since their discovery in the 20th century. In the dairy industry, antibiotic treatment is the key strategy for bovine mastitis treatment. On the contrary, beyond the expenses of its usage, the occurrence of resistance and the non-responsiveness of cattle to antibiotics have developed a serious issue. The random and misuse of antibiotics in bovine mastitis may represent intent difficulties linked with the development of antibiotic resistance and the appearance of resistant bacteria in food chain [153]. Furthermore, bacteria, which are accomplished to exist within the cell and mammary gland, and to form abscesses, are further challenging to combat because of the restriction of their interaction with antibiotics. This is the example of S. aureus where the ratio of remedy by presently permitted antibiotics like pirlimycin is approximately 10–30 % [153]. Bannerman and Wall [154] determined that treatment accessible for the treatment of mastitis formed by S. aureus keep at suboptimal and therefore proven ineffective [148,155]. Therefore, there is an urgent need to find effective tool to apply specific antibiotic which can effectively prevent and control bovine mastitis. Currently, Antibiotic susceptibility testing against microbes is considering as one of the choosing methods to treat bovine mastitis in dairy herds (Table 2).
Antibiotics
Percentage of sensitivity to pathogens
References
S.aureus
CNS staph.
S. agalactiae
S.dysagalactiae
S.uberis
E.coli
K. pneumoniae
Penicillin
86.84%
87.14%
100%
92%
100%
4%
0%
[165], [170], [171]
Ampicillin
8.33%
20%
100
71.4
44%
[165], [166], [170], [172]
Amoxicillin
78.95%
87.14%
100%
71.4%
100%
18%
-
[165], [170]
Tetracycline
70.4%
80%
60%
40%
57.1%
40%
-
[172]
Gentamicin
97.8%
42%
40%
42%
-
94.4%
98.7
[163], [164], [171]
Ciprofloxacin
64%
70%
75%
73%
-
66%
50%
[163]
Neomycin
28%
29%
0%
50%
-
41%
0%
[163]
Amoxicillin + clavulanat
94.74%
98.57%
100%
100%
100%
94%
62.6%
[165], [171]
Cloxacillin
92.11%
97.14%
100%
94.5%
80%
2%
0%
[165], [171]
Streptomycin
71.05%
85.71
66.67%
0%
33.33%
84%
0%
[163], [165], [172]
Enrofloxacin
97.37%
97.14%
100%
75%
100%
100%
33%
[163], [165]
Erythromycin
51.8%
60%
80%
60%
20%
20%
0%
[163], [172]
Ceftifour
94.74%
97.14%
100%
100%
100%
100%
-
[165], [170]
Sulfamethoxazole
99.3%
88%
98.6%
96.2%
96.9%
88.9%
95.9%
[171], [172]
Table 2: Antibiotic sensitivity of different microbial pathogens to different groups of antibiotics causes mastitis in cows.
The practice of these drugs joined with improvements in housing, sanitation, nutrition and the introduction of extensive vaccination programs for key environmental pathogens, has managed to infinite fall in occurrence of mastitis that was previously prevalent and frequently fatal. These advances are now a days extremely threatened by emergence and spread of new microbes those are resistant to economical and effective first-line drugs. Beta-lactamase producing bacteria S. aureus and B. fragilis candiminish the activity of penicillin. Various mastitogenic strains of B. fragilis are unaffected to ampicillin, amoxicillin and cephalotine [134,156], and certain strains yield cephalosporinase, which diminishes them resistant to cephalosporin [157]. The microbe P. aeruginosa is not susceptible to ampicillin, cefoxitin, cephalothin, cefamandole oroxacillin [157]. Thus mastitis caused antibiotic resistant microbes fail to respond to treatment, ensuing constant illness and more threat of cull or death. Presently, there is also evidence of multiple drug resistance in the common bacterial agents liable for mastitis in bovines. Treatment failure also result increase number of infected cows affecting the farm and thus express the whole herd to the danger of contracting a resistant strain of infection [158,159]. Significant concern has been stretched over the last few years regarding the regulation of antibiotic use, due to the worrying rise of bacterial resistance. Antibiotic sensitivity testing is regarded as method of choice to treat the mastitis infected cows in dairy herds.
Antibiotic susceptibility can be done using a series of identical testing techniques [160]. Among them two most commonly used procedures are agar disc diffusion (ADD) also known as the Kirby- Bauer method and the broth microdilution method, called as Sensititre system [161]. Both microdilution delivers a direct extent of the minimum inhibitory concentration (MIC) that inhibits bacterial progression beneath distinct in vitro environments [160]. Antibiotic concentrations are usually diluted by 2-fold serial dilution in broth microdilution technique. In contrast ADD results are specified as inhibition of zone diameter growth measured in millimeters [160]. Consequences of MIC testing are used in founding clinical breakpoints for deciding susceptibility and are associated with ADD procedures for microbial species [160]. Recently, Saini et al. [161] reported that ADD and the “Sensititre” techniques has moderate to high diagnostic accuracy in selecting antibiotics against udder pathogen for mastitis treatment. Susceptibility testing is directed when a clinician is unable to assume antimicrobial susceptibility against a pathogen based on his clinical knowledge [160,162]. Alekish et al. [163], analyzed 205 mastitis cases and isolated 11 different pathogens of which S. aureus had the highest incidence and all isolates were examined for antimicrobial susceptibility test. The highest percentage of sensitivity was to enrofloxacinamongst all of the pathogens, followed by ciprofloxacin, gentamycin and neomycin. However, the highest rate of resistance among the isolates was found against trimethoprim/sulfamethoxazole and penicillin. In another study Chauhan et al. [164] identified antibiotic sensitivity by disc diffusion technique by means of Mueller-Hinton agar, the basis of zone of obstruction and revealed that the highest sensitivity was found in gentamicin followed by Ceftriaxone and Enrofloxacin. The cow was cured with intra-mammary and systemic injections of gentamicin for 5 successive days and exhibited recovery within few days. Moreover, a total of 390 milk samples were analyzed by Idriss et al., [165] and found that highest sensitivity was found against Streptococcus agalactiae and Streptococcusuberis by enrofloxacin, followed by Escherichia coli, coagulase Negative Staphylococci and Staphylococcus aureus using the combination therapy of cefalexin and kanamycin and combination of amoxicillin and clavulanate. Maximum resistance was observed against penicillin and streptomycin. Ikiz et al., [166] revealed investigation to identify the manifestation of contagious mastitis agents Streptococcus agalactiae and Staphylococcus aureus in 270 bovine milk samples collected from 132 dairy cows. Antibiotic susceptibilities of the isolates were examined by disk diffusion method and found that all of the S. aureus isolates were resistant to ceftiofur and all of the S. agalactiae isolates were resistant to streptomycin. The multiple drug resistance amongst the cultures involved in mastitis has also been described by several other investigators [33,148]. Most of the cultures exhibited resistance to the third generation cephalosporins. This excessive advent of resistance to cephalosporin’s is unpleasant owing to its extensive usage against microbes as per adapted by most of the clinicians as the first line of treatment in recent years. Therefore, the probable longterm haphazard usage of these antibiotics has directed to the advent of resistance in mastitogenic bacteria counter to third generation cephalosporin [167,168]. Increase of resistance among bacteria is a matter of noticeable alarm in the field of veterinary medicine specifically when the organisms render an animal unproductive. It is clinically thoughtful to practice susceptibility outcomes to guide treatment results for some pathogens [169]. Thus, constant screening of cultures for antibiotic susceptibility outline will be advantageous for picking a suitable antibiotic and also to recognize the shifting drifts of antibiotic resistance for evolving antibiotic usage strategy and for controlling the indiscriminate practice of influential antibiotics like cephalosporin as primary treatment.
Conclusions
Bovine mastitis is truly important production disease due to its impact in the quality and quantity of milk production. The dairy industry suffers from substantial financial losses due to mastitis all over the world. Practice of hygienic methods during milking, milk collection, lactation and dry cow therapy, dietary supplements and culling are likely to reduce but not control the incidence of both clinical and subclinical mastitis. The effects of mastitis on dairy cattle health and milk production highlight an urgent need to develop effective strategy of prevention and control. Antibiotic therapy is a dynamic component in mastitis control programs. Therefore, it is of fundamental significance to detect what type of disease causing organism are predominant in each dairy farm and what type of medicines can be used to prevent and control mastitis. Antibiotic sensitivity test is thus playing a key role to treat the mastitis case more effectively.
References
- Albenzio M, Taibi l, Muscio A. and Sevi A. Prevalence and etiology of subclinical mastitis in intensively managed flocks and related changes in the yield and quality of milk. Small Ruminant Res. 2002; 43: 219- 226.
- Dua K. Incidence, aetiology and estimated loss due to mastitis in India- An update. Ind Dairy man. 2001; 53: 41-48.
- Singh PJ, Singh PB .A study of economic losses due to mastitis in India. Ind J Dairy Sci. 1994; 47: 265-272.
- Bramley AJ, CullorJS, Erskine RJ, Fox LK, Harmon RJ, Hogan JS, et al. Current concepts of bovine mastitis, 4th Ed. 1996. National Mastitis Council, Madison, WI.
- Fang WH, Hu SH, Jiang CS, Huang IQ, Liu HR. Bovine mastitis - a Chinese approach to its prevalence, causal pathogens, losses and control. Proceedings of the 6th International Symposium on Veterinary Epidemiology and Economics. Ottawa, Canada, 1991.
- Swinkels JM, Hogeveen H, Zadoks RN. A partial budget model to estimate economic benefits of locational treatment of subclinical Staphylococcus aureus mastitis. J Dairy Sci. 2005; 88: 4273-4287
- NAAS. Mastitis Management in Dairy Animals. Policy Paper No. 61, National Academy of Agricultural Sciences, New Delhi, India. 2013.
- Wilson DJ, González RN, Hertl J, Schulte HF, Bennett GJ, Schukken YH, et al. Effect of clinical mastitis on the lactation curve: A mixed model estimation using daily milk weights. J. Dairy Sci. 2004; 87: 2073-2084.
- Bogni C, Odierno L, Raspanti C, Giraudo J, Larriestra A, Reinoso E, Lasagno M, et al. War against mastitis: Current concepts on controlling bovine mastitis pathogens. Science against microbial pathogens: communicating current research and technological advances. A. Méndez-Vilas (Ed.). 2011.
- Dairy Australia. Dairy Australia Strategic Plan. 2011; 2011-2015.
- Tuteja FF, Kapur MP, Sharma A, Vinajaka AK. Studies on bovine subclinical mastitis: Prevalence and microflora. Indian Vet J. 1993; 70: 787-791.
- Brightling P, Mein GA, Malmo J, Ryan DP. Countdown down under: farm guidelines for mastitis control. In Countdown Downunder: Dairy Research and Development Corporation. 1998.
- Seegers H, Fourichon C, Beaudeau F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res. 2003; 34: 475–491.
- Chagunda MGG, Friggens NC, Rasmussen MD, Larsen T: A Model for Detection of Individual Cow Mastitis Based on an Indicator Measured in Milk. J Dairy Sci. 2006; 89: 2980–2998.
- Halasa T, Nielen M, De Roos APW, Van Hoorne R, de Jong G, Lam TJGM, et al. Production loss due to new sub-clinical mastitis in Dutch dairy cows estimated with a test-day model. J Dairy Sci. 2009; 92: 599–606.
- Tiwari JG, Babra C, Tiwari HK, Williams V, Sharon DW, Gibson J, et al. Trends In Therapeutic and Prevention Strategies for Management of Bovine Mastitis: An Overview. J Vaccin. 2013; 4: 2.
- Thomson K, Rantala M, Hautala M, Pyörälä S, Kaartinen l. Cross-sectional prospective survey to study indication-based usage of antimicrobials in animals: Results of use in cattle. BMC Vet. Res. 2008; 4: 15.
- Kaneene JB, Miller R: Description and evaluation of the influence of veterinary presence on the use of antibiotics and sulfonamides in dairy herds. J. Am. Vet.Med. Assoc. 1992; 201: 68-76.
- Sharma N, Gautam A, Upadhyay SR, Hussain K, Soodan JS, Gupta SK. Role of antioxidants in udder health: a review. Indian J. Field Vet. 2006; 2: 73–76.
- Gogoi J. Public health importance of Staphylococcus aureus isolated from cases of bovine mastitis and milkers. MVSC thesis. Assam Agricultural University, India. 1997.
- Zafalon LF, Nader Filho A, Oliveira JV, Resende FD. Subclinical mastitis caused by Staphylococcus aureus: Cost benefit analysis of antibiotic therapy in lactating cows. Arq Bras Med Vet Zootec. 2007; 59: 577-585.
- White DG, McDermott PF. Emergence and transfer of antibiotic resistance. J Dairy Sci. 2001; 84: E151-E155.
- Virdis S, Scarano C, Cossu F, Spanu V, Spanu C, et al. Antibiotic Resistance in Staphylococcus aureus and Coagulase Negative Staphylococci Isolated from Goats with Subclinical Mastitis. Vet Med Int. 2010; 517060.
- Collins NA, Moses M, Moneoang SM, Corneleus B .Antibiotic-resistant Staphylococcus aureus isolated from milk in the Mafikeng Area, North West province, South Africa. South Afr J Sci. 2010; 106: 1-6.
- Sharif A, Umer M, Muhammad G. Mastitis control in dairy production. J Agril Soc Sci, 2009; 5: 102-105.
- Wilson DJ, Gonzalez RN, Das HH. Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J Dairy Sci. 1997; 80: 2592-2598.
- Barkema HW, Schukken YH, Lam TJ, Beiboer ML, Wilmink H, et al. Incidence of clinical mastitis in dairy herds grouped in three categories by bulk milk somatic cell counts. J Dairy Sci. 1998; 81: 411-419.
- Fox LK, Kirk JH, Britten A. Mycoplasma mastitis: a review of transmission and control. J Vet Med B Infect Dis Vet Public Health. 2005; 52: 153-160.
- Watts JL. Etiological agents of bovine mastitis. Vet Microbiol.1988; 16: 41-66.
- Hogeveen H, Pyorala S, Waller KP, Hogan JS, Lam JGMT, Oliver SP, et al. In Proceedings of the National Mastitis Council 50th Annual Meeting: Arlington. Canada, 2011.
- Jones GM. Understanding the basics of mastitis. Virginia Cooperative Extension, Publication No. 404-233, Virginia State University, USA, 2006; 1-7.
- Philpot WN, Nickerson SC. Mastitis: Counter Attack. Westfalia Surge LLC. Illinois. USA. 1999.
- El-Hamid MIA. Bovine Mastitis: Current Concepts and Future Control Approaches. Austin Clin Microbiol. 2016; 12: 1007.
- Bradley AJ, Green MJ. Clinical mastitis in dairy cows after ‘blitz’ therapy. Vet Rec. 1997; 141: 179-180.
- Hogan JS, Smith KL, Hoblet KH, Todhunter DA, Schoenberger PS, et al. Bacterial counts in bedding materials used on nine commercial dairies. J Dairy Sci. 1989; 72: 250-258.
- Boyer PJ. Outbreak of clinical mastitis in dairy cows following ‘blitz’ therapy. Vet Rec. 1997; 141: 55.
- Edmondson PW. Clinical mastitis in dairy cows after ‘blitz’ therapy. Vet Rec. 1997; 141: 108.
- Bradley AJ, Green MJ. Adaptation of Escherichia coli to the bovine mammary gland. J. Clin Microbiol. 2001; 39: 1845-1849.
- Bradley AJ, Leach KA, Breen JE, Green LE, Green MJ. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Vet Rec. 2007; 160: 253-257.
- Dairy Australia. Environmental mastitis increasing. The Land, 2011.
- Harmon RJ. Physiology of mastitis and factors affecting somatic cell counts. J Dairy Sci. 1994. 77: 2103-2112.
- APHIS (Animal and Plant Health Inspection Service) Info sheet. Prevalence of contagious mastitis pathogens on U.S. dairy operations, 2007. USDA. 2008.
- Smith KL, Hogan JS. Epidemiology of mastitis. Proceedings of 3rd International Mastitis Seminar, 1995.Tel Aviv, Israel. 1995; S6: 3-12.
- Barkema HW, Schukken YH, Zadoks RN. Invited Review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J Dairy Sci. 2006; 89: 1877-1895.
- Monecke S, Kuhnert P, Hotzel H, Slickers P, Ehricht R. Microarray based study on virulence-associated genes and resistance determinants of Staphylococcus aureus isolates from cattle. Vet Microbiol. 2007; 125: 128-140.
- BurgtGVD, Main W, Ayling R. Bovine mastitis caused by Mycoplasma bovis. Vet Rec. 2008; 163: 666.
- Workineh S, Bayleyegn M, Mekonnen H, Potgieter LN. Prevalence and aetiology of mastitis in cows from two major Ethiopian dairies. Trop Anim Health Prod. 2002; 34: 19-25.
- Barrett DJ, Healy AM, Leonard FC, Doherty ML. Prevalence of pathogens causing subclinical mastitis in 15 dairy herds in the Republic of Ireland. Ir Vet J. 2005; 58: 333-337.
- Middleton JR. Staphylococcus aureus antigens and challenges in vaccine development. Expert Rev Vaccines. 2008; 7: 805-815.
- Unnerstad HE, Lindberg A, Persson Waller K, Ekman T, Artursson K, et al. Microbial aetiology of acute clinical mastitis and agent-specific risk factors. Vet Microbiol. 2009; 137: 90-97.
- Pereira UP, Oliveira DG, Mesquita LR, Costa GM, Pereira LJ. Efficacy of Staphylococcus aureus vaccines for bovine mastitis: a systematic review. Vet Microbiol. 2011; 148: 117-124.
- Owens WE, Watts JL, Boddie RL, Nickerson SC. Antibiotic treatment of mastitis: comparison of intramammary and intramammary plus intramuscular therapies. J Dairy Sci.1988; 71: 3143-3147.
- Haveri M. Staphylococcus aureus in bovine intramammary infection: molecular, clinical and epidemiological characteristics. Academic dissertation, submitted to Department of Production Animal Medicine, Faculty of Veterinary Medicine, University of Helsinki, Finland. 2008.
- Pyörälä S, Taponen S. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet Microbiol. 2009; 134: 3-8.
- Sordillo LM. Factors affecting mammary gland immunity and mastitis susceptibility. Liv Prod Sci. 2005; 98: 89-99.
- Emanuelson U, Persson E. Studies on somatic cell counts in milk from Swedish dairy cows. I. Non-genetic causes of variation in monthly test-day results. Acta Agri Scand. 1984; 34: 33-44.
- Busato A, Trachsel P, Schällibaum M, Blum JW. Udder health and risk factors for subclinical mastitis in organic dairy farms in Switzerland. Prev Vet Med. 2000; 44: 205-220.
- Sharma H, Maiti SK, Sharma KK. Prevalence, etiology and antibiogram of microorganisms associated with sub-clinical mastitis in buffaloes in Drug, Chhattisgarh state. Intl J Dairy Sci. 2007; 2: 145-151.
- Sharma N, Maiti SK. Incidence, etiology and antibiogram of sub clinical mastitis in cows in drug, Chhattisgarh. Ind J Vet Res. 2010; 19: 45-54.
- Schukken YH, Grommers FJ, Van de Geer D, Brand A. Incidence of clinical mastitis on farms with low somatic cell counts in bulk milk. Vet Rec. 1989; 125: 60-63.
- Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Mastitis. In: Veterinary Medicine, A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. 9thEdn, W B Saunders Co. Philadelphia, USA. 2000; 603–612.
- Rahman AM. Bhuiyan MU, Kamal MM, Shamsuddin M. The Bangladesh Veterinarian. 2009; 26: 54 – 60.
- Dulin AM, Paape MJ, Nickerson SC. Comparison of phagocytosis and chemiluminescence by blood and mammary gland neutrophils from multiparous and nulliparous cows. Am J Vet Res. 1988; 49: 172-177.
- Sarker SC, Parvin MS, Rahman AK MA and Islam MT. Prevalence and risk factors of subclinical mastitis in lactating dairy cows in north and south regions of Bangladesh, Tropical Animal Health and Production. 2013; 45: 1171-1176.
- Rajala PJ, Gröhn YT. Disease occurrence and risk factor analysis in Finnish Ayrshire cows. Acta Vet Scand. 1998; 39: 1-13.
- Grohn YT, Erb HN, McCulloch CE, Saloniemei HS. Epidemiology of mammary gland disorders in multiparous Finnish Ayrshire cows. Prev Vet Med. 1990; 8: 241-252
- Lactation Resource Library. Other Factors Affecting Milk Yield and Composition. 2009.
- Rathore AK. Relationships between teat shape, production and mastitis in Friesian cows. Br Vet J. 1976; 132: 389-392.
- Seykora AJ, McDaniel BT. Udder and teat morphology related to mastitis resistance: a review. J Dairy Sci. 1985; 68: 2087-2093.
- Sharma N, Singh NK, Singh OP, Pandey V, Verma PK. Oxidative stress and antioxidant status during transition period in dairy cows. Asian-Aust J Anim Sci. 2011; 24: 479-484
- Bradley A, Green MJ. Use and interpretation of somatic cell count data in dairy cows. In Pract. 2005; 27: 310-315.
- Berry, D.P., Lee, J.M., Macdonald, K.A., Stafford, K., Matthews, L. and Roche, J.R. Associations among body condition score, body weight, somatic cell count, and clinical mastitis in seasonally calving dairy cattle. J Dairy Sci. 2007; 90: 637-648.
- Plastridge WN. Bovine mastitis: A review. J Dairy Sci. 1958; 41: 1141-1181.
- Peeler EJ, Green MJ, Fitzpatrick JL, Morgan KL, Green LE. Risk factors associated with clinical mastitis in low somatic cell count British dairy herds. J Dairy Sci. 200; 83: 2464-2472.
- Kivaria FM, Noordhuizen JPTM, Kapaga AM. Risk factors associated with sub-clinical mastitis in smallholder dairy cows in Tanzania. Tropic Anim Prod. 2004; 36: 581–592.
- AbdullahiSJ, Jibril A, Mohammed AA, FasinaFO. Sub-clinical mastitis and associated risk factors on lactating cows in the Savannah Region of Nigeria. BMC Vet Res. 2012; 8: 134
- Mgedela RH, Ryoba R, Karimuribo ED, Phiri EJ, Loken T, Reksen O et al. Prevalence of clinical and sub-clinical mastitis and quality of milk on smallholder dairy farms in Tanzania. J South African Vet Assoc. 2009; 80: 163–168.
- Blood DC, Radostits OM .Veterinary medicine. In: textbook of the diseases of cattle, sheep, pigs, goats and horses, (7th edn), WB Saunders Co. Philadelphia, USA.1989; 501.
- Mein GA, Schuring N. Lessons from scrapbooks and scrapheaps of history. Bulletin-International Federation. 2003.
- Reneau J. Gear up for warm weather mastitis management now. Dairy Star. 2012
- Costa EO, Ribeiro AR, Watanabe ET, Melville PA. Infectious bovine mastitis caused by environmental organisms. Zentralbl Veterinarmed B. 1998; 45: 65-71.
- Akyuz A, Boyaci S, Cayli A. Determination of critical period for dairy cows using temperature humidity index. J Anim Vet Adv. 2010; 9: 1824-1827.
- Ribeiro AR, Garinojr F, Silva JAB, Watanabe ET, Vallue CR, et al. Seasonality on the occurrence of environmental bovine mastitis. Nopgama. 2001; 4: 19-22.
- Godden S, Rapnicki P, Stewart S, Fetrow J, Johnson A, et al. Effectiveness of an internal teat seal in the prevention of new intramammary infections during the dry and early-lactation periods in dairy cows when used with a dry cow intramammary antibiotic. J Dairy Sci. 2003; 86: 3899-3911.
- Shathele MS. Weather effect on bacterial mastitis in dairy cows. Intl J Dairy Sci. 2009; 4: 57-66.
- Ranjan R, Gupta MK, Singh KK. Study of bovine mastitis in different climatic conditions in Jharkhand, India. Vet World. 2011; 4: 205-208.
- Heinrichs AJ, Costello SS, Jones CM. Control of heifer mastitis by nutrition. Vet Microbiol. 2009; 134: 172-176.
- Spears JW, Weiss WP. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet J. 2008; 176: 70-76.
- Hogan JS, Weiss WP, Todhunter DA, Smith KL, Schoenberger PS. Efficacy of an Escherichia coli J5 mastitis vaccine in an experimental challenge trial. J Dairy Sci. 1992; 75: 415-422.
- Chamberlain AT, Wilkenson JM. Feeding the dairy cow. Chalcombe Publications. 1996; 90-91.
- Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000; 346: 1-8.
- Erskine RJ, Eberhart RJ, Grasso PJ, Scholz RW. Induction of Escherichia coli mastitis in cows fed selenium-deficient or selenium-supplemented diets. Am J Vet Res. 1989; 50: 2093-2100.
- Grasso PJ, Scholz RW, Erskine RJ, Eberhart RJ. Phagocytosis, bactericidal activity, and oxidative metabolism of milk neutrophils from dairy cows fed selenium-supplemented and selenium-deficient diets. Am J Vet Res. 1990; 51: 269-274.
- Underwood EJ, Suttle NF. In: The Mineral Nutrition of Livestock. Underwood EJ and Suttle NF (eds), CABI Publishing, New York. 1999.
- Sordillo LM, Shafer-Weaver K, DeRosa D. Immunobiology of the mammary gland. J Dairy Sci. 1997; 80: 1851-1865.
- Chew BP. Role of carotenoids in the immune response. J Dairy Sci. 1993; 76: 2804-2811.
- Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004; 37: 1182-1190.
- Bruno DR. Mastitis, Mammary Gland Immunity, and Nutrition. Mid- South Ruminant Nutrition Conference 19 Arlington, Texas. 2010.
- Bogni C, Odierno L , Raspanti C, Giraudo J , Larriestra A , Reinoso E, et al. War against mastitis: Current concepts on controlling bovine mastitis pathogens. Science against microbial pathogens: communicating current research and technological advances. Formatex. 2011.
- Joshi S, Gokhale S. Status of mastitis as an emerging disease in improved and periurbandairy farms in India. Ann New York Acad Sci. 2006; 1081: 74-83.
- Reneau JK. Somatic cell counts: measures of farm management and milk quality. In: Proceedings of National Mastitis Council Ann. 2001. Mtg. p. 29-37. Reno, NV.
- Frundzhyan V, Parkhomenko I, Brovko L, Ugarova N. Improved bioluminescent assay of somatic cell counts in raw milk. J Dairy Res. 2008; 75: 279-83.
- Zadoks R, Schukken Y. Use of molecular epidemiology in veterinary practice. Veterinary Clinics of North America: Food Animal Practice. 2006; 22: 229-261.
- Phuektes P, Browning G, Anderson G, Mansell P. Multiplex polymerase chain reaction as a mastitis screening test for Staphylococcus aureus, Streptococcus agalactiae, Streptococcus dysgalactiaeand Streptococcus uberisin bulk milk samples. J Dairy Res. 2003; 70: 149-155.
- Viguier C, Arora S, Gilmartin N, Welbeck K, O’Kennedy R. Mastitis detection: current trends and future perspectives. Trends in Biotech. 2009; 27: 8-10.
- Du Preez JH. Bovine mastitis therapy and why it fails. Tydskr.S.Afr.vet.Ver. 2000; 71: 201–208.
- Du Preez JH. Reasons why mastitis therapy is unsuccessful. S. Afr J Dairy Sci. 1989; 21: 21–29.
- Barlow J. Mastitis Therapy and Antimicrobial Susceptibility: a Multispecies Review with a Focus on Antibiotic Treatment of Mastitis in Dairy Cattle. J Mammary Gland BiolNeoplasia. 2011; 16: 383–407.
- MacDiarmid S C. Drugs used in the antibacterial therapy of mastitis. Proceedings of a post-graduate short course. 1980, Palmerston North, Massey University, New Zealand. 1980: 103–110.
- Ziv G. Practical pharmacokinetic aspects of mastitis therapy – 1: parental treatment. Agri Practice: 1980a; 227–290.
- Prescott JF, Baggot JD. Antimicrobial therapy in veterinary medicine. Blackwell Scientific Publications, Boston. 1988.
- Du Preez JH, Giesecke WH. Mastitis. In Coetzer JAW, Thomson GR, Tustin RC (eds) Infections diseases of livestock with special reference to southern Africa, Vol. 2. Oxford University Press, Cape Town South Africa. 1994; 1564–1595.
- Du Preez JH. Speenkanaalinfeksies by melkkoeie: diagnose, voorkoms, aard, terapie, omvang en betekenis. DVScthesis, University of Pretoria. 1985.
- Ziv G. Drug selection and use in mastitis: systemic vs local therapy. J Amer Veter Medi Assoc. 1980d; 176: 1109–1115.
- Rasmussen F. Studies on the mammary excretion and absorption of drugs. Carl F Mortensen, Copenhagen. 1966.
- Rasmussen F. Excretion of drugs in milk. In Brodie BB, Gillette J (eds) Handbook of experimental pharmacology. Springer Verlag, New York. 1971; 28: 390–402.
- Sjoqvist E, Borga O, Orme ML. Fundamentals of clinical pharmacology drug treatment. In Avery G S (ed.) Drug treatment. Adis Press, Sydney. 1976: 1–42.
- Miller GE, Banerjee NC, Stowe CM. Diffusion of certain weak organic acids and bases across the bovine mammary gland membrane after systemic administration. J Pharm and Ther. 1967; 157: 245–253.
- Giesecke WH. Bovine mastitis. Department of Agricultural Technical Services, Republic of South Africa, Technical Communication No. 1979; 151: 1–37.
- Sandholm M, Honkanen-Buzalski T, Kaartinen L, Pyörälä S. The bovine udder and mastitis. Gummerus Kirjapaino Oy, Helsink. 1995.
- Wilson CD. Antibiotic therapy in mastitis control. In Bramley A J, Dodd F H, Griffen T K (eds) Mastitis control and herd management. Technical Bulletin 4, National Institute for Research in Dairying, Reading. 1980. 113–127.
- Pyörälä S. Therapy of clinical mastitis. In Sandholm M, Honkanen-Buzalski T, Kaartinen L, Pyörälä S (eds) The bovine udder and mastitis. Gummerus Kirjapaino Oy, Jyväskylä. 1995; 201–208.
- Robinson TC. Therapy for acute and peracute mastitis. In Bramley AJ, Dodd FD, Griffen TR (eds) Mastitis control and herd management. Technical Bulletin 4, National Institute for Research in Dairying, Reading. 1980; 128–134.
- Jackson E, Bramley J. Coliform mastitis. In Practice 5. 1983; 135–146.
- MacDiarmid SC. Antibacterial drugs used against mastitis in cattle by the systemic route. N Zeal Veter J. 1978; 26: 290–295
- Ziv G. Practical pharmacokinetic aspects of mastitis therapy 1: parental treatment. Agri Practice. 1980; 227–290.
- DuPreez JH. Planne teen hoë SST in kuddes. Landbouweekblad. 1999;1094: 24–26.
- Philpot WN, Nickerson SC. Mastitis:counter attack. Babson Bros, Naperville. 1991.
- Wilson CD, Agger N, Gilbert GA, Thomasson CA, Tolling ST. Field trials with cefoperazone in the treatment of bovine clinical mastitis. The Veter Recor. 1986; 118: 17.
- Schalm OW, Carroll EJ, Jain NC. Bovine mastitis. Lea &Febiger, Philadelphia. 1971
- DuPreez JH. Reasons why mastitis therapy is unsuccessful. S Afr J Dairy Sci. 1989; 21: 21–29.
- DuPreez JH. Planne teen hoë SST in kuddes.Landbouweekblad. 1999; 1094: 24–26.
- Moore GA, Heider LE. Treatment of mastitis. Veterinary Clinics of North America: Large Animal Practice. 1984; 6: 323–333.
- DuPreez JH. The role of anaerobic bacteria in bovine mastitis: a review. J S AfrVeter Ass. 1989;60: 159–168.
- Giesecke WH, DuPreez JH, Petzer IM. Practical mastitis control in dairy herds. Butterworths, Durban. 1994.
- Platonow I, Blobel H. Therapeutic failures in chronic staphylococcal mastitis. 1963. 142; 1097.
- Oliver SP, Sordillo LM. Udder health in the periparturient period. J Dairy Sci.1988; 71: 2584-2606.
- Ziv G, Storper M, Saran A. Comparative efficacy of three antibiotic products for the treatment and prevention of subclinical mastitis during the dry period. Vet Q. 1981; 3: 74-79.
- Oliver SP, Mitchell BA. Susceptibility of bovine mammary gland to infections during the dry period. J Dairy Sci. 1983; 66: 1162-1166.
- Todhunter DA, Smith KL, Hogan JS, Schoenberger PS. Gram-negative bacterial infections of the mammary gland in cows. Am J Vet Res. 1991; 52: 184-188.
- JanosiSz, Huszenicza G. The use of the dry cow therapy in the control of bovine mastitis. Vet Med-Czech 2001; 46: 55-60.
- Babra C, Gogoi TJ, Pier G, Thein TH, Sunagar R, et al. The persistence of biofilm-associated antibiotic resistance of Staphylococcus aureus isolated from clinical bovine mastitis cases in Australia. Folia Microbiol. 2013; 58: 1.
- Bramley AJ, Dodd FH. Reviews of the progress of dairy science: mastitis control--progress and prospects. J Dairy Res. 1984; 51: 481-512.
- Hassan Z, Daniel RC, O’Boyle D, Frost AJ. Effects of dry cow intramammary therapy on quarter infections in the dry period. Vet Rec. 1999; 145: 635-639.
- Dehkordi SH, Hosseinpour F, KahrizangiAE. An in vitro evaluation of antibacterial effect of silver nanoparticles on Staphylococcus aureus isolated from bovine subclinical mastitis. Afr J Biotechnol. 2011; 10: 10795–10797.
- Xue FY. Post-antibiotic effect of amoxicillin nanoparticles against main pathogenic bacteria of bovine mastitis in vitro. J Northwest Sci-Tech UnivAgric For. 2009; 37: 1–6.
- Bannerman DD, Wall RJ. A novel strategy for the prevention of Staphylococcus aureus-induced mastitis in dairy cows. Information Systems for Biotechnology (isb) news report. 2005; 1–4.
- Gomes F,Henriques M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr Microbiol. 2016 ; 72: 377–382.
- Hoedemaker M, Korff B, Edler B, Emmert M, Bleckmann E. Dynamics of Staphylococcus aureus infections during vaccination with an autogenousbacterin in dairy cattle. J Vet Med. 2001; 48: 373–383.
- Kazemi J, Ahmadi M, Saei HD, Adibhesami M. Antibacterial effect of silver nanoparticles along with protein synthesis-inhibiting antibiotics on Staphylococcus aureus isolated from cattle mastitis. Biol J Microorg. 2014; 2: 15–22.
- Chibani-Chennoufi S, Dillmann ML, Marvin-Guy L, Rami-Shojaei S, Bru¨ssowH. Lactobacillusplantarum bacteriophage LP65: a new member of the SPO1-like genus of the family Myoviridae. J Bacteriol. 2004; 186: 7069–7083.
- Alluwaimi AM. The cytokines of bovine mammary gland: prospects for diagnosis and therapy. Res Vet Sci. 2004; 77: 211–222.
- White DG, McDermott PF. Emergence and transfer of antibiotic resistance. J Dairy Sci. 2001; 84: E151–E155.
- Bannerman DD, Wall RJ. A novel strategy for the prevention of Staphylococcus aureus-induced mastitis in dairy cows. Information Systems for Biotechnology (isb) news report. 2005; 1–4.
- Atulya M, Mathew AJ, Rao JV, Rao CM. Influence of milk components in establishing biofilm mediated bacterial mastitis infections in cattle: a fractional factorial approach. Res Vet Sci. 2014; 96: 25–27.
- Du Preez JH. Comparison of various criteria for determining the health status of the bovine udder. J S Afr Veter Asso. 1987; 58: 9–14.
- Finegold SM, Rosenblatt JE, Sutter VC, Atteberry HR. Anaerobic infections. In Thomas B A (ed.) Scope monograph on anaerobic infections (3rd edn). The Upjohn Company, Kalamazoo, Michigan. 1976.
- Lipsitch M, Singer RS, Levin BR. Antibiotics in agriculture: when is it time to close the barn door? Proc Natl Acad Sci USA. 2002; 99: 5752–5754.
- Sischo WM. Stakeholder position paper: dairy producer. Prev Vet Med. 2006; 73: 203–208.
- CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: approved standard. M31-A3, 3rd ed. Wayne, PA: CLSI; 2008.
- Saini V, Riekerink RG, McClure JT, Barkema HW. Diagnostic accuracy assessment of Sensititre and agar disk diffusion for determining antimicrobial resistance profiles of bovine clinical mastitis pathogens. J Clin Microbiol. 2011; 49: 1568–1577.
- Walker RD. Antimicrobial susceptibility testing methods and interpretation of results. 4th ed. Antimicrobial Therapy in Veterinary Medicine. Ames, Iowa: Blackwell. 2006.
- Alekish1 MO, Al-qudah KM, Al-saleh A. Prevalence of antimicrobial resistance among bacterial pathogens isolated from bovine mastitis in northern Jordan. Revue Méd. Vét. 2013; 164: 319-326.
- ChauhanPM, Thumar HK,Bhagat A, Sharma VK, Chauhan HC, Patel MR. Comparative efficacy of antibiotic sensitivity tests for management of acute clinical Escherichia Coli mastitis in crossbred cow. J. Livestock Sci. 2016; 7: 41-45.
- Idriss SE, Foltys V, TaninV, Kirchnerová V, TaninovD , Zaujec K. Mastitis pathogens and their resistance against antimicrobial agents in dairy cows in nitra, slovakia. Slovak j. Anim. Sci. 2014; 47: 33-38.
- Ikiz S , Saran BB, Bingöl EB, Çetin Ö, Kasikçi G, Özgur NY, et al. Presence and antibiotic susceptibility patterns of contagious mastitis agents (Staphylococcus aureusand Streptococcus agalactiae) isolated from milks of dairy cows with subclinical mastitis. 2013; 37: 569-574.
- Hornish RE &Kotarski SF. Cephalosporins in veterinary medicine—Ceftiofur use infood animals. Curr top med chem, 2002; 2: 717–731.
- Nora Mestorino and Jorge O. Errecalde. Pharmacokinetic – Pharmacodynamic Considerations for Bovine Mastitis Treatment. A Bird's-Eye View of Veterinary Medicine Edited by Dr. Carlos C. Perez-Marin.www.intechopen.com. 2012.
- Waller KP, HårdemarkV, Nyman AK, Duse A. Veterinary treatment strategies for clinical mastitis in dairy cows in Sweden.Veter Rec. 2016; 178: 240.
- Cameron M, Saab M, Heider L, McClure JT, Rodriguez-Lecompte JC, J Sanchez J. Antimicrobial Susceptibility Patterns of Environmental Streptococci Recovered from Bovine Milk Samples in the Maritime Provinces of Canada. Front Vet Sci. 2016; 15: 79.
- Rocha B,Mendonça D, Niza-Ribeiro J. Trends in Antibacterial Resistance of Major Bovine Mastitis Pathogens in Portugal. RCPV. 2014; 109: 79-88.
- Moges N, Asfaw Y, Belihu K, Tadesse A. Antimicrobial susceptibility of mastitis pathogens from small holder dairy herd in and around Gondar, Ethiopia. J AnimVeter Ad. 2011; 10: 1616-1622.