
Research Article
Austin J Vet Sci & Anim Husb. 2019; 6(2): 1057.
Development and Validation of a Novel ELISA for the Detection of Neospora caninum Antibodies in Bovine Sera
Mansilla FC1*, Gual I2,4, Pereyra WR2,3, Cardoso NP1,2, Turco CS1 and Capozzo AV1,2
1Instituto de Virología, CICVyA, INTA CNIA, Hurlingham, Buenos Aires, Argentina
2Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
3Estación Experimental Agropecuaria INTA Salta, Salta, Argentina
4Estación Experimental Agropecuaria INTA Balcarce, Balcarce, Buenos Aires, Argentina
*Corresponding author: Mansilla FC, INTA-Instituto de Virología, Centro de Investigaciones en Ciencias Veterinarias y Agronómicas, N. Repetto y De los Reseros de S/N, Hurlingham, Buenos Aires, Argentina
Received: May 17, 2019; Accepted: June 19, 2019; Published: June 26, 2019
Abstract
Neospora caninum is a world-wide distributed apicomplexan parasite, causative agent of bovine neosporosis, which is one of the major causes of reproductive losses in cattle affecting both dairy and beef industries. Several techniques are regularly used for the diagnosis of bovine neosporosis. Amongst them, serological techniques are generally preferred, being IFAT the traditionally accepted reference method for diagnosis (gold standard). However, ELISA has become the most eligible method for large-scale screening of specific antibodies against N. caninum in diagnostic laboratories around the world, providing results that can be easily standardized between laboratories, within a few hours at a relative low-cost. There is no local production of commercial ELISA tests nor in Argentina neither in South America, therefore they must be purchased abroad, turning unfeasible the routinely use of ELISA tests. Here we have developed and validated accordingly to OIE specifications a novel ELISA test based on soluble antigens form N. caninum tachyzoites to accurately determine the presence of specific antibodies anti- N. caninum antigens in bovine sera samples, enabling the rapid, objective and low- cost diagnosis of bovine neosporosis in Argentina; replacing IFAT and avoiding delays and high importation taxes.
Keywords: Neospora caninum; ELISA; Bovine Sera Samples; IgG
Introduction
Neospora caninum is a world-wide distributed apicomplexan parasite, closely related to Toxoplasma gondii. It has a heteroxene life cycle, in which dogs and canids in general are the definitive hosts [1]; and a wide range of domestic and wild animals (cattle, buffalos, sheep’s, horses, dogs, cats, etc) behave as intermediate hosts [2].
N. caninum is the causative agent of bovine neosporosis, which is considered one of the major causes of reproductive losses in cattle as it can lead to abortion, stillbirth, reduced milk production and weight gain and increased birth-to-conception intervals and replacement rates [3-6] induced by mid-gestational exposure to the parasite or recrudescence of latent infections [2,7]. Globally, the estimated median losses due to N. caninum induced abortions were valued in US $1,298.3 million per annum (range US $633.4 million to US $2,380.1 million), affecting both dairy and beef industries [8].
Despite several approaches to N. caninum vaccines have been reported [9], currently there is no a preventive method worldwide available to control this disease, and only few management options are employed [10,11]. The culling of infected cattle from the herd has been proposed to be a quite efficacious control method, though it is very expensive [12]. Other lower-cost options include the selective breeding from only sero-negative cows, breeding of sero-positive cows only to beef, and the culling of those cows that have actually aborted; besides strengthening the biosecurity measures to avoid horizontal transmission. In addition to this, it has been reported that seropositivity to N. caninum increases the risk of abortion, with estimates for the increased risk often quoted to range between 1.7 and 7.4-fold [5,13], but decreasing over time with increasing parity 5. In this context, the development of reliable diagnostic tools to accurately detect the spread and circulation of the parasite is paramount.
Several techniques are regularly used for the diagnosis of bovine neosporosis, including histology and Immunohistochemical staining (IHQ), nested-PCR [14], Immunoblotting (IB), Direct Agglutination test (DAT), direct Fluorescent Antibody Test (IFAT) and different Enzyme-Linked Immunoabsorbent Assays (ELISAs) [15,16]. Serological techniques are preferred, being IFAT the accepted reference method for diagnosis and therefore it is generally considered as the “gold standard” technique [17,18]. However, due to the fact that it is easier and less time-consuming to obtain rapid results from large-scale cattle herds with ELISAs than with IFAT, this assay has become the most eligible method for large-scale screening of specific antibodies against N. caninum in diagnostic laboratories around the world [3]. ELISAs can provide results within a few hours at relatively low-cost, do not rely on cell cultures or live parasites and do not need expensive specialized equipment, thus can be performed in almost any laboratory. ELISAs are also useful for determining the route of N. caninum transmission as avidity tests enable to differentiate between acute and chronic infections [19-23]. Furthermore, discrete results obtained by this assay allow the operator to minimize the bias introduced by the subjective analysis of the data achieved by IFAT. This is particularly relevant when comparing results obtained from different laboratories and countries, as the lack of standardization between protocols involved in the diagnosis of neosporosis may interfere with the accurate estimation of its prevalence and with the management strategies described above [24,25].
Despite different commercial ELISA tests are available in many countries, there is no local production of those kind of products in Argentina; therefore they must be purchased abroad [25]. This implies high costs and long waiting periods, turning unfeasible the routinely use of ELISA tests in the diagnosis of bovine neosporosis.
The objective of this study was to develop and validate accordingly to OIE specifications a novel ELISA test based on soluble antigens form N. caninum tachyzoites to accurately determine the presence of specific antibodies anti- N. caninum antigens in bovine sera samples. This would enable the rapid, objective and low-cost diagnosis of bovine neosporosis in Argentina; replacing IFAT and avoiding delays and high importation taxes.
Materials and Methods
Reference sera samples
A total of 634 sera samples gently provided by Dr. Dadin P. Moore from the Animal Health Group (EEA INTA Balcarce) were involved in this study. They correspond to two different panels. One of them consisted of 374 samples from 27 pregnant heifers from a herd with high prevalence of the disease; whose serostatus was monitored by IFAT during gestation. The other panel comprised 260 samples corresponding to 130 heifers at parturition and their calves before colostrum intake.
Indirect fluorescent antibody test (IFAT)
The presence of specific antibodies anti-N. caninum antigens was assessed in each sample employing IFAT as the “gold standard” technique. IFAT was performed using a fluorescein is thiocyanate labeled affinity-purified rabbit anti-bovine IgG antibody (Sigma, St. Louis, USA), as it was previously described [26-28]. Briefly, N. caninum specific antibodies were measured in serum samples using dilutions starting at 1:25. Positive and negative control sera were used. Slides were examined with an epifluorescence microscope (Olympus Bx 51, Olympus Inc, Tokyo, Japan). Antibody titers were expressed as the reciprocal of the highest serum dilution that showed complete peripheral fluorescence of tachyzoites [29]. A titer greater than 1:25 was indicative for N. caninum infection [26].
ELISA procedures
Purification of the antigen: The soluble fraction of a whole tachyzoite lysate (sNcAg), purified as previously described [30,31], was used as capture antigen. Briefly, tachyzoites (Nc1 strain) were partially purified using sephadex columns (Sephadex TM G-25 Medium, GE Healthcare, Sweden); disrupted by ultrasonic treatment and centrifuged. Soluble antigens (sNcAg) were recovered from the supernatant and quantified using a commercial protein assay method (Micro BCA Pierce, Rockford, USA).
Feasibility and standardization: To standardize and optimize the ELISA protocol positive and negative control samples were generated by pooling sera samples from three positive or negative cattle, respectively, according to IFAT titers. Those controls were diluted 1:10 in stabilizing buffer and were used throughout the different steps of the validation process.
Polystyrene microtitre plates (Grenier Bio One, Austria) were coated with different amounts of sNcAg per well (500, 750 and 1000ng) diluted in coating buffer (0.05M carbonate-bicarbonate buffer, pH 9.6). Different dilutions of the sera (1:40; 1:60; 1:120) and the antibody anti-bovine IgG: HRP conjugated (1:2500, 1:5000; Jackson Immuno Research, PA, USA) and two different presentations of the substrate (revealing solution) were evaluated. To reduce the background level, different blocking solutions (with 0.5 to 5% of the blocking agent) and different washing conditions (in PBS-tween 0.05%) were also assessed. Plates were incubated with different blocking solutions for 60 and 90 minutes at 37°C and for 16 hours at 4°C. Several conditions related to the incubation of samples and conjugate were also assessed, ranging from 21 to 37°C and from 10 to 60 minutes.
Accuracy assessment: Repeatability, reproducibility and precision: The repeatability of the assay was evaluated by selecting a strong, mid and weak positive sample (IFAT titers 1:1600, 1:800 and 1:400, respectively) and a negative control (IFAT titer ‹1:25). They were run by ELISA in triplicate in eight independent series within the same plate. The reproducibility was evaluated by the analysis of 20 randomly selected samples by two operators from different laboratories. These samples were run 8 times by each operator in independent assays. A Man-Whitney test was developed for each sample to assess significant differences between operators. The precision of the positive and negative controls was determined running them 20 times in independent assays. For each determination Coefficient of Variation (CVs) were determined. Accuracy tests were approved when CVs between OD values were 30% or lower (according to OIE specifications).
Performance characteristics: To assess the Diagnostic Sensitivity and Specificity (SnD and SpD, respectively) and to determine the optimal threshold value a positive and negative sera samples (according to IFAT titers, considering this technique as the gold standard) were run in different dilutions (1:40, 1:60 and 1:120). Results were normalized as sample/positive control ratios (S/P%). Frequency distribution curves and a Receiver Operating Characteristic analysis (ROC) of positive and negative samples were constructed to determine the area under the curve and positive and negative predicted values. Test agreement, SnD and SpD were calculated using GraphPad Prism 7 software. The level of agreement between the different tests was expressed as Kappa values (Κ). The agreement was considered low, moderate and high, i.e., 0.4 = κ < 0.6; 0.6 = κ =0.8 and 0.8 = κ < 1, respectively. Mann-Whitney test was used to evaluate whether differences between tests were significant or not.
High-yield screening in pooled samples
In order to assess whether this protocol could be applied to the quick high-yielded screening of bovine neosporosis, different pooled samples were generated. For this purpose, a strong, mid and weak positive sample (IFAT titers 1600, 800 and 400, respectively) was mixed with either 25, 50, 75 or 100 negative samples. Samples were run at different dilutions (1:40 and 1:120) and both the limit of detection and the performance characteristics were determined as described above.
Results
IFAT
A total of 634 bovine sera samples were analyzed by IFAT. This technique was considered as the gold standard. From those, 327 samples were classified as negative (titers lower than 1:25) and 307 as positive; with titers ranging from 1:25 to 1:12800.
Feasibility and standardization
In a first attempt to determine the feasibility of the technique, different concentrations of capture antigen (500, 750 and 1000ng/ well), different dilutions of the sera (1:40, 1:60, 1:120) and two different presentations of the revealing solution were evaluated following a standard protocol. To standardize the final conditions of use sera samples at the optimized dilution were incubated 30 or 40 minutes either at 37°C or at room temperature. Different dilutions of the secondary antibody (peroxidase conjugated) were assessed (1:2500 or 1:5000), whether incubated 20 or 30 minutes at 37°C or at room temperature. Optimal conditions finally stablished are summarized on Table 1.
Step
Optimal condition
Coating
750ng/well in coating buffer. Incubate 6h at 4°C
Washing steps
Wash 3x with PBS-tween 0.05%
Blocking
Block ON at 4°C with 0.5% of the blocking agent
Sera samples
Dilution of use 1:120 (1:40 in the confirmatory assay). Incubate 30 min at room temperatura
Conjugate
Dilution of use 1:5000. Incubate 20 min at room temperatura
Revealing solution
Incubate 10 min at room temperatura in a dark place
Stop solution
Add quickly and read immediately at 450nm
Table 1: Optimal conditions selected for the ELISA protocol after feasibility and standardization was assessed.
Accuracy assessment
A strong (Pos+++), mid (Pos++) and weak (Pos+) positive sample and a negative control (Neg) were run by triplicate in eight independent series in order to determine the intra-plate variability (repeatability). Within each series (triplicate) of the same operator the coefficient of variation for each sample was less than 10% for the 3 out of 4 samples analyzed. Higher variation (10-14%) was observed in 4 series of the weak positive control (Table 2, Figure 1). Variation lower than 10% was observed between the 8 series for each sample (Table 3), within the same plate.
Serie
Sample ID
Media (OD450nm)
SD
CV(%)
1
Pos+++
1.347
0.062
4.602
Pos++
1.062
0.034
3.206
Pos+
0.917
0.081
8.788
Neg
0.292
0.009
2.926
2
Pos+++
1.291
0.072
5.608
Pos++
1.026
0.018
1.769
Pos+
0.793
0.082
10.293
Neg
0.279
0.015
5.321
3
Pos+++
1.221
0.079
6.484
Pos++
0.977
0.008
0.828
Pos+
0.804
0.072
8.946
Neg
0.219
0.007
2.678
4
Pos+++
1.131
0.047
4.112
Pos++
0.903
0.070
7.745
Pos+
0.765
0.048
6.226
Neg
0.245
0.008
3.341
5
Pos+++
1.223
0.050
4.112
Pos++
0.952
0.019
0.197
Pos+
0.796
0.084
10.57
Neg
0.221
0.010
4.355
6
Pos+++
1.212
0.029
2.418
Pos++
0.964
0.018
1.869
Pos+
0.806
0.083
10.3
Neg
0.257
0.008
3.026
7
Pos+++
1.241
0.061
4.88
Pos++
1.018
0.010
1.026
Pos+
0.73
0.100
13.63
Neg
0.266
0.009
3.212
8
Pos+++
1.291
0.020
1.567
Pos++
0.992
0.049
4.973
Pos+
0.786
0.034
4.475
Neg
0.265
0.013
4.712
Table 2: The repeatability or intra-operator variability was assessed by running a strong, mid and weak positive sample and a negative control in eight independent series within the same plate. The average OD (450nm) values, SD and coefficient of variation (CV%) within each serie are depicted.
Sample ID
Media (Series 1-8)
SD (Series 1-8)
CV(%) between series
Pos+++
1.245
0.053
4.223
Pos++
0.978
0.028
2.868
Pos+
0.8
0.073
9.123
Neg
0.258
0.01
3.693
Table 3: Repeatability between independent series for the same operator, indicated with the coefficient of variation (CV%).
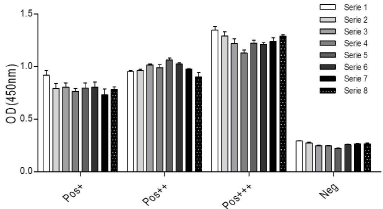
Figure 1: Repeatability was assessed evaluating a strong, mid and weak
positive sample and a negative control (according to IFAT titers). Samples
were run by triplicate in eight independent series within the same plate.
Average values for each sample are shown, different colors indicate different
series.
To assess the reproducibility of the technique, 20 samples were run in 8 independent assays by two operators from different laboratories. The coefficient of variation intra-operator was determined for each sample using raw data (OD values). The mean variation for the operator one was 22.52% and for the operator two was 8.59%, both acceptable levels according to OIE (‹30%, Tables 4a and 4b).
ID
Operator 1 – Independent assays
Media
SD
CV(%)
1
0.48
0.57
0.35
0.65
0.3
0.37
0.4
0.43
0.444
0.12
26.38
2
0.46
0.55
0.36
0.61
0.29
0.38
0.4
0.39
0.429
0.11
24.35
3
0.69
0.77
0.5
0.8
0.49
0.59
0.57
0.68
0.636
0.12
18.5
4
0.77
0.91
0.62
1
0.49
0.65
0.72
0.71
0.732
0.16
22.07
5
1.09
1.21
0.79
1.33
0.68
0.92
1
1.12
1.017
0.22
21.46
6
1.22
1.34
0.91
1.45
0.72
1.01
1.03
1.08
1.094
0.24
21.56
7
1.53
1.66
1.18
1.66
1.16
1.29
1.3
1.33
1.388
0.2
14.37
8
1.55
1.63
1.27
1.9
1.13
1.23
1.32
1.22
1.407
0.27
18.82
9
0.24
0.32
0.22
0.37
0.18
0.23
0.26
0.24
0.258
0.06
23.06
10
0.51
0.55
0.35
0.71
0.37
0.46
0.49
0.49
0.492
0.11
22.93
11
0.27
0.34
0.21
0.36
0.2
0.23
0.24
0.26
0.265
0.06
22.03
12
1.81
2.05
1.61
2.19
1.22
1.33
1.4
1.12
1.59
0.39
24.79
13
0.18
0.23
0.15
0.28
0.12
0.14
0.16
0.22
0.184
0.05
28.75
14
1.29
1.42
0.93
1.55
0.79
0.93
0.98
1.08
1.121
0.27
24.01
15
0.9
1.1
0.66
1.15
0.65
0.75
0.8
0.89
0.864
0.19
21.62
16
0.28
0.35
0.24
0.36
0.2
0.26
0.28
0.29
0.286
0.06
21.83
17
0.3
0.35
0.22
0.52
0.21
0.26
0.28
0.31
0.306
0.1
31.68
18
0.27
0.31
0.2
0.19
0.2
0.21
0.25
0.26
0.236
0.05
18.97
19
0.31
0.37
0.25
0.43
0.24
0.28
0.31
0.33
0.313
0.06
20.36
20
0.28
0.35
0.22
0.41
0.21
0.26
0.28
0.27
0.287
0.07
22.98
Table 4a: Reproducibility test. Twenty randomly selected samples were run in eight independent assays by two operators from different laboratories. The mean OD (450nm) values, SD and CV% for each sample obtained by operator 1 (Table 4a) and operator 2 (Table 4b) is depicted.
ID
Operator 2 – Independent assays
Media
SD
CV(%)
1
0.38
0.34
0.39
0.38
0.36
0.37
0.36
0.36
0.37
0.02
4.1
2
0.36
0.35
0.41
0.45
0.33
0.33
0.32
0.47
0.38
0.06
15.57
3
0.4
0.46
0.43
0.41
0.46
0.43
0.47
0.49
0.44
0.03
6.71
4
0.54
0.51
0.6
0.55
0.53
0.54
0.55
0.54
0.54
0.03
4.78
5
0.79
0.69
0.72
0.71
0.76
0.74
0.77
0.78
0.74
0.03
4.59
6
0.89
0.89
0.83
0.8
0.86
0.84
0.81
0.83
0.84
0.04
4.17
7
1.12
1.1
1.09
0.99
1.13
1.09
1.08
0.98
1.07
0.06
5.26
8
1.06
1.15
1.14
1.1
1.16
1.13
1.12
1.03
1.11
0.05
4.2
9
0.24
0.21
0.27
0.29
0.26
0.26
0.26
0.25
0.25
0.02
8.5
10
0.4
0.36
0.32
0.4
0.4
0.39
0.37
0.38
0.38
0.03
7.34
11
0.24
0.28
0.25
0.28
0.24
0.23
0.22
0.22
0.24
0.02
9.54
12
1.44
1.34
1.35
1.41
1.4
1.37
1.35
1.43
1.39
0.04
2.73
13
0.11
0.19
0.12
0.11
0.13
0.12
0.13
0.13
0.13
0.03
19.75
14
0.8
0.76
0.88
0.81
0.85
0.81
0.84
0.85
0.83
0.04
4.43
15
0.63
0.69
0.64
0.72
0.64
0.67
0.63
0.7
0.66
0.03
5.24
16
0.27
0.29
0.27
0.31
0.26
0.32
0.29
0.28
0.28
0.02
7.63
17
0.26
0.31
0.29
0.22
0.22
0.23
0.27
0.28
0.26
0.03
12.98
18
0.3
0.25
0.22
0.4
0.25
0.27
0.26
0.32
0.28
0.06
19.9
19
0.26
0.3
0.3
0.21
0.25
0.23
0.25
0.27
0.26
0.03
12.22
20
0.27
0.22
0.21
0.28
0.26
0.27
0.28
0.31
0.26
0.03
12.16
Table 4b:
A Mann-Whitney test was carried out to compare the OD values obtained for each sample from operator one (laboratory A) and operator two (laboratory B). No significant differences were observed for 10 out of 20 of the analyzed samples (p>0.05). All of them, however, exhibited acceptable CVs (‹30%).
To assess the precision of the technique the dispersion observed between different series of the negative and positive control samples in 20 independent assays was determined. Results obtained for each sample were very precise, as low level of dispersion was observed, particularly from series 4 to 20 (Figure 2).
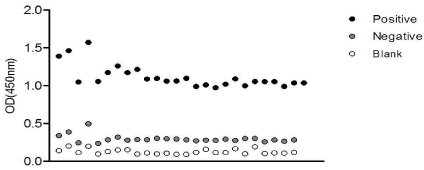
Figure 2: Accuracy test. The dispersion of the positive (black circles) and
negative (grey circles) control and blank values (white circles) from twenty
independent assays is depicted.
Performance characteristics
Cut off values: The identity of the samples was unknown to the laboratory operators to avoid any testing bias. Relative frequency distribution curves (percentages) of positive (n=307) and negative (n=327) samples (final dilution 1:120) were constructed. Two histograms were drawn and their intercept point was considered as the cut-off value (Figure 3). An S/P ratio of 40% was defined as the appropriate cut-off threshold, but as certain overlapping of both curves was observed, samples with S/P values between 30 and 40% were considered doubtful and were run again in a final dilution of 1:40. The cut off value of this confirmatory test was defined as 30% (Figure 4).
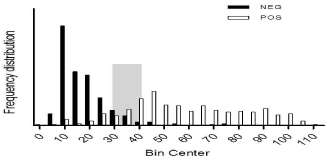
Figure 3: Frequency distribution of the reference positive and negative sera
samples (according to IFAT titers) are shown. The intercept region is depicted
in grey.
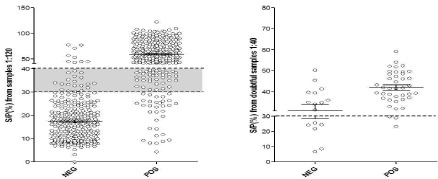
Figure 4: Sensitivity (Sn) and Specificity (Sp) was assessed by a ROC analysis. The doubtful range (grey) and the confirmatory test cut off is shown.
Receiver operating characteristic (ROC) curve analysis: Samples were run in the conditions above described. Starting from 307 positive and 327 negative samples (according to IFAT titers), a total of 285 True Positive (TP), 291 True Negative (TN), 22 False Positive (FP, type I error) and 36 False Negative (FN, type II error) samples were detected. A ROC analysis was assessed to determine the diagnostic Sensitivity (Sn) and Specificity (Sp) of the technique. The area under the curve was 0.9499 (SD=0.009159; p=0.0001; 95% CI). Considering a prevalence of the disease of 30%, the estimated Sn and Sp were 92.50 and 96.81, respectively; with a Positive Predicted Value (PPV) of 0.928 and a Negative Predicted Value (NPP) of 0.889.
Concordance analysis: The estimated accuracy of the technique was 0.908, depicting very good discrimination between N. caninum infected and non-infected cattle. With a 90% of confidence, the Cohen’s kappa coefficient was 0.817, thus showing an almost perfect concordance between ELISA and IFAT. A significant correlation between both techniques was observed (p‹0.0001; IC; 95%; Spearman r=0.7903; data not shown).
High-yield screening in pooled samples
Pooled samples containing one strong (Pos+++), mid (Pos++) or weak (Pos+) positive serum within 25, 50, 75 or 100 negative samples were run at different dilutions (1:120 and 1:40) following the protocol above standardized. One strong or mid positive serum (with IFAT titers ranging from 1:1600 to 1:800) pooled with up to 100 negative samples was detected when samples were diluted 1:40 (Table 5). To assess the SpD and SnD and determine the most suitable cut off value, the total panel of 634 samples was run at this dilution and a ROC analysis was carried out. Under these conditions, pooled samples with S/P values greater than 30% were considered positive, with a SnD of 90.09% and a Sp of 90.10 (95% CI, Figure 5). The area under the curve was 0.9433 (95% IC, 0.9243-0.9623).
Pool ID
OD(450nm)
S/P(%)
0.13
1:40
0.125
1:40
Neg 25
0.27
0.41
15.13
20.13
Neg 50
0.4
0.54
22.62
26.16
Neg 75
0.35
0.5
19.76
24.45
Neg 100
0.57
0.55
32.61
26.59
Pos+++ 25
0.69
1
39.24
48.66
Pos+++ 50
0.57
0.78
32.44
37.87
Pos+++ 75
0.52
0.78
29.93
37.73
Pos+++ 100
0.57
0.85
32.5
41.23
Pos++ 25
0.36
0.83
20.67
40.11
Pos++ 50
0.39
0.75
22.05
36.56
Pos++ 75
0.36
0.8
20.33
38.65
Pos++ 100
0.35
0.71
20.05
34.37
Pos+ 25
0.26
0.47
14.56
22.9
Pos+ 50
0.35
0.59
19.87
28.54
Pos+ 75
0.32
0.6
18.22
29.22
Pos+ 100
0.12
0.61
6.682
29.51
Table 5: OD (450nm) and S/P values obtained in pooled samples diluted 1:120 or 1:40 are shown. The highlighted values indicate the detection range.
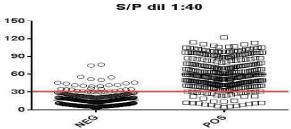
Figure 5: ROC analysis of the high-yield screening assay. The full line
indicates the cut off value.
Discussion
Amongst serological diagnostic tools, ELISAs have become the most preferable technique to detect the presence of specific antibodies against N. caninum in bovine serum samples. When correctly validated, they can provide reliable information allowing the screening of large herds in few hours. Unfortunately, national commercial kits are not available in Argentina and they must be purchased abroad, leading to very high costs due to importation taxes. In this context, local farmers tend to delay the diagnose, underestimating the prevalence and local impact of this disease. What is more, the lack of accuracy in the diagnosis of bovine neosporosis also impacts globally, as there is no a standardized method to assess the global prevalence of this disease [32].
One problem in the validation of N. caninum diagnostic tests is the lack of an appropriate gold standard as, in general, no perfect tests are available for the detection of N. caninum infection [33]. In the absence of routine direct tests in adult cows enabling accurate results, and in the absence of a large panel of fully characterized samples, a no gold standard (e.g. Bayesian) analysis is sometimes advised [34]. However, IFAT has generally been used as a reference test in the evaluation of other diagnostic tests and different versions of IFATs have routinely been used in both diagnostic and research laboratories, despite its subjective reading and substantial between-laboratory variation [33]. To establish appropriate control measures for bovine neosporosis is essential to understand the performance of the different available ELISAs. The difference between these serological tests, as well as the multiple versions of test protocols in use, make it difficult to compare test results from different studies. Therefore, it is essential that proper evaluations are made for the tests used.
Here we have developed and validated an ELISA kit locally produced; which enables the quick, objective and economic diagnosis of bovine neosporosis; avoiding delays and high importation taxes. This technique also shows objective results; as the lecture of the row data does not depend on the operator. Our results showed an almost perfect level of concordance with the gold standard technique; with a very good SeD and SpD. It is also very useful to perform high-yielded screening of large herds quickly; by pooling up to 100 samples.
Acknowledgment
Funding for developing and validating the present assay was from the National Agency for the Promotion of Science and Technology (ANPCyT) and National Council of Scientific and Technical Research (CONICET) from Argentina. FCM is researcher of the National Institute of Agricultural and Livestock Technologies (INTA). AVC and NPC are researchers of CONICET. WRP and IG are doctoral and post-doctoral fellows of CONICET, respectively. CST is postdoctoral fellow of ANPCyT.
References
- McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. 1998; 28: 1473-1478.
- Dubey JP, Schares G. Neosporosis in animals-the last five years. Vet Parasitol. 2011; 180: 90-108.
- Dubey JP, Dubey J. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol. 2003; 41: 1-16.
- Haddad JP, Dohoo IR, VanLeewen JA. A review of Neospora caninum in dairy and beef cattle--a Canadian perspective. Can Vet J. 2005; 46: 230-443.
- Thurmond MC, Hietala SK. Effect of congenitally acquired Neospora caninum infection on risk of abortion and subsequent abortions in dairy cattle. Am J Vet Res. 1997; 58: 1381-1385.
- Thurmond MC, Hietala SK. Effect of Neospora caninum infection on milk production in first-lactation dairy cows. J Am Vet Med Assoc. 1997; 210: 672- 674.
- Innes EA, Wright S, Bartley P, Maley S, Macaldowie C, Esteban-Redondo I, et al. The host-parasite relationship in bovine neosporosis. Vet Immunol Immunopathol. 2005; 108: 29-36.
- Reichel MP, Alejandra Ayanegui-Alcérreca M, Gondim LF, Ellis JT. What is the global economic impact of Neospora caninum in cattle - the billion dollar question? Int J Parasitol. 2013; 43: 133-142.
- Monney T, Hemphill A. Vaccines against neosporosis: What can we learn from the past studies? Exp Parasitol. 2014.
- Reichel MP, Ellis JT. If control of Neospora caninum infection is technically feasible does it make economic sense? Vet Parasitol. 2006; 142: 23-34.
- Reichel MP, Ellis JT. Control options for Neospora caninum infections in cattle-current state of knowledge. N Z Vet J. 2002; 50: 86-92.
- Hall CA, Reichel MP, Ellis JT. Neospora abortions in dairy cattle: diagnosis, mode of transmission and control. Vet Parasitol. 2005; 128: 231-241.
- Moen AR, Wouda W, Mul MF, Graat EA, van Werven T. Increased risk of abortion following Neospora caninum abortion outbreaks: a retrospective and prospective cohort study in four dairy herds. Theriogenology. 1998; 49: 1301- 1309.
- Cabral AD, Camargo CN, Galleti NT, Okuda LH, Pituco EM, Fava CD. Diagnosis of Neospora caninum in bovine fetuses by histology, immunohistochemistry, and nested- PCR. Rev Bras Parasitol Vet. 2009; 18:14-19.
- Atkinson R, Harper PA, Reichel MP, Ellis JT. Progress in the serodiagnosis of Neospora caninum infections of cattle. Parasitol Today. 2000; 16: 110-114.
- Wouda W, Dubey JP, Jenkins MC. Serological diagnosis of bovine fetal neosporosis. J. Parasitol. 1997; 83: 545-547.
- Ghalmi F, China B, Kaidi R, Losson B. First epidemiological study on exposure to Neospora caninum in different canine populations in the Algiers District (Algeria). Parasitol Int. 2009; 58: 444-450.
- Ghalmi F, China B, Jenkins M, Azzag N, Losson B. Comparison of different serological methods to detect antibodies specific to Neospora caninum in bovine and canine sera. J Vet Diagn Invest. 2014; 26: 136-140.
- Björkman C, Näslund K, Stenlund S, Maley SW, Buxton D, Uggla A. An IgG avidity ELISA to discriminate between recent and chronic Neospora caninum infection. J Vet Diagn Invest. 1999; 11: 41-44.
- Björkman C, McAllister MM, Frössling J, Näslund K, Leung F, Uggla A. Application of the Neospora caninum IgG avidity ELISA in assessment of chronic reproductive losses after an outbreak of neosporosis in a herd of beef cattle. J Vet Diagn Invest. 2003; 15: 3-7.
- Björkman C, Gondim LF, Näslund K, Trees AJ, McAllister MM. IgG avidity pattern in cattle after ingestion of Neospora caninum oocysts. Vet Parasitol. 2005; 128: 195-200.
- Björkman C, Alvarez-Garcia G, Conraths FJ, Mattsson JG, Ortega-Mora LM, Sager H, et al. Neospora caninum IgG avidity tests: an interlaboratory comparison. Vet Parasitol. 2006; 140: 273-280.
- Sager H, Gloor M, Björkman C, Kritzner S, Gottstein B. Assessment of antibody avidity in aborting cattle by a somatic Neospora caninum tachyzoite antigen IgG avidity ELISA. Vet Parasitol. 2003; 112: 1-10.
- Dubey JP, Schares G. Diagnosis of bovine neosporosis. Vet Parasitol. 2006; 140: 1-34.
- Campero LM, Minke L, Moré G, Rambeaud M, Bacigalupe D, Moore DP, et al. Evaluation and comparison of serological methods for the detection of bovine neosporosis in Argentina. Rev Argent Microbiol. 2015; 47: 295-301.
- Mansilla FC, Czepluch W, Malacari DA, Hecker YP, Bucafusco D, Franco- Mahecha OL, et al. Dose-dependent immunogenicity of a soluble Neospora caninum tachyzoite-extract vaccine formulated with a soy lecithin/beta-glucan adjuvant in cattle. Vet Parasitol. 2013; 197: 13-21.
- Dubey JP, Hattel AL, Lindsay DS, Topper MJ. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc. 1988; 193: 1259-1263.
- Venturini MC, Venturini L, Bacigalupe D, Machuca M, Echaide I, Basso W, et al. Neospora caninum infections in bovine foetuses and dairy cows with abortions in Argentina. Int J Parasitol. 1999; 29: 1705-1708.
- Paré J, Hietala SK, Thurmond MC. An enzyme-linked immunosorbent assay (ELISA) for serological diagnosis of Neospora sp. infection in cattle. J Vet Diagn Invest. 1995; 7: 352-359.
- Moore DP, Echaide I, Verna AE, Leunda MR, Cano A, Pereyra S, et al. Immune response to Neospora caninum native antigens formulated with immune stimulating complexes in calves. Vet Parasitol. 2011; 175: 245-251.
- Mansilla FC, Franco-Mahecha OL, Lavoria M, Moore DP, Giraldez AN, Iglesias ME, et al. The immune enhancement of a novel soy lecithin/β- glucans based adjuvant on native Neospora caninum tachyzoite extract vaccine in mice. Vaccine. 2012; 30: 1124-1131.
- Benavides J, Collantes-Fernández E, Ferre I, Pérez V, Campero C, Mota R, et al. Experimental ruminant models for bovine neosporosis: what is known and what is needed. Parasitology. 2014:1-18.
- Frössling J, Bonnett B, Lindberg A, Björkman C. Validation of a Neospora caninum is com ELISA without a gold standard. Prev Vet Med. 2003; 57: 141-153.
- Roelandt S, Van der Stede Y, Czaplicki G, Van Loo H, Van Driessche E, Dewulf J, et al. Serological diagnosis of bovine neosporosis: a Bayesian evaluation of two antibody ELISA tests for in vivo diagnosis in purchased and abortion cattle. Vet Rec. 2015; 176: 598.