
Review Article
J Vet Sci & Anim Husb. 2017; 4(2): 1035.
Review on Bovine Babesiosis and Its Economical Importance
Jemal Jabir Yusuf*
School of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia
*Corresponding author: Jemal Jabir Yusuf, School of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia
Received: May 10, 2017; Accepted: June 28, 2017; Published: July 05, 2017
Abstract
Babesiosis is a tick-borne disease of cattle caused by the protozoan parasites. The causative agents of Babesiosis are specific for particular species of animals. In cattle: and B. bigemina are the common species involved in babesiosis. Rhipicephalus (Boophilus) spp, the principal vectors of B. bovis and B. bigemina, are widespread in tropical and subtropical countries. Babesia multiplies in erythrocytes by asynchronous binary fission, resulting in considerable pleomorphism. Babesia produces acute disease by two principle mechanism; hemolysis and circulatory disturbance. Affected animals suffered from marked rise in body temperature, loss of appetite, cessation of rumination, labored breathing, emaciation, progressive hemolytic anemia, various degrees of jaundice (Icterus). Lesions include an enlarged soft and pulpy spleen, a swollen liver, a gall bladder distended with thick granular bile, congested dark-coloured kidneys and generalized anemia and jaundice. The disease can be diagnosis by identification of the agent by using direct microscopic examination, nucleic acid-based diagnostic assays, in vitro culture and animal inoculation as well as serological tests like indirect fluorescent antibody, complement fixation and Enzyme-linked immunosorbent assays tests. Babesiosis occurs throughout the world. However, the distribution of the causative protozoa is governed by the geographical and seasonal distribution of the insect vectors. Recently Babesia becomes the most widespread parasite due to exposure of 400 million Cattle to infection through the world, with consequent heavy economic losses such as mortality, reduction in meat and milk yield and indirectly through control measures of ticks. Different researches conducted in Ethiopia reveal the prevalence of the disease in different parts of the country. The most commonly used compounds for the treatment of babesiosis are diminazene diaceturate, imidocarb and amicarbalide. Active prevention and control of Babesiosis is achieved by three main methods: immunization, chemoprophylaxis and vector control.
Keywords: Babesia; Protozoa; Tick; Vector control
Introduction
Ethiopia has a high livestock population, but productivity is low as a result of diseases, malnutrition and other management problems. Parasitism is one of the major problems to livestock development in the tropics [1].
Babesiosis is a dangerous, invasive disease of humans and animals. Probably the first described case of an epidemic caused by the Babesia genus was a cattle mortality described in the biblical Book of Exodus [2]. In 1888, Victor Babes described intraerythrocytic microorganisms responsible for the death of 50 thousand cattle in Romania and classified them as Bacteria. In 1893, Kilborne and Smith described a factor of Texas cattle fever, giving them the rank of genus and name Babesia as classifying them as Protozoans [3]. Parasites of the genus Babesia infect a wide variety of domestic and wild mammals as well as man [4].
B. bovis and are the common species that affect cattle. Both species belong to the phylum Apicomplexa with B. bovis causing more severe disease than [5]. In 1981 Purnell wrote “Bovine babesiosis caused by B. divergens, also known as redwater fever, is considered the most important tick-transmitted disease in cattle” [6].
Bovine babesiosis is caused by protozoan parasites of the genus Babesia, order Piroplasmida, phylum Apicomplexa. Tick species are the vectors of Babesia [7]. Tick-borne pathogens affect 80% of the world’s cattle population and are widely distributed throughout the world, particularly in the tropics and subtropics [8]. A major tick vector in Australia and Africa for both species is Boophilus microplus, while B. bigeminais also transmitted by B. decloratus and Rhipicephalus spp. in Africa [9].
Disease is characterised by fever, weakness, ataxia, haemoglobinuria, anaemia and presence of intra-erythrocytic parasites [10]. Giemsa stained blood smear examination is the most widely used method for the diagnosis of tick-transmitted diseases. Microscopic examination of blood smear is relatively inexpensive and less time consuming but this method is less sensitive and specific. The effective diagnosis depends on personal ability and experience [11].
However, the major impact occurs in the cattle industry and the species affecting bovines are the most studied, including Babesia bovis, and B. divergens [12]. Since the times of Babes, Smith and Kilborne, Bovine babesiosis has had a huge economic impact due to loss of meat and beef production of infected animals and death. Nowadays to those costs there must be added the high cost of tick control, disease detection, prevention and treatment [13].
Control of Bovine babesiosis can be either by tick management, immunization, and anti-Babesia drugs or by a combination of these approaches [14]. Chemotherapy of babesiosis is important for controlling the disease either to treat field cases or to control artificially induced infections [15]. In the past, treatment of cattle babesiosis was less important than disease eradication, principally in countries were the goal was to eradicate the tick vector; however, chemotherapy has been important to control and prevent babesiosis in some areas of the world [16].
The objective of this seminar paper is to review available literature on Bovine babesiosis with special attention to B.bovis and B.bigemina.
Bovine Babesiosis
Etiology
The genus Babesia belongs to the phylum Apicomplexa, class Sporozoasida, order Eucoccidiorida, suborder Piroplasmorina and family Babesiidae [17]. Criado-Fornelio et al. [18] used the 18s rRNA gene for phylogenetic analysis and divided Babesia species from ungulates as: B. caballi, B. bigemina, B. bovis and Babesia sp. From cattle (proposed name for the group, without taxonomic value: Ungulibabesids) (Table 1).
Babesia species
Major ixodid vectors
Known distribution
Boophilus microplus
Boophilus decoloratus
Boophilus annulatus
Boophilus geigyi
Africa, Asia, Australia, Central and
South America and Southern Europe
Babesia bigemina
Babesia bovis
Rhipicephalus evertsi
Boophilus microplus,
Boophilus annulatus,
As for Babesia bigemina, but less
widespread in Africa due to B. microplus competition with, B. decoloratus
Babesia divergens
Boophilus geigyi
Ixodes ricinus
Ixodes persulcatus
North-west Europe, Spain,
Great Britain, Ireland
Babesia major
Haemaphysalis punctata
Europe, North west Africa, Asia
Babesia ovis
Haemaphysalis longicornis
Eastern Asia
Table 1: Major Babesia species infective to cattle, their ixodid tick vectors and geographical distribution (Adapted from [72]).
The causative agents of Babesiosis are specific for particular species of animals. In cattle: B. bovis, B bigemina, B. divergens and B. major [19]. B. ovis and B. motasi are known to be pathogenic agents in sheep and goats [20]. B. bovis and are the common species that affect cattle. Both species belong to the phylum Apicomplexa with B. bovis causing more severe disease than B.bigemina [5].
Babesia bovis is a small parasite, usually centrally located in the erythrocyte. It measures approximately 1– 1.5 μm long and 0.5–1.0 μm wide, and is often found as pairs that are at an obtuse angle to each other. Babesia bigemina is a much longer parasite, and is often found as pairs at an acute angle to each other. Babesia bigemina is typically pear-shaped, but many diverse single forms are found. It is 3–3.5 μm long and 1–1.5 μm wide, and paired forms often have two discrete red-staining dots in each parasite (B. bovis and B. divergens always have only one) [21].
Life cycle
Babesia multiplies in erythrocytes by asynchronous binary fission, resulting in considerable pleomorphism. This replication eventually gives rise to gametocytes that are ingested by the vector tick. Conjugation of gametocytes occurs in the tick gut followed by multiplication by multiple fission and migration to various tissues including the salivary glands. Further development occurs in the salivary glands before transmission. The ovaries are also invaded, which leads to transovarial transmission [22]. The host gets the infection when the larva sucks blood. After one moulting the larva transforms into nymph which also infect as larva. Nymph transforms into adult after moulting and they transmit infection in similar way [23]. The infective stage of Babesia, sporoziote, enters in to the host when the tick sucks blood [24] (Figure 1).
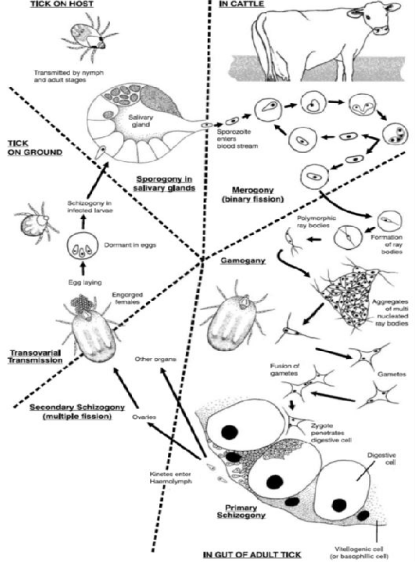
Figure 1: The development life cycle of Babesia bigemina in cattle and the ixodid tick vector Boophilus microplus as currently understood (adapted; [73]).
Pathogenesis
Babesia produces acute disease by two principle mechanism; hemolysis and circulatory disturbance [25]. During the tick bite, sporozoites are injected into the host and directly infect red blood cells. In the host, Babesia sporozoites develop into piroplasms inside the infected erythrocyte resulting in two or sometimes four daughter cells that leave the host cell to infect other erythrocytes [26].
It invades erythrocyte and cause intravascular and extravascular hemolysis [25].The rapidly dividing parasites in the red cells produce rapid destruction of the erythrocytes with accompanying haemoglobinaemia, haemoglobinuria and fever. This may be so acute as to cause death within a few days, during which the packed cell volume falls below 20% which will lead to anemia. The parasitaemia, which is usually detectable once the clinical signs appear, may involve between 0.2% upto 45% of the red cells, depending on the species of Babesia [27].
Cytokines and other pharmacologically active agents have an important function in the immune response to Babesia. The outcome is related to the timing and quantity produced, but their overproduction contributes to disease progress causing vasodilatation, hypotension, increased capillary permeability, oedema, vascular collapse, coagulation disorders, endothelial damage and circulatory stasis [28].
Although stasis is induced in the microcirculation by aggregation of infected erythrocytes in capillary beds, probably the most deleterious pathophysiological lesions occur in the brain and lung. This can result in cerebral babesiosis and a respiratory distress syndrome associated with infiltration of neutrophils, vascular permeability and oedema. Progressive haemolyticanaemia develops during the course of B. bovis infections. While this is not a major factor during the acute phase of the disease, it will contribute to the disease process in more protracted cases [29].
Clinical sign
Affected animals suffered from marked rise in body temperature, loss of appetite, cessation of rumination, labored breathing, emaciation, progressive hemolytic anemia, various degrees of jaundice (Icterus) from paleness in mild cases to severe yellow discoloration of conjunctival and vaginal mucous membranes in more progressive cases; haemoglobinuria, accelerated heart and respiratory rates, ocular problems and drop in milk production. The fever during infections in some cases cause abortion to pregnant cattle [30]. Coffee colored urine is the characteristics clinical feature of Babesiosis [23]. Babesia parasites cause both acute and persistent subclinical disease in cattle [31].
Babesia bovis infections are characterized by high fever, ataxia, anorexia, general circulatory shock, and sometimes also nervous signs as a result of sequestration of infected erythrocytes in cerebral capillaries. Anaemia and haemoglobinuria may appear later in the course of the disease. In acute cases, the maximum parasitaemia (percentage of infected erythrocytes) in circulating blood is less than 1%. This is in contrast to infections, where the parasitaemia often exceeds 10% and may be as high as 30%. In infections, the major signs include fever, haemoglobinuria and anaemia. Intravascular sequestration of infected erythrocytes does not occur with infections. The parasitaemia and clinical appearance of B. divergens infections are somewhat similar to infections [32].
The acute disease generally runs a course of 3 to 7 days and fever (>40°C) is usually present for several days before other signs become obvious. This is followed by inappetence, depression, increased respiratory rate, weakness and a reluctance to move. Haemoglobinuria is often present; hence, the disease is known as redwater in some countries. Anaemia and jaundice develop especially in more protracted cases. Muscle wasting, tremors and recumbency develop in advanced cases followed terminally by coma [33]. Cerebral babesiosis is manifested by a variety of signs of central nervous system involvement and the outcome is almost invariably fatal [33].
Pathological lesions
Lesions include an enlarged soft and pulpy spleen, a swollen liver, a gall bladder distended with thick granular bile, congested darkcoloured kidneys and generalized anaemia and jaundice. Other organs may show congestion or petechial haemorrhages and occasionally there will be pulmonary oedema. The grey matter surface of the brain can appear pink. Acute cases will show haemoglobinuria, but this may be absent in subacute or chronic cases. Clinical pathology centres on a haemolytic anaemia, which is characteristically macrocytic and hypochromic. Haematological, biochemical and histopathological changes are described by de Vos & Potgieter [33].
Diagnosis
Identification of the agent
Direct microscopic examination: The traditional method of identifying the agent in infected animals is by microscopic examination of thick and thin films stained with Giemsa, a Romanowsky type stain (10% Giemsa in phosphate buffered saline (PBS) or Sorenson’s buffer at pH 7.4). The sensitivity of thick films is such that it can detect parasitaemias as low as 1 parasite in 106 red blood cells (RBCs) [34]. Species differentiation is good in thin films but poor in the more sensitive thick films. This technique is usually adequate for detection of acute infections, but not for detection of carriers where the parasitaemias are mostly very low. Parasite identification and differentiation can be improved by using a fluorescent dye, such as acridine orange, instead of Giemsa [34].
Nucleic acid-based diagnostic assays: Nucleic acid-based diagnostic assays are very sensitive particularly in detecting B. bovis and in carrier cattle [35]. Polymerase chain reaction (PCR-based techniques are reported to be as much as 1000 times more sensitive than microscopy for detection of Babesia spp., with detection at parasitaemia levels ranging from 0.001% to 0.0000001% (1 parasite in 109 RBCs) [35]. A number of PCR techniques have been described that can detect and differentiate species of Babesia in carrier infections [36]. PCR assays to differentiate isolates of B. bovis have also been described. The application of the reverse line blot procedure, in which PCR products are hybridised to membrane-bound, speciesspecific oligonucleotide probes, to Babesia [37] and, more recently, two quantitative PCR methods [38] have enabled the simultaneous detection of multiple species, even in carrier state infections. However, current PCR assays generally do not lend themselves well to largescale testing and at this time are unlikely to supplant serological tests as the method of choice for epidemiological studies. PCR assays are useful as confirmatory tests and in some cases for regulatory testing.
In vitro culture: In vitro culture methods have been used to demonstrate the presence of carrier infections of Babesia spp. [39], and B. bovis has also been cloned in culture. The minimum parasitaemia detectable by this method will depend, to a large extent, on the facilities available and the skills of the operator [34], but could be as low as 10–10 [40], making it a very sensitive method, with 100% specificity, for the demonstration of infection.
Animal inoculation: Confirmation of infection in a suspected carrier animal can also be made by transfusing approximately 500ml of jugular blood intravenously into a splenectomised calf known to be Babesia-free, and monitoring the calf for the presence of infection. This method is cumbersome and expensive, and obviously not suitable for routine diagnostic use. Mongolian gerbils (Merione sunguiculatus) have been used to demonstrate the presence of B. divergens [32].
Serological tests: The indirect fluorescent antibody (IFA) test was widely used in the past to detect antibodies to Babesia spp., but the test has poor specificity. Cross-reactions with antibodies to B. bovis in the B. bigemina IFA test were a particular problem in areas where the two parasites coexist. The IFA test also has the disadvantages of low sample throughput and subjectivity [41]. The complement fixation (CF) test has been described as a method to detect antibodies against B. bovis and [42]. This test has been used to qualify animals for importation into some countries.
Enzyme-linked immunosorbent assays (ELISA) have largely replaced the IFA as the diagnostic test of choice for Babesia spp. because of the objectivity in interpretation of results and capacity to process high numbers of samples daily. An ELISA for the diagnosis of B. bovis infection that uses a whole merozoite antigen has undergone extensive evaluation [43].
Other serological tests have been described in recent years, and include a dot ELISA [44], a slide ELISA [45], latex and card agglutination tests [46,47] and an immunochromatographic test [48].
Differential diagnosis: Other conditions that should be considered and may resemble babesiosis are anaplasmosis, trypanosomiasis, theileriosis, leptospirosis, bacillary hemoglobinuria, haemobartonellosis and eperythrozoonosis.
Epidemiology
Babesiosis occurs throughout the world (Fahar et al.2012). However, the distribution of the causative protozoa is governed by the geographical and seasonal distribution of the insect vectors [19]. The vector of Babesia, Boophilus microplus is wide spread intropics and subtropics [49].
Bovine babesiosis associated with and B. bovis is an important disease of tropical and sub tropical regions of the world. Both species are transmitted transovarially by Boophilus ticks, but only tick larvae transmit B, bovis, where as nymphs and adults transmit and B. divergens. Bovine babesiosis by Ixodes ricinus is wide spread [50].
Risk factors: Host factor: Bos indicus breeds of cattle are more resistance to Babesiosis than Bos Taurus [51]. This is a result of evolutionary relationship between Bosindicus cattle, Boophilus species and Babesia [19]. Because of natural selection pressure, indigenous populations, having lived for a long time with local ticks and tick-borne diseases, have developed either an innate resistance or an innate ability to develop a good immune response to the tick or tick-borne hemoparasitic disease in question. Sheep were highly susceptible to B. ovis than goats [52]. It is frequently stated that there is an inverse age resistance to Babesia infection in that young animals are less susceptible to Babesiosis than older animals; the possible reason is passive transfer of maternal antibody via colostrum. The severity of the clinical Babesiosis increases with age [53].
Pathogen factor: Many Intra-erythrocyte hemoparasites survive the host immune system through rapid antigenic variation which has been demonstrated for B. bovis and [19]. B. bovis is the most pathogenic organism, resulting in high mortality rates among susceptible cattle [54].
Environmental Factor: There is a seasonal variation in the prevalence of clinical Babesiosis, the greatest incidence occurring soon after the peak of the tick population. Of the climatic factors, air temperature is the most important because of its effect on tick activity; higher temperatures increase its occurrence. Heaviest losses occur in marginal areas where the tick population is highly variable depending on the environmental conditions [19]. Babesiosis infection in cattle mostly reaches peak in summer [30].
Economic importance
Recently Babesia becomes the most widespread parasite due to exposure of 400 million Cattle to infection through the world, with consequent heavy economic losses such as mortality, reduction in meat and milk yield and indirectly through control measures of ticks. Babesiosis, especially in cattle has great economic importance, because unlike many other parasitic diseases, it affects adults more severely than young cattle, leading to direct losses through death and the restriction of movement of animals by quarantine laws [55,56].
The disease is also a barrier to improving productivity of local cattle by cross-breeding due to the high mortality of genetically superior but highly susceptible cattle, especially dairy cattle, imported from Babesia-free areas [57]. The consequence is that the quality of cattle in endemic areas remains low, therefore impeding the development of the cattle industry and the wellbeing of producers and their families [58].
Previous studies in Ethiopia
In a cross-sectional study conducted to assess the prevalence of Bovine babesiosis in Teltele District, North West Borena Zone, Southern Ethiopia [59]. In this study the overall prevalence of Bovine babesiosis was found 16.9% (65/384) using microscopic examination of Geimsa stained blood smear. Four PAs were assessed and the lowest prevalence was recorded in Fulotole (9.4%) followed by Hatuse (13.6%), Kulcha (18.2%) while the highest was recorded in Billakebele (27.85%). Higher prevalence was recorded in female (17.5%) than male (16.3%) with significant difference. Age wise prevalence showed the highest prevalence among old animals (23.5%) followed by adult (15%) and young animals (13.2%). Body condition of the animal was significantly associated and highest prevalence recorded in animals with poor body condition (35.96%). Two species of Babesia identified were B. bovis (9.9%) followed by (7%) [59].
In other study that conducts to assess the prevalence of Bovine babesiosis in and around Jimma town, the prevalence of the disease is moderate. A total of 400 blood samples collected from cattle and examined by thin smear using Giemsa stained; and packed cell volume for determination of anemia. Out of the total blood samples examined, the Giemsa stained blood smears revealed an overall prevalence rate of Bovine babesiosis as 23% (92/400). From the study areas the lowest prevalence was recorded in dairy farm of Jimma University (11.6%) while the highest was recorded in Mantina (34.5%). Prevalence of the disease was recorded in both sexes; age and breeds with in all cases insignificant difference were detected. However; association was made between the species of B. bovis and B. bigemina; and presence tick in the study areas. Therefore, strong statistically significant difference was observed. In conclusion the results of this study have indicated that Bovine babesiosis was moderate in the study area (Lemma et al., 2016).
According to sebele et al, the prevalence of Babesia bigemina as well as B. bovis was 0.3% in and around Deber-Zeit [60].
Treatment
Control of Bovine babesiosis can be either by tick management, immunization, anti-Babesia drugs or by a combination of these approaches [14]. Chemotherapy of babesiosis is important for controlling the disease either to treat field cases or to control artificially induced infections [15].
In the past, treatment of cattle babesiosis was less important than disease eradication, principally in countries were the goal was to eradicate the tick vector; however, chemotherapy has been important to control and prevent babesiosis in some areas of the world [16].
In endemic areas, sick animals should be treated as soon as possible with an anti-parasitic drug. The success of the treatment depends on early diagnosis and the prompt administration of effective drugs [61]. A large number of chemical compounds have been reported to be effective against bovine Babesia parasites.
Some of them were very specific and effective [61]. But many have been withdrawn for several reasons. In addition, supportive therapy such as blood transfusions, anti inflammatory drugs, tick removal, iron preparations, dextrose, vitamins (B complex), purgatives, and fluid replacements, may be necessary in severe cases of babesiosis [32].
The first specific drug used against Bovine babesiosis was trypan blue, which is a very effective compound against B. bigemina infections, however, it did not have any effect on B. bovis and it had the disadvantage of producing discoloration of animal’s flesh, so it is rarely used [16].
The most commonly used compounds for the treatment of babesiosis are diminazene diaceturate (3-5 mg/kg), imidocarb (1-3 mg/kg), and amicarbalide (5-10 mg/kg); however, the quinuronium and acridine derivatives are also effective. For many years, the babesiacides: quinuronium sulfate, amicarbalide, diminazeneaceturate and imidocarbdiproprionate were used against Bovine babesiosis in most of Europe; however, quinuronium sulfate and amicarbilide were withdrawn because of manufacturing safety issues, and diminazene, which is widely used in the tropics as both a babesiacide and a trypanocide, was withdrawn from Europe for marketing reasons [61]; in addition, the product was also withdrawn from the market in Japan recently and is not approved by the Food and Drug Administration in U.S.A [62].
The indiscriminate use of anti-Babesia prophylactic agents including the administration of the drug at sub lethal blood levels to animals, can produce the development of drug resistant parasites, a problem that will require the development of new drugs [61]. New drugs with a chemotherapeutic effect against babesiosis, with high specificity to the parasites and low toxicity to the hosts are desired to control the disease [63]. Identification of novel drug targets is usually based upon metabolic pathways and cell structure [61]. Babesia spp. are Apicomplexa parasites that invade erythrocytes and multiply asexually with a reproductive phase [64]. which differ from other Apicomplexa that are able to invade and replicate within nucleated cells. However, Babesia is closely related to Plasmodium protozoa, which also proliferate within erythrocytes and some drugs can be useful for both of these erythrocyte-invading parasites [61].
In addition, most members of the phylum Apicomplexa harbor a semiautonomous plastid like organelle called apicoplast, which was derived via secondary endo-symbiotic events from an eukaryotic alga [65]. The apicoplast is essential for long term parasite viability and has been an attractive target for development of parasiticidal drug therapies [66].
In fact, the genome of B. bovis has been sequenced and provides a greater understanding of B. bovis metabolism and potential avenues for drug therapies [67] (Table 2).
Compound
Chemical name
species
Dose
Route
Current use
Imidocarb
3,3’-bis(2-imidazolin-2-yl)-carbanalidae
B. bovis
B. bigemina
B. divergens
B. caballi
1-3 mgkg-1
IM, SC
yes
Diminazeneaceturate
4,4´(azoamino) dibenzamidine
B. bovis
B. bigemina
B. divergens
B. caballi
3-5 mgkg-1
IM
Yes
Nerolidol
cis-3,7,11-Trimethyl-
1,6,10-dodecatrien-3-ol
B. bovis
B. bigemina
B. ovata
B. caballi
10μM
25μM
-
On research
Triclosan
2’,4’,4’-tricloro-2’hydroxyphenil ether
B. bovis
B. bigemina
B. caballi
100μgml-1
50μgml-1
-
On research
Epoxomicin
a´,β´-epoxyketone
B. bovis
B. bigemina
B. ovata
B. caballi
B. microti
10nM
5nM
0.05-0.5 mgkg-1
-
On research
Gossypol
1,1’,6,6’,7,7’-hexahydroxy-5,5’-diisopropyl- 3, 3‘dimethyl [2,2’-binaphthaleneI8,8‘ dicarboxaldehyde
B. bovis
100μM
-
On research
Atovaquone
1,4-hydroxynaphthoquinone
B. divergens
1mgkg-1
-
On research
Table 2: Chemical Drugs Used to Treat Babesiosis. IM Intramuscular, SC Subcutaneous [58].
Prevention and control
Epidemiological surveillance is the important aspect to control Babesiosis [68]. Active prevention and control of Babesiosis is achieved by three main methods: immunization, chemoprophylaxis and vector control. Ideally, the three methods should be integrated to make the most cost-effective use of each and also to exploit breed resistance and the development and maintenance of enzootic stability [69].
Chemotherapy/chemoprophylaxis: several groups of compounds have been used in the chemical control Babesiosis. Of these, only imidocarb dipropionate, diminazeneaceturate and tetracycline antibiotics remain freely available in most endemic countries. Chemoprophylaxis is not a viable long-term alternative to effective immunization, but imidocarb and diminazene have been used to protect cattle for several months against Babesiosis [69]. At dosage of three mg/kg, imidocarb provides protection for Babesiosis for around four weeks from carrier animals [70].
Immunization: Using blood from carrier animals has been practiced for many years in tropical areas and more recently in Australia [71]. Vaccination has been done with varying degree of success with live and dead whole parasite and isolated parasite antigen. Several findings support the development of vaccines against Babesiosis First; cattle which recover from a primary Babesia infection or that have been immunized with attenuated parasites are resistant to challenge infection. Second, immunization of cattle with native Babesia antigen extracts or culture-derived supernatants containing secreted Babesia antigens elicit protective immunity against both homologous and heterologous challenge [19].
Vector control: it is done by repeated treatment of cattle with acaricides in areas of high challenge; such treatment may require to be carried out twice weekly in order to kill the tick before the infective sporozoite develop in the salivary gland [27]. Significant factors currently affecting the control of Babesiosis include increased resistance to acaricides by ticks and the numerous draw backs of the current live vaccines [14].
Conclusion and Recommendations
Babesiosis is a dangerous, invasive disease of cattle’s. Babesiosis is caused by any one of many Babesia species that infect wide variety of vertebrate hosts, including domestic and wild animals, as well as man. Despite the diagnostic and preventive advances resulting from extensive research and a greater understanding of the disease, babesiosis continues to have significant medical and economic impact. Diagnostic advances, like the development of PCR assays, have resulted in increased sensitivity for detection as well as the discovery and characterization of new babesial species. Further studies using the molecular tools now available and those to be developed will lead to a better understanding of the natural history of these organisms, including the transmission cycle and the potential role of Babesia parasites themselves as immunomodulators.
For years, babesiosis treatment has been based on the use of very few drugs like imidocarb or diminazeneaceturate. Recently, several pharmacological compounds were developed and evaluated, offering new options to control the disease. Babesiosis impair the export and import trade of live animal and animal products (Meat, milk, bide and skin) by down grading their quality and fear of the co-traders. Control of tick borne diseases is crucial in improving livestock health services and animal productivity. There are different control strategies which vary from region to region as well as from area to area. Potential control methods for tick borne pathogens include tick control, vaccines (Against ticks and parasites) and drugs (Against ticks and parasites).
• Therefore, based on this conclusion the following recommendations are forwarded:Economic losses from Babesiosis are very high so that concerned organization should give attention to control and eradicate them.
• Since chemical control can result in resistance and environmental contamination, environmentally friend control mechanisms like vaccination and biological methods should be further developed.
• Awareness should be created on mode of transmission, control and prevention methods of babesia to livestock owners.
• Proper identification and characterization of ticks involved in the transmission of disease should be done to implement particular control strategy.
• New vaccines and drugs should be designed that eliminates carrier states.
References
- Kassai T. Veterinary helmintology. University of veterinary science, Oxford: Butter Worth Heinemann. 1999; 9.
- Homer MJ, Aguilar-Delfin I, Telford SR, Krause PJ, Persing DH. Babesiosis. Clinical Microbiology Reviews. 2000; 13: 451–469.
- Kjemtrup AM, Conrad PA. Human babesiosis: an emerging tick-borne disease. International Journal of Parasitology. 2000; 30: 1323–1337.
- Penzhorn BL. Babesiosis of wild carnivores and ungulates. Veterinary Parasitology. 2006; 138: 11-21.
- deVos AJ, Bock RE. Vaccination against bovine babesiosis. Ann. N Y Acad. Sci. 2000; 916: 540-545.
- Purnell RE. Tick-borne diseases of British livestock. Vet. Med. Rev. 1981; 1: 58–69.
- Bock R, Jackson L, De Vos AJ and Jorgensen W. Babesiosis of cattle. In: Ticks: Biology, Disease and Control, Bowman AS. and Nuttall PA, eds. Cambridge University Press, Cambridge. UK. 2008; 281–307.
- De Castro JJ, James AD, Minjauw B, Di Giulio GU, Permin A, Pegram RG, et al. Long-term studies on the economic impact of ticks on Sanga cattle in Zambia. Experimental & applied acarology. 1997; 21: 3-19.
- Friedhoff KT. Transmission of Babesia. In: M. Ristic (Ed) Babesiosis of Domestic Animals and Man. CRC Press, Florida. 1988; 23-52.
- Wright IG, Goodger BV, Buffington GD, Clark IA, Parrodi F, Waltisbuhl DJ. Immunopathophysiology of babesial infections.Trans R Soc. Trop. Med. Hyg. Suppl. 1989; 83:11-13.
- Salih DA, El Hussein AM, Seitzer U and Ahmed JS. Epideniiological studies on tick-borne diseases of cattle in Central Equatoria State, Southern Sudan. Parasitology Research. 2007; 101: l035-1044.
- Bock R, Jackson L, De Vos A and Jorgensen W. Babesiosis of cattle. Parasitology. 2004; 129: 247-269.
- Pérez de León AA, Strickman DA, Knowles DP, Fish D, Thacker E, La Fuente JD, et al. "One Health approach to identify research needs in bovine and human babesioses: workshop report." Parasites & vectors 3: 1-10.
- Suarez CE and Noh S. Emerging perspectives in the research of bovine Babesiosis and Anaplasmosis. Veterinary Parasitology. 2011; 180: 109-125.
- Rodriguez RI and Trees AJ. In vitro responsiveness of Babesia bovis to imidocarb dipropionate and the selection of a drug-adapted line. Veterinary parasitology. 1996; 62: 35-41.
- Kuttler KL. Chemotherapy of babesiosis: A review. In Babesiosis. 1981; 65-85.
- Allsopp MT, Cavalier-Smith T, De Waal DT and Allsopp BA. Phylogeny and evolution of the piroplasms. Parasitology. 1994; 108: 147–152.
- Criado-Fornelio A, Martinez-Marcos A, Bulingsarana A and Barba- Carretero JC. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part II. Phylogenetic analysis and evolutionary history. Veterinary Parasitology. 2003; 114: 173–194.
- Radostits OM, Gay GC, Hinchiff KW and Constable PO. Veterinary Medicine: A text book of the disease of cattle, sheep, goat, pigs and horses. 10th London: Saunders Elsevier. 2007; 1110-1489, 1527-1530.
- Fakhar M, Hajihasani A, Maroufi S, Alizadeh H, Shirzad H, Piri F, et al. An epidemiological survey on bovine and bovine Babesiosis in Kurdistan Province, western Iran. Tropical Animal Health and Production. 2012; 44: 319-322.
- Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Sixth Edition, Volume 2. 2008; 612.
- Gray J, Zintl A, Hildebrandt A, Hunfeld K and Weiss L. Zoonotic Babesiosis: Overview of the disease and novel aspects of pathogen identity. Ticks and Tick-borne Diseases. 2010; 1: 3-10.
- Mandal SC. Veterinary Parasitolgy. 2nd India: Panacea Computer. 2012; 355-365.
- Lefevre PC, Blancous J, Chermitte R and Uilenberg G. Infectious and Parasitic Diseases of Livestock. 1st France: Lavoisier. 2010: 1247-1256, 1819-1861.
- Carlton WW and McGavin MD. Thomson’s Special Veterinary Pathology. 2nd USA: Mosby Year Book Incorparted. 1995; 292-294.
- Hunfeld KP, Hildebrandt A and Gray JS. Recent insights into Babesiosis. International Journal for Parasitology. 2008; 38: 1219-1237.
- Urquhart GM, Armour J, Duncan JL, Dunn AM and Jennings FW. Veterinary Parasitology. 2nd USA: Blackwell Science Incorporated. 1996; 242-253.
- Ahmed JS. The role of cytokines in immunity and immunopathogenesis of pirolasmoses. Parasitology Research. 2002; 88: 48–50.
- Brown WC and Palmer GH. Designing blood-stage vaccines against Babesia bovis and bigemina. Parasitology Today. 1999; 15: 275–281.
- ElMoghazy HM, Ebied MM, Abdelwahab and El Sayed AA. Epidemiological studies on bovine Babesiosis and Theileriosis in Qalubia governorate. BenhaVeterinary Medical Journal. 2014; 27: 36-48.
- Abdel Aziz KB, Khalil WKB, Mahmoud MS, Hassan NHA, Mabrouk DM and Suarez CE. Molecular characterization of Babesiosis infected cattle. Global Veterinaria. 2014; 12: 197-206.
- Zintl A, Mulcahy G, Skerrett HE, Taylor SM, and Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clinical microbiology reviews. 2003; 16: 622-636.
- De Vos AJ and Potgieter FT. Bovine babesiosis. In Infectious Diseases of Livestock (ed. Coetzer, J. A. W., Thomson, G. R. & Tustin, R. C.), 1994; 278–294.
- Bose R, Jorgensen WK, Dalgliesh RJ, Friendhoff KT and De Vos AJ. Current state and future trends in the diagnosis of babesiosis. Vet. Parasitol. 1995; 57: 61–74.
- Criado-Fornelio A. A review of nucleic acid-based diagnostic tests for Babesia and Theileria, with emphasis on bovine piroplasms. Parassitologia(Rome). 2007; 49: 39–44.
- Buling A, Criado-Fornelio A, Asenzo G, Benitez D, Barba-Carretero JC and Florin-Christensen M. A quantitative PCR assay for the detection and quantification of Babesia bovis and bigemina. Vet. Parasitol. 2007; 147: 16–25.
- Gubbels JM, De Vos AP, Van Der Weide M, Viseras J, Schouls LM, De Vries E, et al. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin.Microbiol. 1999; 37: 1782–1789.
- Criado-Fornelio A, Buling A, Asenzo G, Benitez D, Florin-Christensen M, Gonzalez-Oliva A, et al. Development of fluorogenic probe-based PCR assays for the detection and quantification of bovine piroplasmids. Vet. Parasitol. 2009; 162: 200–206.
- Holman PJ, Waldrup KA, Droleskey RE, Corrier DE and Wagner GG. In vitro growth of Babesia bovis in white-tailed deer (Odocoileusvirginianus) erythrocytes. J. Parasitol. 1993; 79: 233–237.
- Friedhoff K and Bose R. Recent developments in diagnostics of some tick-borne diseases. In: Use of Applicable Biotechnological Methods for Diagnosing Haemoparasites. Proceedings of the Expert Consultation, Merida, Mexico, 4–6 October 1993.
- Ticks and Tick-borne Disease Control: A Practical Field Manual. Vol II Tick-borne Disease Control. Food and Agriculture Organisation of the United Nations (FAO), Rome, Italy. 1984.
- Compliment fixation test for detection of antibodies to Babesiabigemina and Babesiabovis– Microtitration test. United States Department of Agriculture (USDA), Animal and Plant Health Inspection Service, Veterinary Services, National Veterinary Services Laboratories, Ames, Iowa, USA. 2006.
- Molloy JB, Bowles PM, Bock RE, Turton JA, Katsande TC, Katende JM, et al. Evaluation of an ELISA for detection of antibodies to Babesia bovis in cattle in Australia and Zimbabwe. Prev. Vet. Med. 1998; 33: 59–67.
- Montenegro-James S, Guillen T and Toro M. Dot-ELISA paradiagnostico serologico de la anaplasmosis y babesiosis bovina. Rev. Cientifica[FCV de Luz]. 1992; 2: 23.
- Kung’u MW and Goodger BV. A slide enzyme-linked immunosorbent assay (SELISA) for the diagnosis of Babesia bovis infections and for the screening of Babesia-specific monoclonal antibodies. Int. J. Parasitol. 1990; 20: 341–345.
- Blandino T, Alvarez M, Larramendi R, Gomez E, and Alonso M. Elaboracion y evaluacion de un antigen de Babesiabovispara la prueba de aglutination en latex. Rev. Salud Animal. 1991; 13:177–179.
- Madruga CR, Kessler RH, Schenk MAM, Honer MR and Miquita M. Analise de testes de conglutinacaorapidaparadeteccao de anticorpos contra Babesia bovis Babesia Arq. Bras. Med. Vet. Zoot. 1995; 47: 649–657.
- Chul-Min K, Blanco LBC, Alhassan A, Iseki H, Yokoyama N, Xuan X, et al. Development of a rapid immuno chromatographic test for simultaneous serodiagnosis of bovine babesioses caused by Babesia bovis and Babesia bigemina. Am. J. Trop. Med. Hyg. 2008; 78: 117–121.
- Chaudhry ZI, Suleman M, Younus M and Aslim A. Molecular detectionof Babesi bigemina and Babesia bovis in crossbred carrier cattle through PCR. Pakistan Journal of Zoology. 2010; 42: 201- 204.
- Esmaeilnejad B, Tavassoli M, Asri-Rezaei S, Dalir-Naghadeh B, Mardani K, Golabi M, et al. Determination of prevalence and risk factors of infection with Babesia bovis in small ruminants from west Azerbaijan province, Iran by Polymerase chain reaction. Journal Arthropod-Borne Disease. 2015; 9: 246-252.
- Kamani J, Sannusi A, Egwu OK, Dogo GI, Tanko TJ, Kemza S, et al. Prevalence and significance of haemoparasitic infections of cattle in north- central, Nigeria. Veterinary World. 2010. 3: 445-448.
- Rahbari S, Nabian S, Khaki Z, Alidadi N and Ashrafihelan J. Clinical, hematologic and pathologic aspects of experimental bovine Babesiosis in Iran. Iranian Journal of Veterinary Research. 2008; 9: 59-64.
- Taylor MA, Coop RL and Wall RL. Veterinary Parasitology. 3rd Blackwell Publishing, USA. 2007; 103-115.
- Brown WC, Norimine J, Goff WL, Suarez CE and Mcelwain TF. Prospects for recombinant vaccines against Babesia bovis and related parasites. Parasite Immunology. 2006; 28: 315-327.
- Onoja II, Malachy P, Mshelia WP, Okaiyeto SO, Danbirni S and Kwanashie G. Prevalence of Babesiosis in cattle and goats at Zaria Abattoir, Nigeria. Journal of Veterinary Advances. 2013; 3: 211-214.
- Osman FA and Gaadee HIM. Evaluation of antioxidant response mechanism in fattening cattle calves suffering from Babesiosis in New Valley Governorate. Egypt. Journal of Veterinary Advances. 2013; 3: 232-237.
- Hong Y, Wenshun L and Jianxun L. Babesiosis in China. Tropical Animal and HealthProduction. 1997; 29: 11-15.
- Mosqueda J, Olvera-Ramírez A, Aguilar- Tipacamú G and Cantó GJ. Current advances in detection and treatment of Babesiosis. Current Medicinal Chemistry. 2012; 19: 1504-1518.
- Hamsho A, Tesfamarym G, Megersa G, and Megersa M. A Cross-Sectional Study of Bovine Babesiosis in Teltele District, Borena Zone, Southern Ethiopia. Journal of Veterinary Science & Technology, 2015.
- SebeleTaddese, Zewedu Freaulai and Girma Afewerek. A study of the prevalence of hemoparasites of ruminants in and around Debre-Zeit, Central Ethiopia. International Scholars Journals. 2015; 2: 66-71.
- Vial HJ, and Gorenflot A. Chemotherapy against babesiosis. Veterinary Parasitology. 2006; 138: 147-160.
- Goo YK, Terkawi MA, Jia H, Aboge GO, Ooka H, Nelson B, et al. Artesunate, a potential drug for treatment of Babesia Parasitology international. 2010; 59: 481-486.
- AbouLaila M, Sivakumar T, Yokoyama N, and Igarashi I. Inhibitory effect of terpene nerolidol on the growth of Babesia Parasitology international. 2010; 59: 278-282.
- Gohil S, Kats LM, Sturm A, and Cooke BM. Recent insights into alteration of red blood cells by Babesia bovis: moovin’forward. Trends in Parasitology. 2010; 26: 591 -599.
- Wilson RJ, and Williamson DH. Extrachromosomal DNA in the Apicomplexa. Microbiology and Molecular Biology Reviews. 1997; 61: 1-16.
- Fichera ME, and Roos DS. A plastid organelle as a drug target in apicomplexan parasites. Nature. 1997; 390: 407-409.
- Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007; 3: 148.
- Chowdhury S, Hossain MA, Barua SR and Islam S. Occurrence of common blood parasites of cattle in Sirajgonj Sadar area of Bangladesh. Bangladesh Journal of Veterinary Medicine. 2006; 4: 143-145.
- International Laboratory for Research on Animal Diseases, Recent developments in the control of Anaplasmosis, Babesiosis and Cowdriosis, Proceedings of a workshop held at Nairobi, Kenya. 1991; 1-174.
- Kahn CM. The Merck Veterinary Manual. 9th USA: Merck and Company Incorporated. 2005; 18-32.
- Atif FA, Khan MS, Roheen T, Muhammad F, Younus M, Avais M, et al. Seroprevalence of Anaplasma marginale infection among cattle from three districts of the Northern Punjab, Pakistan. J. Anim. Plant Sci. 2013; 23: 995-998.
- Uilenberg G. Significance of tick-borne hemoparasitic diseases to world animal health. Veterinary Parasitology. 1995; 57: 19-41.
- Gough JM, Jorgensen WK, Kemp DH. Development of tick gut forms of Babesia bigemina in vitro. Journal of Eukaryotic Microbiology. 1998; 45:298–306.