
Review Article
Austin J Vet Sci & Anim Husb. 2019; 6(1): 1054.
Aflatoxicosis and Occurrence of Aflatoxin M1 (AFM1) in Milk and Dairy Products: A Review
Ahmed Seid* and Amana Mama
Wolaita Sodo University, School of Veterinary Medicine, Wolaita Sodo, Ethiopia
*Corresponding author: Ahmed Seid, Wolaita Sodo University, School of Veterinary Medicine, Wolaita Sodo, Ethiopia
Received: February 27, 2019; Accepted: April 09, 2019; Published: April 16, 2019
Abstract
Aflatoxicosis is the most important food borne mycotoxins and a condition caused by aflatoxin in both animal and humans. Aflatoxins (AFs) are toxic and carcinogenic metabolites produced by a variety of fungi. Risk of aflatoxin contamination of commodities in the world, especially in Africa is increasing. Milk is a highly nutritious food, and it is a source of necessary macro- and micronutrients for the growth, development and maintenance of human health. However, it may also be a source of natural food contaminants that may cause disease. Aflatoxin M1 (AFM1) is the major carcinogenic type frequently found in milk and dairy products, thus posing a significant impact on human health. Milk and dairy products contamination with aflatoxin M1 is important problem worldwide especially for developing countries for the last ten to twenty years. The presence of this mycotoxin in these products is important issue, especially for children and infants, who are more susceptible than adults. Control of aflatoxins requires an integrated approach, whereby aflatoxins are controlled at all stages from the field to the table, is required for reduction in risk. Strict regulations and adapting good storage practices in developed countries have minimized the contamination of AFM1 in milk and dairy products. The current advancements in analytical techniques have helped the law enforcement agencies to implement strict regulations. Furthermore, the improvement in analytical facility and increasing the awareness related to the health effects of AFM1 in milk and dairy products could minimize its occurrence level in developing countries.
Keywords: Aflatoxin; Cancer; Dairy; Milk; Mycotoxin; Public Health
Introduction
Aflatoxins are a group of naturally occurring carcinogens that are known to contaminate different animal and human food stuffs. Aflatoxins are poisonous by-products from soil-borne fungus Aspergillus, which is responsible for the decomposition of plant materials [1]. The occurrence of aflatoxins in foods and food products vary with geographic location, agricultural and agronomic practices. The susceptibility of food product to fungal attack occurs during preharvest, transportation, storage, and processing of the foods [2]. The problem of aflatoxin contamination of the food products is a common problem in tropical and subtropical regions of the world especially in the developing countries such as the sub-Saharan countries with poor practices and where the environmental conditions of warm temperatures and humidity favors the growth fungi [3]. The various food products contaminated with aflatoxins include cereals like maize, sorghum, pearl millet, rice and wheat; oilseeds such as groundnut, soybean, sunflower and cotton; spices like chillies, black pepper, coriander, turmeric and zinger; tree nuts such as almonds, pistachio, walnuts and coconut; and milk and milk products [4].
Animals are exposed to mycotoxins such as the aflatoxins by consumption of feeds contaminated by mycotoxin producing molds during growth, harvest and/or storage. When lactating cows consume aflatoxin B1 contaminated feed, aflatoxin B1 is metabolized to form the monohydroxy derivative, AFM1, which is expressed in the cow’s milk. The sources of aflatoxin contamination in feed vary from country to country. Consequently, the incidence and occurrence of AFM1 contamination in milk and dairy products depend on the country of origin [5]. Milk and dairy products are principal components in human diet that may be the way for entrance of mycotoxins into the human body posing risk for consumers, in particular, infants and young children [6,7].
Mycotoxins, principally produced by filamentous fungi, are naturally occurring secondary metabolites [8]. They constitute a major problem for food industries due to their effect on productivity, welfare and health of dairy animals, as well as, they impair food safety for humans and animals [9]. Global concerns about mycotoxins have been evoked due to their association with certain animal and human disorders such as acute toxicity and sometimes they are linked with certain types of cancer [10].
Aflatoxins (AFs) produced by Aspergillus flavus, Aspergillus parasiticus and rarely by Aspergillus nomius are the most carcinogenic and toxic type of mycotoxins. They can contaminate cattle feed, fruits, vegetables, different types of food and cereals [11]. Twenty AFs including AFB1, AFB2, AFG1 and AFG2 belong to a larger group of toxic compounds named difuranocoumarins, are the most concerned types that naturally contaminate food [12]. Of them, AFB1 is the most toxic and carcinogenic and classified according to the International Agency for Research on Cancer (IARC) as class 1 human carcinogen [13].
The monohydroxy derivative (AFM1) of AFB1 is produced after metabolization following consumption of contaminated feed by lactating cows. After ingestion of contaminated feed meal, AFM1 is present in cow’s milk with the percentage of 1-6% of AFB1 from a few hours after the ingestion of contaminated meal and up to two days after suspension of the feeding diet [14]. The occurrence and incidence of AFM1 contamination in milk and dairy products varies according to the country of origin [5]. Contamination of milk and dairy products is of public health concern mainly in growing infants because they depend on milk as a major source of nutrients [15].
Aflatoxin occurs worldwide. The recent estimates suggest that there are more than five billion people worldwide at risk of chronic exposure to aflatoxins. But it occurs more frequently in tropical countries because of high temperature, moisture, unseasonal rains. Most of the time Aflatoxicosis is a consequence of ingestion of contaminated feed with aflatoxins. That is continued to be problems of significant public health concern as long as people consumed contaminated animal products and considered as public health important. In the developing world seriously affect people’s health and livelihoods, as freedom of choice in food is limited for a poor and food-insecure population [16,17].
Therefore, the objective of this paper are:
• To review on Aflatoxin and its occurrence in milk and milk products
• To highlight the health effects of aflatoxins on human and animals and as well as its prevention
Aflatoxin and Its Occurrence in Milk and Milk Products
The name ‘‘Aflatoxin” derives from the first letter of the word Aspergillus and the first three letters of flavus. Structurally, Aflatoxins (AFs) are difurocoumarin derivatives with specific fluorescence under ultraviolet light. Depending on the colour of the fluorescence, AFs are divided into aflatoxin B1 and B2 (AFB1, AFB2) for blue florescence, and G1 and G2 (AFG1, AFG2) for green florescence. Aflatoxin M1 and M2 (AFM1, AFM2), known as milk-AFs, are the metabolites of AFB1 and AFB2. Other metabolites of AFB1 are aflatoxin Q1 (AFQ1) and aflatoxicol. Aflatoxin is the most studied mycotoxin, due to both its toxicity to animals and people and its high carcinogenic potential. Out of AFs group, AFB1 is the most toxic and is classified as human carcinogen [18].
Aflatoxins are a group of mycotoxins mainly produced by several fungus species in the genus Aspergillus. It includes A. flavus and A. parasiticus, A. pseudotamarii, and A. nomis species. Among these species A. flavus and A. parasiticus are well known. These organisms invade crops and grow on foods during storage if temperature and humidity levels are favorable. The relative proportions and amounts of the various aflatoxins on food crops depend on the Aspergillus species present, pest infestation, growing and storage conditions, and other factors. Although these species have similar geographical ranges, A. parasiticus is less widely distributed and A. flavus is the most widely reported fungus in foodstuffs. Aflatoxins are metabolized in ruminants by the liver and excreted in the bile. The major aflatoxins produced in feed stuffs are B1, B2, G1, G2, M1 and M2. Both A. flavus and A. parasiticus produce aflatoxins B1 and B2, and A. parasiticus also produces aflatoxins G1 and G2 [19].
There are several different types of aflatoxins strains. The most common naturally produced are B1, B2, G1, and G2 and two additional strains, M1 and M2 are the metabolic products of contaminated food or feed and are found in milk and other dairy products [4]. Among these several type of aflatoxin strain, Aflatoxin B1 is the most potent mycotoxin (toxic substance produced by a mold). This type of toxin increases the apparent protein requirement of cattle and is a potent cancer causing agent (carcinogen). When significant amounts of aflatoxin B1 are consumed, the metabolite M1 appears in the milk within 12 hours [20].
Properties of aflatoxins
Aflatoxins are produced by fungi in the genus Aspergillus that grow on grains and other agricultural crops. They exist as color less to pale-yellow crystals at room temperature. They are slightly soluble in water and hydrocarbons, soluble in methanol, acetone, and chloroform, and insoluble in non- polar solvents. Aflatoxins are relatively unstable in light and air, particularly in polar solvents or when exposed to oxidizing agents, ultraviolet light or solutions with a PH below 3 or above10. Aflatoxins decompose at their melting points, which are between 237°C (G1) and 299°C (M1), but are not destroyed under normal cooking conditions. They can be completely destroyed by autoclaving in the presence of ammonia or by treatment with bleach. Physical and chemical properties of aflatoxins are listed in the following [21].
Physical properties of aflatoxins: Aflatoxins are crystalline odorless solids when isolated and the color range from pale white to yellow. The melting points range from 268°C for B1 down to 190°C for G2 [22]. The optimal water activity for growth of A. flavus is high (about 0.99). The maximum is at least 0.998 whereas the minimum water activity for growth has not been defined. In general, production of toxins appears to be favored by high water activity. Aspergillus flavus is reported to grow within the temperature range 10-43°C. The optimal growth rate occurs at a little above 30°C, reaching as much as 25mm per day. The aflatoxins are produced by A. flavus over the temperature range 15-37°C. It is not possible to specify an optimum temperature for the production of the toxins, although production between 20-30°C is reported to be significantly greater than at higher and lower temperature [23].
Chemical properties of aflatoxins: Aflatoxins belong to the group of difuranocoumarins. The compounds are usually soluble in methanol, chloroform, acetone and acetonitrile which are slightly polar but insoluble in non-polar solvents. Aflatoxins react with alkaline solutions causing the hydrolysis of the lactones moiety. This hydrolysis is reversible since it has been shown that recyclization occurs following acidification of basic solution containing aflatoxin. At higher temperatures above 100°C, ring opening followed by decarboxylation occurs and the reaction may proceed further, leading to the loss of methoxy group from the aromatic ring [24].
In the presence of mineral acids aflatoxins B1 and G1 are converted into aflatoxin B2A and G2A, due to acid catalyzed addition of hydroxyl group across the double bond in the furan ring. In the presence of acetic anhydride and hydrochloric acid, the reaction proceeds further to acetoxy derivative. Similar adducts of aflatoxin B1 and G1 are formed with formic acid-thionyl chloride and trifluroacetic acid. Many oxidizing agents, including sodium hypochlorite, potassium permanganate, chlorine, hydrogen peroxide, ozone and sodium per borate, react with aflatoxin molecule in some way as indicated by the loss of fluorescence in ultraviolet light at 365nm [25].
Hydrogenation of aflatoxin B1 and G1 yields aflatoxins B1 and G1 respectively. Further reductions of aflatoxin B1 by three moles of hydrogen yields tetra hydroxyl aflatoxin. Reduction of aflatoxin B1 and B2 with sodium boro hydride yields aflatoxin R-B1 and R-B2 respectively. These arise as a result of opening of the lactones ring followed by reductions of the acid group and reduction of the keto group in the cyclopentene ring [22].
Factors affecting aflatoxin production
The production of aflatoxin is equally influenced by physical and biological factors. They are reported to be produced between 25°C - 35°C optimum temperature and acidic PH. Relative humidity between 83%-88% and appropriate level of CO2 & O2 has also been reported to influence the mold growth and aflatoxin production. For instance 20% CO2 and 10% O2 in air depress the aflatoxin production [1]. As biological factors, the preferred carbon sources for aflatoxin production are glucose, sucrose or fructose. Also, zinc and manganese are essential for aflatoxin biosynthesis. But a mixture of cadmium and iron depress the mold growth and hence aflatoxin production [26].
Epidemiology
Aflatoxins can affect a wide range of commodities including cereals, oilseeds, spices, and tree nuts as well as milk and milk products. Reports from different part of the world indicated incidence of aflatoxins vary from 40 to 92%. Especially developing countries located in the tropical regions have greatest risk. Their climate is favorable to growth of aflatoxin. Where dietary food stuffs and Staple food source commodities is highly contaminated with aflatoxins [27]. Aflatoxicosis is the most important food borne mycotoxins, which occurred especially in milk and dairy products. It has greatest significance in tropical developing countries [28,29]. Epidemiological studies carried out in several parts of Africa and Asia indicates a correlation between exposure to aflatoxins and primary liver cancer [30]. Besides several epidemiologic investigations is shown that increased aflatoxin ingestion correlates with increased risk of hepatocellular carcinoma in humans [31]. Aspergillus flavus and aflatoxin forms sclerotia which allow it to survive in soil for extended periods of time [32]. The sclerotia are the principal sources of primary inoculums. They are also found in foodstuffs and are not destroyed by normal industrial processing or cooking since they are heat-stable. Conditions such as high temperatures and moisture, unseasonal rains during harvest and flash floods lead to fungal proliferation and production of mycotoxins [33]. Poor harvesting practices, improper storage and less than optimal conditions during transportation, marketing and processing can also contribute fungal growth and increase the risk of mycotoxins production [34]. Some of their metabolites are still toxic and may be involved in human diseases. The toxic effects of aflatoxins on organs like liver, kidney and mainly their carcinogenic effects are mostly known causes of morbidity and mortality [35].
Occurrence of aflatoxin in milk and milk products
AFM1 is a hepatocarcinogen found in milk of animals that have consumed feeds contaminated with AFB1, the main metabolite produced by fungi of the genus Aspergillus in particular A. flavus, A. parasiticus and A. nomius [36] AFM1 remain stable after pasteurization, sterilization, preparation and storage of various dairy products [37]. In the assessment of cancer risk, the infants are more exposed to the risk because the milk is a major constituent of their diet. Therefore the presence of AFM1 in milk and milk products is undesirable and a particular risk for human [38]. The amount of AFM1 excreted as a percentage of AFB1 in feed is usually 1-3%. 12- 24 h after the first AFB1 ingestion, the toxin can be detected in the milk. When the intake of AFB1 is stopped, the AFM1 concentration in the milk decreases to an undetectable level after 72 hours [39]. The contamination of milk and milk products with AFM1 display variations according to geography, country and season. The pollution level of AFM1 is differentiated further by hot and cold seasons, due to the fact that grass, pasture, weed, and rough feeds are found more commonly in spring and summer than in winter. At the end of summer, greens are consumed more than concentrated feed, causing a decreased level of AFM1 in milk and milk products [40]. Aflatoxins are highly toxic, immunosuppressive, mutagenic, teratogenic, and carcinogenic compounds. The main target organ for their toxicity and carcinogenicity is the liver. Milk and milk products are major nutrient for humans, especially children. For this reason, AFM1 in milk and dairy products should be controlled systematically [41].
Absorption, distribution, metabolism, excretion and mechanisms of action of aflatoxins
Aflatoxins are highly lip soluble compounds and are readily absorbed from the site of exposure usually through the gastrointestinal tract and respiratory tract into blood stream [42,43]. Animal and humans get exposed to aflatoxins by two major routes (a) direct ingestion of aflatoxin contaminated foods or ingestion of aflatoxins carried over from feed into milk and milk products like cheese and powdered milk as well as other animal tissues mainly as AFM1 [42] (b) by inhalation of dust particles of aflatoxins especially AFB1 in contaminated foods in industries and factories [44]. After entering the body, the aflatoxins are absorbed across the cell membranes where they reach the blood circulation. They are distributed in blood to different tissues and to the liver, the main organ of metabolism of xenobiotics. Aflatoxins are mainly metabolized by the liver to a reactive epoxide intermediate or hydroxylated to become the less harmful aflatoxin M1 [45]. In humans and susceptible animal species, aflatoxins especially AFB1 are metabolized by cytochrome P450 (CYP450) microsomal enzymes to aflatoxin-8, 9-epoxide, a reactive form that binds to DNA and to albumin in the blood serum, forming adducts and hence causing DNA damage [46].
Various CYP450 enzymes isoforms occur in the liver and they metabolize aflatoxin into a reactive oxygen species (aflatoxin-8, 9-epoxide), which may then bind to proteins and cause acute toxicity (Aflatoxicosis) or to DNA and induce liver cancer [45]. The predominant human CYP450 isoforms involved in human metabolism of AFB1 are CYP3A4 and CYP1A2. Both enzymes catalyze the biotransformation of AFB1 to the highly reactive exo- 8, 9-epoxide of AFB1 [47]. CYP1A2 is also capable of catalyzing the epoxidation of AFB1 to yield a high proportion of endo-epoxide and hydroxylation of AFB1 to form aflatoxin M1 (AFM1), which is a poor substrate for epoxidation [47] and less potent than AFB1 [48]. This is generally considered as the major detoxification metabolic pathway for aflatoxins. The CYP3A4 is the major CYP450 enzyme responsible for activation of AFB1 into the epoxide form and also form AFQ1, a less toxic detoxification metabolite. The CYP3A5 metabolizes AFB1 mainly to the exo-epoxide and some AFQ1 [49]. However, polymorphism studies with CYP3A5 have indicated that, this enzyme isoform is not expressed by most people especially in Africans [48]. Studies in Gambian children showed that aflatoxin cross the placenta and transported to the fetus and the newborn where they can cause detrimental effects [48]. The CYP3A7 is a major CYP450 enzyme isoform in human fetal liver and metabolizes AFB1 to the 8, 9-epoxide that may cause fetal defects to the developing fetus [50].
The epoxidation of AFB1 to the exo-8, 9-epoxide is a critical step in the genotoxic pathway of this carcinogen. The binding of AFB1 to DNA and DNA adduction by AFB1 exo-8, 9 epoxide has been reported to cause a functional changes of DNA conformation [51]. The epoxide is highly unstable and binds with high affinity to guanine bases in DNA to form afltoxin-N7-guanine [52]. The aflatoxin-N7- guanine has been shown to be capable of forming guanine (purine) to thymine (pyrimidine) transversion mutations in DNA and hence affecting the p53 suppressor gene in the cell cycle [53]. The p53 gene is important in preventing cell cycle progression when there are DNA mutations, or signaling apoptosis. The mutations have been reported to affect some base pair locations more than others especially in the third base of codon 249 of the p53 gene in the region corresponding to the DNA binding domain of the corresponding protein [54] and this appears to be more susceptible to aflatoxin- mediated mutations than nearby bases [55]. AFB1 induces the transversion of base G to base T in the third position of codon 249 and similar mutations have been observed in Hepatocellular Carcinoma (HCC) in high AFB1 contaminated food in regions in East Asia and Africa [56].
Epoxide hydrolase and Glutathione-S-Transferase (GST) are both involved in hepatic detoxification of activated AFB1, but the GST-catalyzed conjugation of glutathione to AFB1-8, 9-epoxides is thought to play the most important role in preventing epoxide binding to target macromolecules like DNA and various cell proteins [57]. Glutathione pathway is reported to play a vital role in the detoxification of AFB1 [58]. The AFB1 8, 9 exo and endoepoxides are conjugated by glutathione to form AFB-mercapturate and the reaction is catalyzed by Glutathione S-Transferase (GST) [58]. The glutathione-aflatoxin conjugate is transported from the cells with an ATP-dependent multidrug-resistance protein through an accelerated process [58]. Despite a preference for conjugating the more mutagenic AFB1 exo-epoxide isomer, the relatively low capacity for GST-catalyzed detoxification of bio-activated AFB1 in lung may be an important factor in the susceptibility of the lung to AFB1 toxicity [59]. The exo and endo epoxide can also be converted non-enzymatically to AFB1-8, 9-dihydrodiol which in turn can slowly undergo a basecatalysed ring opening reaction to a dialdehyde phenolate ion [47]. AFB1 dialdehyde can form Schiff bases with lysine residues in serum albumin forming aflatoxin- albumin complex [60]. Also the aflatoxin dialdehyde are reduced to a dialcohol in a NADPH-dependent catalyzed reaction by Aflatoxin Aldehyde Reductase (AFAR) [61]. However the guanine alkylation by aflatoxin B1 produces exo-8, 9-epoxide which is the reactive form and a carcinogen to the liver and the reaction is more than 2000 times more efficient in DNA than in aqueous solution [62] (Figure 1).
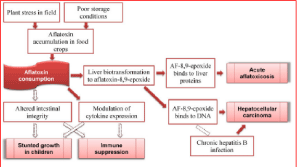
Figure 1: Aflatoxin disease pathways in humans [46].
Effect of aflatoxins on mitochondrial DNA: The reactive aflatoxin-8, 9-epoxide preferentially binds to mitochondrial DNA (mitDNA) during hepatocarcinogenesis as compared to nuclear DNA that hinder ATP production and FAD/NAD linked enzymatic functions and this causes the disruption of mitochondrial functions in the various parts of the body that require production of energy in the form of ATP [63]. Aflatoxin damage to mitochondria can lead to mitochondrial diseases and may be responsible for aging mechanisms [63]. It is reported that certain mitochondrial diseases result from the ability of the nucleus to detect energetic deficits in its area. The nucleus attempts to compensate for the ATP shortages by triggering the replication of any nearby mitochondria but unfortunately, the response promotes replication of the very mitochondria that are causing the local energy deficit hence aggravating the problem [64]. The AFB1 also binds to DNA and cause structural DNA alterations that lead to gene mutations as well as changes in the length of the telomeres and the check points in the cell cycle [65]. The binding of AFB1 to DNA at the guanine base in liver cells corrupt the genetic code that regulates cell growth, thereby leading to formation of tumors [66]. The damage to mitDNA is caused by adduction and mutations of mitochondrial membranes leading to increased cell death (apoptosis) as well as disruption of energy production (production of ATP) [67]. The reactive aflatoxin-8, 9-epoxide can affect the mitotic (M) phase, growth process (G1 and G2 phase) and DNA synthesis (S phase) in the cell cycle by disrupting the various check points that regulate the cell cycle development and proliferation leading to deregulation of the cell and hence cancer development [68] (Figure 2).
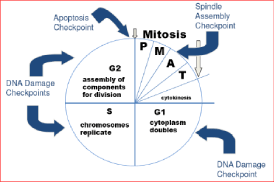
Figure 2: Various check points that can be damaged by binding of aflatoxins and AF-8, 9-epoxide causing the deregulation of the cell cycle; P-Prophase,
M-Metaphase, A-Anaphase, T-Telophase, S-Synthetic DNA phase, G1 and G2-Gaps (growth phase) [69].
However in resistant rodents, their mitDNA is protected from aflatoxins from DNA adducts that effect mitochondrial transcription and translation [64]. The mycotoxin alters energy linked functions of ADP phosphorylation and FAD- and NAD-linked oxidizing substrates and a-ketoglutarate-succinate cytochrome reductases [66].
Effect of aflatoxins on mitochondrial structure: AFB causes ultrastructural changes in mitochondria [65] and also induces mitochondrial directed apoptosis thus reducing their function [68]. Also the aflatoxins may affect the telomere length and the various check point in the cell cycle causing further damage to the regulatory processes of the cell cycle [68]. Also the extent of aflatoxin binding to DNA and its damage, the level of different proteins changes from cell cycle and apoptotic pathways such as c-Myc, p53, pRb, Ras, Protein Kinase A (PKA), Protein Kinase C (PKC), Bcl-2, NF-kB, CDK, cyclins and CKI contribute to the life or death decision making process that may contribute to the deregulation of the cell proliferation leading to cancer development [69] (Figure 2).
Role of glutathione in detoxification of aflatoxins and their metabolites: However like in hepatic detoxification of aflatoxins and other chemicals, GSH act as antioxidant and has many functions in membrane maintenance and stability as well as in reducing oxidative stress factors and the high Reactive Oxygen Species (ROS) produced from the process of lipid peroxidation [70]. The increased depletion of GSH leads to abnormally high levels of ROS found in cells affected by aflatoxin due to uncoupling of metabolic processes resulting from the lack of GSH for GSH-peroxidase catalysis of O2 to H2O2 leading to lipid peroxidation and compromised cell membranes. Its reduction further enhances the damage to critical cellular components (DNA, lipids, proteins) by the 8, 9 epoxides. However the most serious adverse effects of the AFB1-8, 9-epoxide metabolite is that it reacts with amino acids in DNA and forms an adduct [71]. The adduct are fairly resistant to DNA repair processes and this causes gene mutation that leads to liver cancers especially the hepatocellular carcinomas [72].
The role of cytoplasmic reductase in detoxification of AFB1: Also in the hepatocytes, AFB1 are converted to other different classes of metabolites by cytoplasmic reductase such as aflatoxicol and by microsomal mixed-function oxidase system to form AFM1, AGFQ1, AFP1 and AFB1 -epoxide (the most toxic and carcinogenic derivative) and these metabolites may be deposited in various body tissues as well as in edible animal products [73]. These metabolites other than the AFB1 are less toxic and are conjugated with other molecules that enhance their rapid elimination from the body [42]. The metabolite AFQ1 has very little cancer-causing potential and they are usually excreted in urine with little effect on the body [74].
Effect of aflatoxins on protein synthesis: The aflatoxin binds and interferes with enzymes and substrates that are needed in the initiation, transcription and translation processes involved in protein synthesis. They interacts of with purines and purine nucleosides and impair the process of protein synthesis by forming adducts with DNA, RNA and proteins [75]. Aflatoxin also inhibits RNA synthesis by interacting with the DNA-dependent RNA polymerase activity and thus causes degranulation of endoplasmic reticulum. Also the reduction in protein content in body tissues like in skeletal muscle, heart, liver and kidney could be due to increased liver and kidney necrosis [76]. AFB1 is a potent mutagenic, carcinogenic, teratogenic, and immunosuppressive and all these may interfere with normal process of protein synthesis as well as inhibition of several metabolic systems thus causing damages to various organs especially the liver, kidney and heart [77,78].
Role of aflatoxins in cancer: Aflatoxins especially AFB1, AFG1 and AFM1 are the most toxic, naturally occurring carcinogens known with AFB1 the most hepatocarcinogenic compound, causing various cancers of the liver and other body organs in humans and animals [79]. Aflatoxin’s cancer causing potential is due to its ability to produce altered forms of DNA adducts. The primary disease associated with aflatoxin intake is hepatocellular carcinoma (HCC, or liver cancer). This disease is the third-leading cause of cancer death globally [78], with about 550,000-600,000 new cases each year. The incidence of liver cancer has been consistently higher in men than in women with a sex ratio ranging from 2 to 3 in most countries [63]. Eighty-three percent of these cancer deaths occur in East Asia and sub-Saharan Africa [80]. Hepatocellular Carcinoma (HCC) is one of the most common cancers worldwide with extremely poor prognosis. The majority of cases occur in south-east Asia and sub-Saharan Africa where the major risk factors of chronic infection with hepatitis B and C viruses (HBV and HCV) as well as dietary exposure to aflatoxins are a problem [45]. Aflatoxin B1, the most commonly occurring and potent of the aflatoxins is associated with a specific AGG to AGT amino acid transversion mutation at codon 249 of the p53 gene in human HCC, providing mechanistic support to a causal link between exposure and disease [81]. Liver cancer has an increasing incidence that parallels the rise in chronic Hepatitis B (HBV) and Hepatitis C (HCV) infection [82]. Chronic infection with Hepatitis B Virus (HBV) or Hepatitis C Virus (HCV) can progress to advanced liver disease, including cirrhosis and Hepatocellular Carcinoma (HCC), a form of primary liver cancer [83]. HCC is the third leading cause of cancerrelated mortality worldwide [84]. The data show that individuals positive for the hepatitis B virus and exposed to aflatoxin in the diet are about 60 times of risk for developing hepato-biliary carcinoma or liver cancer [46] especially in poor developing countries worldwide [82]. Reports have shown that a number of interactions exist between HBV and aflatoxins in development of hepatocellular carcinoma in humans. They may include the fixation of AFB1-induced mutations in the presence of liver regeneration and hyperplasia induced by chronic HBV infection, the predisposition of HBV infected hepatocytes to aflatoxin induced DNA damage, an increase in susceptibility to chronic HBV infection in aflatoxin exposed individuals and oxidative stress exacerbated by co-exposure to aflatoxins and chronic hepatitis infection [78] (Figure 3).
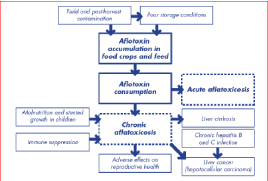
Figure 3: Aflatoxin disease pathways in humans [86,110].
Health effects of aflatoxins on human and animals (aflatoxicosis)
Aflatoxicosis is a condition caused by aflatoxins in both humans and animals. It occurs in two general forms (1) the acute primary aflatoxicosisis produced when moderate to high levels of aflatoxins are consumed. Specific acute episodes of disease may include hemorrhage, acute liver damage, edema, alteration in digestion, absorption and/or metabolism of nutrients, and possibly death [3]. Acute dietary exposure to AFB1 has been implicated in epidemics of acute hepatic injury [85]. Evidence of acute Aflatoxicosis in humans has been reported worldwide especially in the third world countries like Taiwan, Uganda, India, Kenya and many others [86]. (2) The chronic primary aflatoxicosis results from ingestion of low to moderate levels of aflatoxins [86]. The effects are usually subclinical and difficult to recognize. Some of the common symptoms are impaired food conversion and slower rates of growth with or without the production of an overt aflatoxin syndrome [2]. The chronic forms of Aflatoxicosis include (1) teratogenic effects associated with congenital malformations (2) mutagenic effects where aflatoxins cause changes (mutations) in the genetic code, altering DNA and these changes can be chromosomal breaks, rearrangement of chromosome pieces, gain or loss of entire chromosomes, or changes within a gene (3) the carcinogenic effect in which the carcinogenic mechanisms have been identified such as the genotoxic effect where the electrophilic carcinogens alter genes through interaction with DNA and thus becoming a potential for DNA damage and the genotoxic carcino-gens that are sometimes effective after a single exposure, can act in a cumulative manner, or act with other genotoxic carcinogens which affect the same organs [67].
Chronic effects of aflatoxin has been reported to impair the normal body immune function by either by reducing phagocytic activity or reduce T cell number and function as observed immunological suppression in animal model. Aflatoxins have also been reported to interfere with nutrition in a dose response relationship between exposure to aflatoxin and rate of growth in infants and children [87]. Aflatoxins also causes nutrient modification like vitamin A or D in animal models and thus making them unavailable for the normal body physiology and hence leads to nutritional deficiencies [88].
The contamination of foods and feeds with aflatoxin can cause serious consequences in human and animal health. It is estimated that more than 5 billion people in developing countries worldwide are at risk of chronic aflatoxin exposure due to consumption of aflatoxin contaminated foods and of these more than 4 billion people develop aflatoxin related liver cancer especially the hepatocellular carcinoma [20]. Aflatoxin exposure is mainly a problem in poor and developing countries with poor regulatory authorities in food processing and storage as well as with high levels of malnutrition. Aflatoxins have also been linked with kwashiorkor and marasmus in most of the sub- Saharan countries in children [88]. Many people in these countries experience chronic Aflatoxicosis associated with long-term exposure to low to moderate levels of aflatoxin in the food supply chain. AFB1, AFB2 and AFM have been detected in liver, gall bladder, spleen, heart, muscle and kidney [89]. Aflatoxin B1 exposure results in both steatosis and accumulation of fat and necrosis or cell death of liver cells. The amount of aflatoxins consumed contributes to the mutagenic, carcinogenic, teratogenic, and immunosuppressive health effects in the body. The adverse effect of aflatoxins in humans ranges from acute hepatic toxicity to chronic disease such as liver cancer, haemorrhages, oedema, and even immediate death. Prolonged consumption of aflatoxins has also been reported to cause impaired immune function and malnutrition and stunted growth in children and a number of disabilities and death [90]. Human studies have reported that aflatoxins cause an increase in circulating alpha tumor necrosing factor, suggesting that these mycotoxins are also immunotoxic in humans. Due to the aflatoxin body immunosuppressant, it has been associated with HIV and tuberculosis [81] (Figure 1). Aflatoxins also pose a threat to developing fetuses and they are transferred from mother to infant in breast milk. Aflatoxins have been reported to be associated with a Reye-like Syndrome in Thailand, New Zealand, Czechoslovakia, the United States, Malaysia, Venezuela, and Europe [67].
All species of animals are susceptible to Aflatoxicosis and the susceptibility of individual animals to Aflatoxicosis varies considerably depending on dose, duration of exposure, species, age, sex and nutrition. AFB1, AFB2 and AFM have been detected in liver, gall bladder, spleen, heart, muscle and kidney of growing swine when protein and protein-free portions of the diet were separately fed [89]. Chronic exposure of aflatoxins to animals causes immunosuppression and also interferes with protein metabolism and multiple micronutrients that are critical to health due to adduct formation. These adduct are responsible for mutations, cancer, immunosuppression, lung injury and birth defects [64]. In animals, the aflatoxins cause liver damage, decreased milk production, reduced reproductively and suppressed immunity in animals consuming low dietary concentrations. The Aflatoxicosis syndrome in animals may also be characterized by vomiting, abdominal pain, pulmonary oedema, convulsions, coma, and death with cerebral edema and fatty involvement of the liver, kidneys, and heart. In dairy and beef cattle, the signs of acute toxicities include anorexia, depression, dramatic drop in milk production, weight loss, lethargy, gastrointestinal dysfunctions such as ascitis, icterus, tenesmus, abdominal pain, bloody diarrhoea, decreased feed intake and efficiency; weight loss, jaundice, abortion, hepatoencephalopathy, blindness, walking in circles, ear twitching, frothy mouth, photosensitization, bleeding and death [91]. In poultry, beside inappetance, weight loss, decreased egg production, leg and bone problems, poor pigmentation, fatty liver, kidney dysfunction, bruising and death, suppression to natural immunity and susceptibility to parasitic, bacterial and viral infections can occur [42] (Figure 3).
Diagnosis of aflatoxins
Aflatoxicosis in milking cows is readily evident from milk samples. However, diagnosis in nonlactating cattle is more difficult because of the variation in clinical signs, gross pathology, and presence of other diseases due to suppression of the immune system. More than one mold or toxin can further complicate diagnosis as well. By the time overt symptoms are noticed, the prognosis is poor. Aflatoxins have usually been detected by their photo physical properties, such as by absorption and emission spectra. For instance, aflatoxins show characteristic absorption at 360nm, which is the absorption maximum of the aflatoxin ring [92].
Different methods of detection and quantification of aflatoxins are: ELISA, electrochemical immunosensors, chromatography and fluorescence. ELISA is widespread technique. Competitive direct enzyme linked immune sorbent assay test is one of the most common tests conducted in immune biochemistry that allows the user to obtain exact concentration in parts per billion. But it has the disadvantage of requiring well equipped laboratories, well trained professional, harmful solvents and several hours to complete an assay [93]. The detection and quantification of aflatoxins by using electrochemical immune sensor has proven to be efficient, easy to use and able to detect very low levels of aflatoxin. Fluorescence detection is a very good alternative to the conventional techniques. It has a very high sensitivity. But it is inexpensive [94].
Rapid detection techniques are optical fiber, electrochemical transduction, low injection monitoring and biosensors. Most of these still present a lack of applications because of their practical inconveniences except biosensors. The biosensors have been designed to overcome the drawbacks that the common tools employed to detect and quantify aflatoxins presents. Apparently then measurement of aflatoxins in the future tends to be the combination of optical, immunochemical and fluorescence techniques [95].
Economic impacts
The economic consequences of aflatoxicosis are the major areas of concern. Aflatoxins have negative impact on human health, animal productivity and trade. Generally, when susceptible animals are fed contaminated feeds it results in reduced growth rates, illness, and death; moreover, their meat and milk may contain toxic biotransformation products. Livestock owners often take farmers and feed companies to court legal battles can involve considerable amounts of money [96]. The direct economic impact of aflatoxin contamination in crops results mainly from a reduction in marketable by rejection of products from the international market and losses incurred from livestock disease, consequential morbidity and mortality which leads to volume and value loss in the national markets which is huge economic loss [34]. Recommended sanitary and phytosanitary standards set for aflatoxins adversely affect grain trade in developing countries, specifically in the international market, products that do not meet the aflatoxin standards are either rejected at the border, rejected in channels of distribution, assigned a reduced price [97].
The crops contaminated with high levels of aflatoxins are sometimes diverted to animal feed, which resulting in reduced growth rates and illness of animals consuming toxic contaminated feeds. Many countries have established regulations to limit exposure to aflatoxin, typically expressed in parts per billion (ppb). These regulations can result in foregone trade revenues arising from increased cost of meeting the standards including cost of testing, rejection of shipments and even eventual loss of admissibility into foreign markets [98]. Toxigenic fungal pathogens are important constraints to the production of the crop, affecting the quality of the seeds through spoilage, however, aflatoxin contamination is the most important quality problem in Ethiopia with serious health consequences for human and livestock for example groundnut plays an important role as a food as well as a cash crop in Ethiopia. Currently the crop is becoming one of the high value crops that are growing in the dry land areas of the Tigray region, Northern Ethiopia, but the groundnut production highly attack by Aflatoxicosis [33].
Controls and prevention
Control of aflatoxins requires an integrated approach, whereby aflatoxins are controlled at all stages from the field to the table, is required for reduction in risk. Such an approach includes targeted plant breeding practices, enhancement of host plant resistance, and biological control methods, coupled with post-harvest technologies such as proper drying and storage of potentially affected crop products, as well as development of appropriate alternative uses to retain at least some economic return on value of damaged crop. Therefore by removing the sources of contamination, promoting better agricultural and storage techniques, ensuring adequate resources are available for testing and early diagnosis, enforcing strict food safety standards, informing and educating consumers and (small/subsistence) farmers, promoting better livestock feeding and management, and creating general awareness about personal protection, are some of the ways in which national authorities can help to control aflatoxins [99].
Aflatoxicosis can be prevented by feeding rations free of aflatoxin. Preventing aflatoxin contamination requires on-going and thorough sampling and testing program. It requires purchasing feed from reputable persons and companies experienced in aflatoxin prevention and who have a proven record of properly monitoring their feed products. A reliable feed company will also carry insurance to cover misfortunes with aflatoxins or other problems. A good deal on feed prices can be the most expensive buy a dairy farmer ever makes if it proves to contain aflatoxin. Storing feed at proper moisture levels and developing a systematic inspection and clean-up program to keep bins, delivery trucks and other equipment free of adhering or caked feed ingredients are also the most important options. Minimizing dust accumulation in milling and mixing areas and treating grains contaminated with aflatoxins by ammonia have also been successfully inclusive method of preventing the disease but it is expensive and dangerous to do [100].
The presence and growth of Aspergillus on pre-harvested crops is dependent on the environment. Agricultural practices including proper farm system and pest management can reduce aflatoxin contamination [101]. Controlling or reducing infection by regulating the factors that increase the risk of aflatoxin contamination in the field contributes extensively in managing aflatoxin [102]. Management practices that reduce the incidence of aflatoxin contamination in the field include timely planting, maintaining optimal plant densities, proper plant nutrition, avoiding drought stress, controlling other plant pathogens, weeds and insect pests and proper harvesting. Another potential means for aflatoxin control is the bio control of fungal growth in the field. Numerous organisms have been tested for biological control of aflatoxin contamination including bacteria, yeasts, and non-toxigenic (toxigenic) strains of the causal organisms of which only toxigenic strains have reached the commercial stage [103].
Soil testing, field crop rotation, antifungal chemical treatments (for example, proprionic and acetic acids), and adequate insect and weed prevention. Harvesting strategies include use of functional harvesting equipment, clean and dry collection/transportation equipment, and appropriate harvesting conditions (low moisture and full maturity). Postharvest measures include use of drying as dictated by moisture content of the harvested grain, appropriate storage conditions, and use of transport vehicles that are dry and free of visible fungal growth [104]. Sorting out of physically damaged and infected grains from the intact commodity can result in 40-80% reduction in aflatoxin levels. The advantage of this method is that it reduces toxin concentrations to safe levels without the production of toxin degradation products or any reduction in the nutritional value of the food. This could be done manually. Market practices such as grading have also been shown to reduce levels of aflatoxin [105]. Clearing the remains of previous harvests and destroying infested crop residues are basic sanitary measures that are also effective against storage deterioration. Cleaning of stores before loading in the new harvests was correlated with reduction in aflatoxin levels. Wild hosts, which constitute a major source of infestation for storage pests, should also be removed from the vicinity of stores. These improved postharvest technologies have been used successfully to reduce the blood aflatoxin [106]. Aflatoxin regulatory programs are already in place in most countries to the export side these regulatory programs are strictly enforced to protect the export market of agricultural commodities, otherwise the importing countries would reject the commodities resulting in a loss of valuable foreign exchange earnings. Domestic regulatory measures on aflatoxin have received very little attention and are enforced, with no incentives given for the aflatoxin free produce and no heavy penalty on the violators of aflatoxin regulations [16].
To reduce the losses due to aflatoxin contamination risks must be solved through using sanitary measures during pre- harvest, harvest postharvest handling of food and feeds of animals. Multi-sectorial approaches programs are required at all levels to control aflatoxin contamination and improve the quality of product to security and health assessments of communities. Aflatoxin control program will include complementary components such as relevant information standards regulations policy to distribute safe and high quality food to fulfill consumer’s demands [107].
Many chemical preservatives have been used for the control of Aspergillus food contamination such as fungicides, herbicides and insecticides for healthier plants that resist fungus [108]. The widespread use of chemical preservative has significant drawbacks including increased cost, handling hazards, concern about residues of pesticide on food and threat to human health and environment. There is no specific antidote for toxicity of aflatoxins. However; timely administration of l-methionine (200mg/kg) and sodium thiosulfate (50mg/kg) at eight-hour intervals is proven to be of therapeutic value. Supplementation with increased levels of protein, vitamins and antioxidants can also be rewarding [109,110].
Conclusion and Recommendations
In general, chronic consumption of aflatoxin contaminated foods is a common problem in both animals and humans worldwide especially in poor developing nations, where there is poor food harvesting, processing and storage of food and food products thus allowing the growth of mold on them. Dairy products play a significant role in human diet since they are rich sources of bioavailable calcium and proteins. The occurrence of AFM1 in milk and milk products is a public health concern. Aflatoxin M1 in milk and dairy products could be a risk to human as well as animal health. High contamination in feed may result in a significant AFM1 level in milk when animals are fed with highly contaminated foodstuffs. Meeting the demands for higher milk yields and striving for increased milk production may create such situations. AFM1 appears to be a natural contaminant in milk and dairy products. For this reason, milk and dairy products have to be inspected and controlled continuously for AFM1 contamination and animal feeds should be checked regularly for AFB1 and storage conditions of feeds must be taken under strict control. However, analytical methods that can simultaneously detect and quantify a broad number of mycotoxins in milk with low limits of detection and quantification are needed to reduce analytical costs and to allow more frequent monitoring of mycotoxins in milk. In short, adopting good harvesting practices, improving analytical facilities, and implementing strict regulations would avoid or reduce these natural contaminants in milk and ensure the safety of milk and milk products as human food.
In line with the above conclusion, the following recommendations are forwarded:
• Natural contaminants of food chain with aflatoxin should be reduced by using sanitary measures and a multi face awareness approach.
• There should be new strategies to address effectively within developing countries, where aflatoxin exposure is intertwined with the issues of food insecurity and insufficiency.
• Collaboration between the agricultural and public health communities, between the local, regional, national, and international governing bodies, and between different disciplines within public health and agricultural is necessary to reduce aflatoxin exposure.
Acknowledgements
Greatly appreciated thanks to God, by whom I passed all the constraints of life and reach the final stage of the first degree graduation.
In addition, I would like to thank all of my families those are supporting me economically and morally in all my educational levels.
Finally, I have a great thanks to my classmates and lecturers of Wolaita Sodo University, School of Veterinary Medicine for their valuable effort and supports.
References
- Bankole SA, Adebanjo A. Review of mycotoxins in food in West Africa: current situation and possibilities of controlling it. African Journal of Biotechnology. 2003; 2: 254-263.
- WHO. Hazardous Chemicals in Humans and Environmental Health: International Programme on Chemical safety, Geneva, Switzerland. World Health Organization. 2000; 7-9.
- Thrasher JD. Aflatoxicosis in animals. Aflatoxins and Health. 2012.
- Lopez C, Bulacio L, Ramadan S, Ramos L. Rodriguez Aflatoxin B1 content in patients with hepatic diseases. Medicine. 2002; 62: 313-316.
- Prandini A, Tansini G, Sigolo S, Filippi L, Laporta M, Piva G. On occurrence of aflatoxin M1 in milk and dairy products. Food Chem Toxicol. 2009; 47: 984-991.
- Galvano F, Galofaro V, DeAngelis A, Galvano M, Bognanno M, Galvano G. Survey of the occurrence of aflatoxin M1 in dairy products marketed in Italy. J Food Protection. 1998; 61: 738-741.
- Colak H. Determination of aflatoxin M1 levels in Turkish white and kashar cheeses produced from experimentally contaminated raw milk. J. Food and Drug Anal. 2007; 15: 163-168.
- Iqbal SZ, Asi MR, Ari&nTilde;o A. Aflatoxin M1contamination in cow and buffalo milk samples from the North West Frontier Province (NWFP) and Punjab provinces of Pakistan. Food Addit Contam: Part B. 2011; 4: 282-288.
- Bryden WL. Mycotoxin contamination of the feed supply chain: implications for animal productivity and feed security. Anim Feed Sci Technol. 2012; 173: 134-158.
- Castegnaro M, McGregor D. Carcinogenic risk assessment of mycotoxins. Rev MedVet. 1998; 149: 671-678.
- Asi MR, Iqbal SZ, Ari&nTilde;o A, Hussain A. Effect of seasonal variations and lactation times on aflatoxin M1contamination in milk of different species from Punjab, Pakistan. Food Control. 2012; 25: 34-38.
- Dohnal V, Wu Q, Kuca K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch Toxicol. 2014; 88: 1635-1644.
- IARC (International Agency for Research on Cancer): Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, Lyon, France. 1993; 56: 1-391.
- Gürbay A, Aydin S, Girgin G, Engin AB, Sahin G. Assessment of aflatoxin M1 levels in milk in Ankara, Turkey. Food Control. 2006; 17: 1-4.
- Elkak A, Atat OEl, Habib J, Abbas M. Occurrence of a flatoxin M1 in cheese processed and marketed in Lebanon. Food Control. 2012; 25: 140-143.
- Williams H, Phillips D, Jolly E, Stiles K, Jolly M, Aggarwal D. Human Aflatoxicosis in developing countries a review of toxicology exposure potential Health consequences and interventions. Am J Clin Nutr. 2004; 80: 1106-1122.
- WHO. Public health Strategies for Preventing Aflatoxin exposure. Work group report for the international Mycotoxin Workshop. World Health Organization, Geneva, Switzerland. 2005; 1-26.
- Talebi E, Khademi M, Rastad A. An Over Review on Effect of Aflatoxin in Animal Husbandry; Asian J. Exp. Biol. Sci. 2011; 2: 754-757.
- Ramesh V, Siruguri V. Food safety in food security and food trade Mycotoxin Food Safety Risk in Developing Countries. Journal of Food Microbiology. 2003; 7: 219-225.
- Shephard GS. Risk assessment of aflatoxins in food in Africa. Food Additives & Contaminants: Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment. 2008; 25: 1246-1256.
- International Agencies for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, Lyon, France. 1993; 56: 245-395.
- Waliyar F, Reddy S. Immunochemical methods of analysis. International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) strategy. 2003; 3: 79-102.
- Pitt J, Miscamble B. Water relations of as per gillusflav us and closely Related species. Food Protection. 1995; 58: 86-90.
- Scott L, King W, Richard L, Greer T. Analytical scale super critical fluid extraction of aflatoxin B1 from field inoculated corn. Journal of agricultural and Food chemistry. 1993; 8: 910-913.
- Health cote G, Hibert R. Aflatoxins: Chemical and Biological aspects. Journal of Food Science. 2005; 5: 30-130.
- Gilbert J, Anklam E. Validation of analytical methods for determining mycotoxins in foodstuffs. Trends Anal Chem. 2002; 21: 468-486.
- Strosnider H, Baumgartner E, Banziger M, Bhat V. Work group report public Health strategies for reducing aflatoxin exposure in developing countries. Environ Health Perspect. 2006; 114: 1898-1903.
- Kumar V, Basu S, Rajendran T. Mycotoxin Research and Mycoflora in some commercially important agricultural commodities. Crop Protection. 2008; 27: 891-905.
- World Health Organization. Mycotoxins in African foods: Implications to food safety and Health AFRO Food Safety Newsletter World Health Organization Food Safety Issue. 2006.
- Diaz G, Rojas Y, Perilla S. Occurrence of Aflatoxins in selected Colombian foods. Mycotoxin Res. 2001; 17: 15-20.
- Bhatti B, Sardar R, Talat T. Estimation of aflatoxin B1 in feed ingredients and compound poultry feeds. KPakistan Veterinary Journal. 2001; 21: 57-60.
- Scheidegger K, Payne G. unlocking the secrets behind secondary metabolism: A Review of Aspergillus flavus from pathogenicity to functional genomics Journal of Toxicology. 2003; 22: 423-459.
- Bhat R, Vasanthi S. Mycotoxin food safety risks in developing countries Agriculture and Environment Focus. 2003; 10: 1-2.
- Wagacha J, Muthomi J. Mycotoxin problem in Africa current status implications to food safety and Health and possible management strategies. Int Food Microbiol. 2008; 124: 1-12.
- Smith J, Solomon G, Lewis C, Anderson J. Mycotoxins in human Nutrition and Health. European Commission Publication. 2001; 10: 670-700.
- Tajkarimi M, Aliabadi-Sh F, Salah Nejad A, Poursoltani H, Motallebi AA, Mahdavi H. Aflatoxin M1 contamination in winter and summer milk in 14 states in Iran. Food Control. 2008; 19: 1033-1036.
- Nilchian Z, Rahimi E. Aflatoxin M1 in Yoghurts, Cheese and Ice-Cream in Shahrekord- Iran. World Applied Sciences Journal. 2012; 19: 621-624.
- Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: A review. Critical Reviews in Toxicol. 2011; 41: 1-16.
- Azizollahi Aliabadi M, Issazadeh K, Kazemi Darsanaki R, Laleh Rokhi M, Amini A. Determination of Aflatoxin M1 Levels in White Cheese Samples by ELISA in Gilan Province, Iran. Global Veterinaria. 2012; 8: 707-710.
- Celik TH, Sarimehmetoglu B, Kuplulu O. Aflatoxin M1 contamination in pasteurised milk. Veterinarski Arhiv. 2005; 75: 57-65.
- Akkaya L, Birdane YO, Oguz H, Cemek M. Occurrence of aflatoxin M1 in yogurt samples from afyonkarahisar, Turkey. Bull Vet Inst Pulawy. 2006; 50: 517-519.
- Agag BI. Mycotoxins in foods and feeds: Aflatoxins. Association of Universal Bullettin of Environmental Research. 2004; 7: 173-191.
- Larsson P, Tjalve H. Intranasal instillation of Aflatoxin B1 in rats: Bio activation in the nasal mucosa and neuronal transport to the olfactory bulb. Toxicological Science. 2000; 55: 383-391.
- Coulombe RA Jr. Nonhepatic disposition and effects of aflatoxin B1. The Toxicology of Aflatoxins: Human Health, Veterinary and Agricultural Significance. 1994; 89-101.
- Wild CP, Montesano R. A model of interaction: Aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Letters. 2009; 286: 22-28.
- Wu F, Khlangwiset P. Health economic impacts and cost-effectiveness of aflatoxin reduction strategies in Africa: Case studies in biocontrol and postharvest interventions. Food Additives & Contaminants. 2010; 27: 496- 509.
- Guengerich FP, Johnson WW, Shimada T, Ueng YF, Yamazaki H, Langouët S. Activation and detoxication of aflatoxin B1. Mutatation Research. 1998; 402: 121-128.
- Wild CP, Turner PC. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis. 2002; 17: 471-481.
- Wang H, Dick R, Yin H, Licad-Coles E, Kroetz DL, Szklarz G, et al. Structurefunction relationships of human liver cytochromes 450A: aflatoxin B1 metabolism as a probe. Biochemistry. 1998; 37: 12536-12545.
- Kitada M, Taneda M, Ohi H, Komori M. Mutagenic activation of aflatoxin B1 by 450HFLa in human fetal livers. Mutation Research. 1998; 227: 53-58.
- Raney VM, Harris TM, Stone MP. DNA conformation mediates aflatoxin B1- DNA binding and the formation of guanine N7 adducts by aflatoxin B1 8, 9- exo-epoxide. Chemical Research in Toxicology. 1993; 6: 64-68.
- Guengerich FP. Forging the links between metabolism and carcinogenesis. Mutation Research. 2001; 195-209.
- Bailey EA, Iyer RS, Stone MP, Harris TM, Essigmann JM. Mutational properties of the primary aflatoxin B1-DNA adduct. Proceedings of the National Academy of Sciences, USA. 1996; 93: 1535-1539.
- Sudakin DL. Dietary aflatoxin exposure and chemoprevention of cancer: A clinical review. Journal of Toxicology and Clinical Toxicology. 2003; 41: 195- 204.
- Aguilar F, Hussain SP, Cerutti P. Aflatoxin B1 induces the transversion of G-->T in codon 249 of the 53 tumor suppressor gene in human hepatocytes. PNAS. 1993; 90: 8586-8590.
- Mace K, Aguilar F, Wang JS, Vautravers P, Gómez-Lechón M, Gonzalez FJ, et al. Aflatoxin B1-induced DNA adduct formation and 53 mutations in CYP450-expressing human liver cell lines. Carcinogenesis. 1997; 18: 1291- 1297.
- Sherratt PJ, Hayes JD. Glutathione S-transferase. Enzyme systems that metabolize drugs and other xenobiotics. 2001; 9: 320-351.
- Farombi EO, Nwaokeafor IA. Anti-oxidant mechanisms of kolaviron: studies on serum lipoprotein oxidation, metal chelation and oxidative membrane damage in rats. Clin; inical and Experimental Pharmacology and Physiology. 2005; 32: 667-674.
- Whitlow LWW, Hagler MJ, Diaz DE. Mycotoxins in in feeds. Feedstuffs. 2010; 74.
- Sabbioni G, Wild CP. Identification of an aflatoxin G1-serum albumin adduct and its relevance to the measurement of human exposure to aflatoxins. Carcinogenesis. 1991; 12: 97-103.
- Knight LP, Primiano T, Groopman JD, Kensler TW, Sutter TR. cDNA cloning, expression and activity of a second human aflatoxin B1 metabolizing member of the aldo-keto reductase superfamily, AKR7A3. Carcinogenesis. 1999; 20: 1215-1223.
- Brown KL, Bren U, Stone MP, Guengerich FP. Inherent Stereo specificity in the Reaction of Aflatoxin B1 8, 9-Epoxide with Deoxyguanosine and Efficiency of DNA Catalysis. Chemical Research in Toxicology. 2009; 22: 913-917.
- WHO. World Health Statistics. World Health Organization, Geneva. 2008.
- Wallace DC. Mitochondrial DNA in aging and disease. Scientific American. 1997; 40-47.
- Ezekiel CN, Alabi OA, Anokwuru CP, Oginni O. Studies on Dietary Aflatoxininduced Geno toxicity using two In vivo bioassays. Archives of Applied Science Research. 2011; 3: 97-106.
- Vermeulen K, Berneman ZN, Bockstaele DRV. Cell cycle and apoptosis. Cell proliferation. 2003; 36: 165-175
- Thrasher JD, Crawley SL. Neurotoxicity of Mycotoxins. 2012.
- Hornsby PJ. Senescence: As an Anticancer Mechanism. Journal of Clinical Oncology. 2007; 25: 1852-1857.
- Jacotot E, Ferri KF, Kroemer G. Apoptosis and cell cycle: distinct checkpoints with overlapping upstream control. Pathological Biology (Paris). 2000; 48: 271-279.
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochemistry Journal. 2007; 401: 1-11.
- Schubert D, Piasecki D. Oxidative Glutamate Toxicity Can Be a Component of the Excitotoxicity Cascade. The Journal of Neuroscience. 2001; 21: 7455- 7462.
- Verma RJ. Aflatoxin Cause DNA Damage. International Journal of Human Genetics. 2005; 4: 231-236.
- Zhang X, Zhang QY, Liu D, Su T, Weng Y, Ling G, et al. Expression of cytochrome 450 and other biotransformation genes in fetal and adult human nasal mucosa. Drug Metabolism and Disposition. 2005; 33: 1423-1428.
- Bbosa GS, Kitya D, Lubega A, Jasper Ogwal-Okeng, Anokbonggo WW, Kyegombe DB. Review of the Biological and Health Effects of Aflatoxins on Body Organs and Body Systems. Aflatoxins - Recent Advances and Future Prospects. 2013; 240-265.
- Clifford JI, Rees KR. The Interaction of Afiatoxins with Purines and Purine Nucleosides. Biochemistry Journal. 1967; 103: 467-471.
- Sharma V, Sharma C, Paliwal R, Pracheta. Ameliorative Effects of Curcuma Longa and Curcumin on Aflatoxin B1 Induced Serological and Biochemical Changes In Kidney of Male Mice. Asian Journal of Biochemical and Pharmaceutical Research. 2011; 1: 338-351.
- Mohammed AM, Metwally NS. Antiaflatoxicogenic activities of some aqeous plant extracts against AFB1 induced Renal and Cardiac damage. Journal of Pharmacology and Toxicology. 2009; 4: 1-16.
- Beckingham IJ. ABC of liver, pancreas and gall bladder. British Medical Journal. 2001; 1-49.
- Kitya D, Bbosa GS, Mulogo E. Aflatoxin levels in common foods of South Western Uganda: a risk factor to hepatocellular carcinoma. European Journal of Cancer Care. 2009; 1-6.
- Kirk GD, Bah E, Montesano R. Molecular epidemiology of human liver cancer: Insights into etiology, pathogenesis and prevention from The Gambia. Carcinogenesis. 2006; 27: 2070-2082.
- Groopman JD, Kensler TW, Wild CP. Protective Interventions to Prevent Aflatoxin-Induced Carcinogenesis in Developing Countries. Annual Review of Public Health. 2008; 29: 187-203.
- Liu Y, Wu F. Global Burden of Aflatoxin-Induced Hepatocellular Carcinoma: A Risk Assessment. Environmental Health Perspectives. 2010; 118: 818- 824.
- Henry SH, Bosch XF, Bower JC. Mycotoxins and food safety. Advances in Experimental Medicine and Biology. 2002; 504: 229-233.
- Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxinrelated liver cancer: Systematic review and meta-analysis. European Journal of Cancer. 2012.
- Farombi EO. Aflatoxin contamination of foods in developing countries: Implications for hepatocellular carcinoma and chemopreventive strategies: Review. African Journal of Biotechnology. 2006; 5: 001-014.
- USAID. Aflatoxin: A Synthesis of the Research in Health, Agriculture and Trade. Feed the Future: The Office of Regional Economic Integration USAID East Africa Regional Mission Nairobi, Kenya. 2012; 10-15.
- Wangikar PB, Dwivedi P, Sinha N, Sharma AK, Telang AG. Teratogenic effects in rabbits of simultaneous exposure to ochratoxin A and aflatoxin B1 with special reference to microscopic effects. Toxicology. 2005; 215: 37-47.
- Peraica M, Radic B, Lucic A, Pavlovic M. Toxic effects of mycotoxins in humans. Bulletin of the World Health Organization. 1999; 754-766.
- Murthy TRK, Jemmali M, Henry Y, Frayssinet C. Aflatoxin B1, B2 and M were detected in liver, gall bladder, spleen, heart, muscle and kidney of growing swine when protein and protein- free portions of the diet were separately fed. Journal of Animal Science. 1975; 41: 1339-1347.
- Barrett JR. Liver Cancer and Aflatoxin: New Information from the Kenyan Outbreak. Environmental Health Perspectives. 2005; 113: A837-A838.
- Fapohunda SO, Awoyinka OA, Olufunmiso OO, Ezekiel CN. Enzyme-related aflatoxin production in vital organs of rats fed with Aspergillus speciesinoculated rat chow. Journal of Biology and Environmental Science. 2007; 1: 1-41.
- Korde A, Banerjee S, Pillai M, Venkatesh M. Preparation and evaluation of AflatoxinB1. Journal of Radioanal Nuclear Chemistry. 2001; 250: 231-237.
- Dallasta C, Ingletto G, Corradini R, Galaverna G, Marchelli R. Fluorescence enhancement of aflatoxins using native and substituted cyclodextrins. Journal of Inclusion Phenomena and Macrocyclic Chemistry. 2003; 45: 257- 263.
- Cavaliere C, Foglia P, Pastorini E, Samperi R, Lagana A. Liquid chromatography/tandem mass spectrometric confirmatory method for determining aflatoxin M1 in cow milk: Comparison between electro spray and atmospheric pressure photo ionization sources. J Chromatogr A. 2006; 1101: 69-78.
- Carlson M, Bargeron C, Benson R, Fraser A, Phillips T, Velky JT, et al. An automated, handheld biosensor for aflatoxin. Biosens Bioelectron. 2000; 14: 841-848.
- Pier A, Richard L, Cysewski S. Implications of mycotoxins in animal Disease. Journal of American Veterinary Medicine Association. 1990; 176: 719-724.
- Gebrehiwet Y, Kirsten J, Qangweni W. Quantifying the trade effect of sanitary and phytosanitary regulations of Organization for Economic Cooperation and Development (OECD) countries on South African food exports Agrekon. 2007; 46: 23-38.
- Cooper J, Dobson H. The benefits of pesticides to mankind and the Environment crop protection. 2007; 23: 1337-1348.
- World Health Organization (WHO) Aflatoxins. Department of food safety and zoonosis. 2018; 1-5 De.
- Ellen R. Aflatoxins and Dairy Cattle. Educational programs of the Texas Agri Life Extension Service. 2010; 3: 1-4.
- Smela M, Sophie S, Curier E, Bailey A, John M. The chemistry and biology of aflatoxinB1.Carcinogenesis. 2001; 22: 535-545.
- Bruns A. Controlling Aflatoxin and fumonisin in maize by crop management Journal of Toxicology Review. 2003; 22: 153-173.
- Yin Y, Jiang H, Ma H. Biological control of aflatoxin contamination of crops Journal of Zhejiang University. 2008; 9: 787-792.
- Codex Alimentarius Commission (CAC). Code of practice for the prevention and reduction of mycotox in contamination in cereals. 2003.
- Mutegi C, Hendriks S, Jones R, Okello J, Ngugi H. Role of collective action and handling practices on aflatoxin contamination of ground nuts. Proceedings of the African Crop Science conference. 2007; 8: 1779-1782.
- Hell KB, Yopadhyay R, Cardwell, Ketal. Pre and post -harvest management of aflatoxin in maize. Wallingford, Unite Kingdom. 2008; 210-219.
- Hassan A, Aziz N. Influence of moisture content and storage temperature on the production of aflatoxins by A. flavusinmaize after exposure to Gamma radiation. Journal of food safety. 1998; 3: 159-171.
- Maier E, Fuch D, Jenny M, Kurz K, Schennach H. Food preservatives. Food Chemistry and Toxicology. 2010; 48: 1950-1956.
- Nollet L, Rathore H. Handbook of Pesticides Methods. Pesticide Residues Analysis, USA. 2010; 25-30.
- Wu F, Tritscher A. Aflatoxins a global public health problem: Aflatoxins health impact, Jan 2011. World Health Organization. 2011; 1-18.